Abstract
The complexity of the effects of greenhouse gases and especially nitrogen emission on atmospheric aerosols and nitrogen deposition has been puzzling for decades. Moreover, their effects on public health and biodiversity have increased the notion of urgency for drastic reforms and, as a result, have increased the complexity of the debate. This paper focusses on the interaction between agricultural sources of N-consumption and emissions and other anthropogenic activities as well as on the role of N-deposition on plant physiology and biodiversity impairment. Finally, the urgency of farmland reforms is situated in a socio-economic and historical context.
Keywords: Atmospheric aerosol formation; Anthropogenic effects; Agricultural N-emission and N-deposition; Biodiversity paradigm; Public Health; History of farmland reforms
Introduction
The negative impacts on the Earth’s troposphere and biosphere of anthropogenic emissions of greenhouse gases [1-3], of anthropogenic aerosol formation [4,5] and the vertical distribution and degradation of stratospheric ozone [6-8] has been known and is well-documented already for several decades. Various modeling studies have addressed the role of cloud formation, rainfall and meteorological phenomena in the formation, vertical distribution and geographical spread of these chemical substances and of aerosols in particular [9-14]. Also the specific contributions of human traffic, industrial and agricultural activities on the emissions of S- and N-containing chemicals have been extensively estimated or measured at global and local scales, leaving little doubt as to the anthropogenic origin of these effects [15,16]. There is some consensus about the detrimental effects of smog, mineral dust and aerosols on public health [17].
The impact of nitrogen-deposition on (plant) toxicology and pollution also has been demonstrated long ago [18]. Moreover, nitrogen (N) has been designated as the limiting nutrient for many ecosystems, and, as a result, even a small increase in N-supply might in some cases lead to significant negative changes of ecosystem resilience (and biodiversity) [16]. In terrestrial ecosystems, ammoniaderived N may be takeup directly through the plant leaves or indirectly through the root as N-rich soil water [16,18]. Indirectly, saturation of terrestrial ecosystems with N leads to a washout of soil nitrate (NO3-) and a slow acidification of the soil and loss of other soil-bound ions [16]. As a result, in European rural areas, agriculture-derived N-deposition is designated as the primary source for biodiversity loss [16].
Important questions remain about the role of biogenic emissions in marine environments [19], and the relative contribution of agricultural, industrial and traffic-born emissions on aerosol formation in relation to the weather and/or meteorological conditions.
Most puzzling, however, is the question why the political doctrine of industrially scaled meat production and the use of N-rich fertilizers, feeding with N-rich livestock fodder and disposal of livestock manor on farmland has been continuously promoted for decades, in countries where the emission and N-deposition were known to be too high. In the words of the special mediator appointed for the nitrogencrisis in the Netherlands (2022), Johan Remkes, former deputy prime minister of the Netherlands, a radical fifty % reduction of the national livestock, and a complete stop of peak pollutants nearby natural reserves will be inevitable, to prevent the country going into an economic lock-down. It means that either an economic lock, i.e. all other economic activities taking part into nitrogen-emission have to be frozen down to zero, or, a dramatic reduction of all activities related to animal farming is mandatory to ‘unlock’ the country. The urgency question of farmland reforms will be discussed later in this paper (see 7. The urgency of farmland reforms and political decisionmaking).
How could the situation come this far?
The deposition of N-rich mineral particles mainly is the result of the following reactions, the uptake and neutralization of gaseous ammonia (NH3) with sulfuric acid (H2SO4) (basically the reaction occurs in two steps) or with nitric acid (HNO3) [16]:
2 NH3 + H2SO4 → (NH4)2SO4
NH3 + HNO3 ↔ NH4NO3
The reaction with nitric acid is reversible (in contrast to the reaction with sulfuric acid). The atmospheric concentration of nitrogen oxides (NOx) has a dominant part originating from anthropogenic sources other than agriculture, namely traffic, industry, tourism, etc. Moreover, the formation of nitric oxide in the troposphere largely depends on the presence of ozone (O3) and radical molecules that originate from O3 breakdown, NO emission and NO formation. The fraction of NO in the total emission of nitrogen oxides (NOx) plays a key-role in the oxidation processes that are in favor of the formation of O3 and HOx radicals (20) (see 3. Atmospheric aerosol formation and anthropogenic activities). Water-borne radicals indeed form an very important link in the photo-oxidative destruction of ozone [19].
The deposition of N-rich aerosols thus is largely dependent on the meteorological conditions. An important distinction has to be made between dry and wet deposition [16]. Average deposition data form the usual instruments to calculate the relative contribution of various anthropogenic sources in relation to the total N-deposition [21]. Hereby, the notion of the half-live of aerosol particles and the average deposition radius from the emission source may become important key elements in the analysis of various contributions (see 4. Agricultural NH3 emission and deposition).
In order to understand the impact of N-emission and deposition on ecosystem biodiversity, it is equally important to understand the mechanisms of ecosystem development and biodiversity resilience (see 5. The biodiversity paradigm). Recent meta-analyses of multi-decadal biodiversity trends in Europe have revealed an amalgam picture of the effect of changing land use and climate change upon the major biodiversity indexes in the European continent [22]. For instance, on the one hand a decreased abundance of terrestrial invertebrates is found, but an increased richness of birds and marine invertebrates. Also a decreased diversity in benthic algae, but increased diversity in birds and aquatic invertebrates, as well as an increased turnover in plants (following an influx of new species) were the result of the metaanalysis [22]. Previously, models to incorporate different taxonomic and heuristic levels of biodiversity analysis have been presented [23,24]. In the present paper, we will discuss the implications of the nitrogen issues (as summarized above) on biodiversity trends and will investigate the key determinants of the urgency paradigm of a potential ecosystem collapse.
Atmospheric Aerosol Formation and Anthropogenic Activities
In the troposphere, a complex and highly variable interplay has been discovered between the processes causing the physical transport and chemical destruction of ozone (O3) and the nitrogen oxides and other precursors of O3-formation [20] (Figure 1). Whereas water (H2O) and sunlight are important for the photolysis of O3, resulting in the production of various free radical molecules (like hydroxyl [OH] and peroxyl- [HO2]radicals), these radicals together with nitrogen oxides (NOx) are determining the production of O3 too. The resulting production and decay cycles of tropospheric ozone form a multileveled dynamic equilibrium system, in which the ratio of NO2 to NO concentrations is crucial for the photo-stationary state between NO, NO2 and O3 [20]. It appears that there is a sink for ozone (namely the O[1D] reaction with H2O) [25] and a source for ozone, resulting from the coupling between HOx and NOx pathways [20]. Moreover, radicals of the so-called ‘odd hydrogen family’ (defined as HOx = [OH] + [HO2]) play a key role in the oxidation of SO2, NO2, as well as in the breakdown of hydrogen peroxide (H2O2) and also in the formation of so-called peroxyacetylnitrate (PAN). PAN is a secondary pollutant present in photochemical smog, originating from various carbonyl compounds present in polluted air, like acetaldehyde (CH3CHO) and acetone (CH3COCH3) [20]. Interestingly, an important observation made by aircraft observations was the much higher [OH] radical concentrations above clouds compared to clear skies [26,27]. It remains to be investigated whether the importance of these photochemical processes will augment in a changing climate with increasing average temperatures and increased water volume transport during precipitation [28].
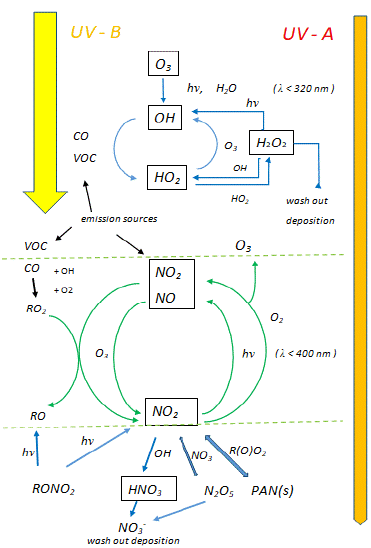
Figure 1: Scheme representing the chemical processes affecting the
distribution of tropospheric ozone, with special emphasis to the roles of nitric
oxides (NOx) (lower half), hydroxyl radicals (OH) and other members of the
odd hydrogen family (HOx) (upper half) as well as the roles of ultraviolet
A- and B-radiation (modified after S.A. Penkett, et al. [20]; courtesy of S.A.
Penkett, University of East Anglia, Norwich, England). VOC = Volatile Organic
Carbons (for other abbreviations and chemical formulas see main text).
Schematically, the key chemical reactions are summarized here:
1) regarding the photolysis of nitric oxide and the formation/ degradation of ozone, due to the coupling of the following reactions :
NO2 (+ hν) → NO + O (λ< 400 nm) (k1)
NO + O3 → NO2 + O2 (k2)
O + O2+ M → O3 + M
d[O3]/dt = k1[NO2] / k2 [NO]
2) regarding the photolysis of ozone in the presence of water, carbon monoxide (CO) and radicals of the ‘odd hydrogen family’ [20]:
O3 (+ hν) → O (¹D) + O2 (λ< 320 nm)
O (¹D) + H2O → OH + OH
OH + CO → H + CO2
H + O2 + M → HO2 + M
HO2 + O3→ 2 O2 + OH
The latter reaction occurs at low NOx concentrations, whereas in the presence of methane (CH4) and higher concentrations of NOx the removal of OH and HO2 radicals may occur through the following chains of reactions [20]:
3) formation of methane radical and oxidation of this radical to formaldehyde and HO2:
OH + CH4 → H2O + CH3
CH3 + O3→ HCHO + HO2
(It has been suggested that the high OH radical concentrations lead to the formation of high O3 loads during the (wet) summer period, due to the OH-induced destruction of hydrocarbons and resulting reaction chains [16]).
4) formation of hydrogen peroxide and nitric acid:
2 HO2→H2O2 + O2 (removal of HO2)
OH + NO2 + M →HNO3 + M
Reactive nitrogen oxides are an important source for larger aerosol molecules, like peroxyacetyl nitrate (PAN), which can become an additional source for NO2 through decomposition of PAN, or photolysis and oxidation of HNO3 [20]:
5) formation of PAN from acetaldehyde (CH3CHO) or acetone(CH3COCH3), through the reaction with oxygen and methane and/or hydroxyl radicals:
CH3CHO + OH (+O2) → CH3C(O)OO + H2O
CH3COCH3 (+O2) → CH3C(O)OO + CH3
CH3C(O)OO + NO2 ↔ CH3C(O)OONO2 (PAN)
The latter equation describes the dynamic equilibrium for the formation of PAN. There is some similarity with the formation of ammonium nitrate (see 2. Introduction ), although in the atmosphere, in the photo-oxidative chemical processes described above, ammonia (NH3) doesn’t take much part, if any at all [20]. In the presence of ozone, NH3 is completely oxidized to nitrate, at least in wastewater [29]. Meteorological measurements of the vertical distributions of ammonia and related N-gases in aerosols revealed an upward NH3 flux of 0.12 μgm-2s-1 [30]. Moreover, a close correlation between the concentrations of NH3 and HNO3 gases was found, based on the concentration product [NH3 (g)] x [HNO3 (g)] (at temperatures above 0°C and relative humidity below 80 %). However, in other cases, higher NH3 and/or HNO3 concentrations in the gas phase were measured, deviating from the theoretically predicted values [30].
One of the most puzzling phenomena is that although HNO3 has a much shorter half-life in the atmosphere compared to the halflife of NH3 (which therefore could be transported over much longer distances), it nevertheless has been estimated that about 50% of the NH3 emission is deposited within 50 km of the emission source [16].
The multitude of chemical reactions, both of anthropogenic or natural processes, are not the only cause of aerosol formation. The presence of enormous amounts of fine particles, of organic or inorganic source, from concentrated sea salt droplets to desert dust and plant pollen, they all create a diversified array of habitats to generate atmospheric aerosols [31]. Many of these processes are indirectly related to climate warming, to increased water evaporation or directly related to human activities (such as deforestation and desertification). Most of these effects have a negative impact on the climate, although a significant counteractive effect on radiative forcing (through greenhouse gases) has been demonstrated too [32]. As aerosol particles are strongly coupled with the gas-phase chemistry of the atmosphere including the clouds, in order to understand their properties and effects on climate and health, the gases, aerosol particles and clouds are regarded as one continuum, a single system, with scales ranging from molecules and nanometersized aerosol particles to frontal cloud systems spanning hundreds of squared kilometers [31]. These systems can react as sinks for reactive species and therefore may decrease or increase radiation-driven photochemical effects [31]. The influence of clouds on OH radical formation, as mentioned above [26,27], may extend as well to the much larger and diverse group of atmospheric chemicals (mentioned as well as others). For reasons of manageability and biological impact, we will hereafter focus on the lower troposphere and the impact of agricultural NH3 emission and deposition (see 4.Agricultural NH3 emission and deposition).
Agricultural NH3 Emission and Deposition
It is important to note that most of the data in the present paragraph are derived from European studies and are best related to the European situation in agricultural and other forms of land use [18]. There is ample evidence that terrestrial NH3-emission is predominantly derived from agricultural activities and these have (or had) a strong negative effect on plant biodiversity [16,18]. In Europe, there is also quite some seasonal variation in NH3-emission which obviously follows the seasonal variation in agricultural activities. Field data in Denmark (referring to the period 1989-2003) have revealed a change of the peak emissions in spring and/or summer following the utilization of animal manure for crop fertilization, as a result of changes in the local legislation [16]. Peak NH3-pollutions that are strongly correlated with animal production farms are reported in several European countries, although these emission data haven’t been easily accessible to the public in previous decades.
However, a most important distinction has to be made regarding the dry and wet deposition of N-rich chemicals. Herein, it is obvious that the meteorological conditions play a decisive role too, in the proportion and source of agricultural versus other sources of N-deposition [16] (Figure 2). Whereas, nitrate-derived nitrogen is assimilated in plants, often in synergistic collaboration with fungal mycorrhiza [33], ammonia-derived nitrogen first needs to be nitrified (i.e. oxidized) through specific bacteria and other soil microbial organisms. NH3 appears to stick to plant leaves and other surfaces. Adverse effects on vegetation occur when the rate of foliar uptake (through leaves) exceeds the capacity for detoxification of living plants [18]. In higher plants NH3 uptake occurs mostly through the shoots, whereas NH4+ uptake occurs through both shoots and roots [18].
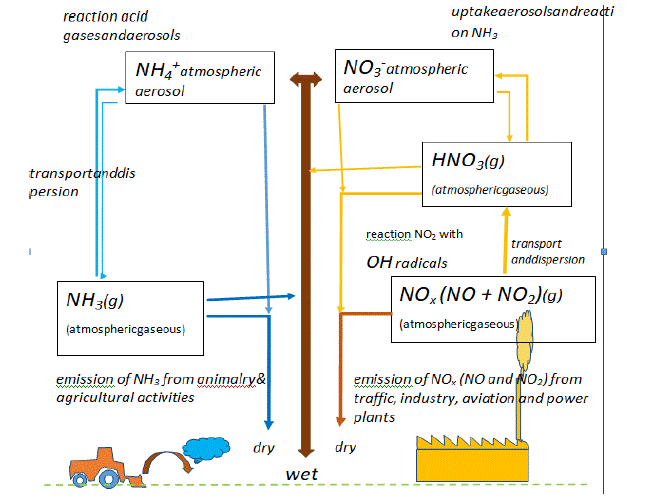
Figure 2: Scheme representing the chemical interactions in the lower atmosphere between agricultural N-emissions (molecules of the NHy group, left) and other
sources of N-emission (molecules of NOx group, right) (modified after O. Hertel, et al. [16]; Danish National Environmental Research Institute, Roskilde, Denmark).
In the assimilation and detoxification of the couple NH3↔NH4+ (shortly denoted as NHy), a significant role is played by the Glutamine Synthase (GS) – glutamate synthase (GOGAT) circular pathway [34]. Besides this, also glutamate dehydrogenase activity has an important contribution to the regulation of glutamate in higher plants [35]. The GS-COGAT pathway moreover is coupled to the Krebs cycle, through the reaction of 2-oxoglutaric (also known as a-ketoglutaric acid), formed from citric acid in the Krebs cycle, with glutamine resulting in the formation of glutamate (Figure 3). This reaction also depends on the presence of the Nicotinamide-Adenine-Dinucleotide- (Phosphate)-enzyme (NAD(P)H) – Ferredoxin (Fd) enzyme complex, which is present in the chloroplast and root cell plastids of higher plants [18]. As a result, NHy uptake and assimilation results in enhanced amino acid and protein metabolism. In a number of studies [18], for instance in coniferous trees, increased concentrations of free N-rich amino acids (especially arginine) were found in pine needles [36]. In the latter study, in the coniferous trees studied, also a correlation of high N-availability with the occurrence of the parasitic fungus Sphaeropsis sapinea was detected [36]. Other distortions like an altered crop to weed ratio would result from too high NHy assimilation [18]. When the relation with epiphytic lichens and the air pollution with SO2 is considered, it appears that ‘nitrophytic’ lichens that are more sensitive to SO2, respond more rapidly than others to sudden decreases in SO2 and consequently nitrophytic species may colonize new sites more rapidly [37]. N-deposition therefore may interfere with the species balance in ecosystems and consequently may have an impact on biodiversity (see 5. The biodiversity paradigm).
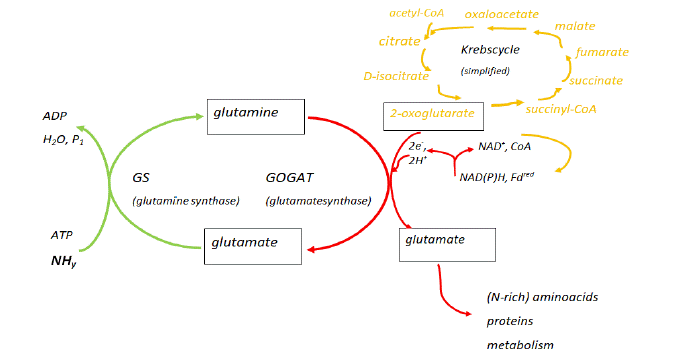
Figure 3: Scheme representing the interaction of NHy assimilation in plants with the glutamine synthase (GS) - glutamate synthase (GOGAT) pathway and Krebs
cycle. (modified after S.V. Krupa [18]; Department of Plant Pathology, University of Minnesota, USA) (for abbreviations and chemical formulas see main text).
Taking into account the adverse effects of NH3-assimilation, including the complex mechanisms of N-saturation through NH4+ and NO3- retention and/or NO3- washout from forest soils, studied in North-American [38,39] and European forests [40], a critical load for total N-deposition was repeatedly suggested [41]. Such critical N-deposition loads have been adopted at a Convention on Long- Range Transboundary Air Pollution [18,42]. It was suggested that a critical load of 5-10 kg ha-1 yr-1 of total N-deposition (both dry and wet deposition combined of all atmospheric N substances) would be sufficient to protect the most vulnerable terrestrial ecosystems [18]. In forests, a somewhat higher critical load of 10-20 kg ha-1 yr-1, depending on the soil conditions, was estimated (depending on the N-saturation and competition for N-availability in the tree species) [18].
Meanwhile in the Netherlands, the total N-deposition has decreased from above 2700 mol N/ha in 1990 to about 1500 mol N/ ha in 2010 [43]. Between 2010 and 2020, however, the N-depostion showed no further decline, due to the lack of further lowering of the reduced (NHy-derived) nitrogen from agricultural activities [43]. The oxidized (NOx-derived) nitrogen on the other hand, showed a prolonged steady decline, also as a result of the use of better NOx- free combustion engines in traffic, with even an increased decline during the corona lockdown. The corresponding mass data of NOx- deposition, roughly equals a reduction from ca. 62 kg ha-1 yr-1 to ca. 18 kgha-1 yr-1(of which, in the case of nitrate, roughly 22, 6 mass % is attributable to N), versus a steady 20 to 25 kg ha-1 yr-1 for the reduced NHy-derived deposition (of which roughly 80 mass % is N). So, despite the enormous efforts to diminish the total N-deposition, the effects of agricultural reduction measures on N-deposition have been much more modest. The socio-economic consequences of this failed reduction policy and measures will be the main topic of paragraph 7 (see 7. The urgency of farmland reforms and political decisionmaking).
The Biodiversity Paradigm
The worldwide reduction of 69% (since 1970) of the number and abundance of wild animals, as reported by the World Wildlive Fund [44], clearly shows the urgency of the biodiversity question. But, despite the international consensus and agreement resulting in authoritative declarations and manifestos [45,46], the aim to achieve a significant reduction of worldwide biodiversity loss by 2010, was not corroborated by the recent WWF-conclusions [44]. A lot of emphasis has been given to different rates of biodiversity reduction, especially when correlated to the local as well as global trends in deforestation and in particular the devastating effects of destroying tropical rain forests. Also in the moderate climates of Europe, Asia and North America, the biological diversity is under pressure. Regarding the effects of intensive, N-enriching agricultural activities, it has been estimated that, for instance in the Netherlands, several tens to hundreds of plant species have disappeared from rural areas since the introduction of modern fertilization techniques [47] (see also 7. The urgency of farmland reforms and political decision-making).
However, a serious methodological problem may hamper the implementation of the biodiversity convention recommendations [45], namely concerning the question how to estimate biodiversity at a local scale. It is not only because numerous new species are discovered at remote areas and habitats that are difficult to investigate, because local estimates of a reduced biodiversity can be directly or indirectly inferred from the total of human activities [48]. Neither it is because of the complex interactions between trophic levels and the role of migration and long-range interactions that affect the survival of species [23,49,50]. An abridged definition of biodiversity that is contained within one or two trophic levels, is by definition insufficient to explain multiple interactions between trophic levels (or ecosystem niches) and the chemical composition of the environment [24,51]. This is especially of concern in the case of N-induced biodiversity impairment. Moreover, the multitude of factors affecting the diversity of biological life forms, from local nitrogen deposition to global warming and other climate effects, has revealed an amalgam picture, at least when considering the recent decades in Europe [22]. They encompass divergent effects of both local increases as well as decreasing numbers and diversity estimates, a higher turnover rate of plant and other species groups (e.g. influx of bacteria, fungi and plant species from warmer climate zones) [22].
The biodiversity paradigm therefore forms a challenging paradigm for the creed to ‘act locally and think globally’. When translated into terms of socio-economic change, and especially when defining the urgency of these measures, it seems inappropriate not to consider the full complexity of the biodiversity problem [51]. Moreover, to ‘act locally’ shouldn’t be interpreted too rigorously, for minor measures to mitigate the negative effects of e.g. deforestation, may not suffice to counteract the gross impact of habitat fragmentation. On the other hand, biodiversity hot spots consisting of highly miniaturized biological habitats (within larger ecosystems) are easily destroyed when restoration measures are organized at a scale that is too coarse, because of the use of too heavy material or for economic reasons.
Effects on Human Health
In addition to the biodiversity impairment, the effects of N-deposition and anthropogenic aerosols on human well-being and health should not be under-estimated [52]. A lot of progress has been made in medical diagnosis and monitoring, since the first discoveries of serious health impairment due to polluted air inhalation in smog areas [53], already long before the Great Smog of London in 1952, when some 4000 deaths were counted in only one week [54]. In the past decades however, the problem of air pollution has travelled from the London city to the megacities and densely populated, industrialized and heavy traffic zones of the globe, especially in the developing countries and in China [55,56]. Air pollution was found to be the cause and established aggravating factor of a number of respiratory diseases like COPD, asthma [57,58] and lung cancer [59,60].
The list of detrimental health effects accompanying the industrialized meat production and meat consumptive habits has been extended recently, including the food contamination with dioxins and PCBs (polychlorinated biphenyls), micro-plastics, et cetera. However, one may ask whether such an orchestrated action against animal farming is really accountable to the agricultural activities and the contribution thereof to the environmental problems. On the other hand, the aggravating effects of a weakened respiratory system has proven to dramatically augment the morbidity in large groups of the population, during the recent corona pandemic(s) [61]. The pandemonium of health effects therefore emphasize the urgency of measures to counteract the nitrogen emission and aerosol pollution all the more.
The Urgency of Farmland Reforms and Political Decision-Making
It is well documented that the Netherlands occupy a frontrunner position in Europe, with respect to the consumption of nitrogen in agriculture, namely about 166 kg ha-1 yr-1, versus an average N-consumption of 48 kg ha-1 yr-1 in Europe (reference year 2019) [62]. These amounts of N-consumption are mainly due to the use of N-rich fodder and N-rich fertilization of farmland of which - again (!) - about 58 % is livestock-derived manure, the other part being derived from fertilizers [63]. From this yearly total N-load of about 669 million kg for the whole of the Netherlands (including some 18 million kg N-deposited from air)(reference year 2020), roughly 13,2% or 88 million kg nitrogen yr-1 is lost to the air, where as 32,7% is lost to the soil (corresponding to a national total of 307 million kg yr-1) [63].
These problems aren’t new, and the recent national farmer protests and upheaval of certain protest groups may seem strange to the fellow inhabitants of the world. But these reactions are not so strange, if they are put in a political and historical context. Not fortuitously, it was a Dutch minister of agriculture, Sicco Mansholt (1908-1995), who proposed the European Commission in 1952 an ambitious plan to re-organize European agriculture [64]. Mansholt became the first Commissioner for agriculture in the European Commission in 1958. The Mansholt-plan consisted of harmonizing the conditions of agricultural production, the settling of commercial prices (and fixing minimum prices to protect the farmer’s income) and the regulation of import, and export of agricultural products [64].
The fact that the Netherlands became a very ‘successful’ export nation for both meat and crop products, was not in the least a byproduct of the exploitation - since the early 1960s - of gas resources resulting in the availability of ‘cheap’ hydrogen. This hydrogen also became available for industrialized ammonia and ammoniumnitrate production for the fertilizer market. The discoveries of chemical techniques for ammonia and fertilizer production by the Germans Justus von Liebig (1803-1873), Fritz Haber (1868-1934) and Carl Bosch (1874-1940) (the so-called Haber-Bosch process for NH3 production) in the early twentieth century, had enabled these chemical and economic breakthroughs in a previous era. However, the negative fame of these chemists, mainly due to their association with the chemical industries involved in toxic gas production, did not prevent the Nobel committee to award to Haber the Nobel prize for chemistry in 1918. According to some historical sources, both von Liebig and Mansholt afterwards deplored the impact of their chemical discoveries/agricultural reforms, respectively. Von Liebig warned against the large scale usage of chemical fertilizers already in 1861 [65]. Mansholt allegedly was also very disappointed, but the agricultural reforms would become irreversible, possibly due to the influential pressure of agricultural lobbyists.
It is obvious that the present conditions of 2022, both politically as regarding the ‘cheap’ exploitation of gas reserves in the Groningen area [66], have come to an immanent turning point. The largest gas reserves on the European continent, allegedly some 300 billion cubic meters [67], no longer are regarded as protecting the Dutch welfare state economy, since the gas exploitations are also causing billions of damages to housing, due to the numerous earthquakes in the Groningen area [66]. Within these socio-economic and environmental contexts, the recent political adaptations are more easily apprehended, but not at all justified.
Political adaptations require decisive choices to be made, but sometimes it is rather about avoiding the wrong choices. The choice of augmenting the conditions for food production in the postwar rural areas of Europe, e.g. given the limited availability of phosphorus in Dutch peaty soils [47], apparently is an illustrative example given the present N-crisis. Re-allotment of farmland was considered a necessary step in consolidating the up-scaling of agricultural activities, following the Mansholt reforms. But it also resulted in a dramatic loss of linear landscape elements and the impairment of biodiversity hot spots. The risks of forced collectivization of private farms, as experienced in other regions of the world before, were also mentioned by Mansholt in his Paris speech [64]. Replacing agricultural activities by other economic activities that could augment N-deposition, is understandable for the those who are eager to unlock the economy (see 2. Introduction). However, it can hardly be called a solution for the nitrogen crisis to replace one nitrogen emission source for another.
The urgency of the deterioration of the environmental conditions and the overall biodiversity decline (see also 5. The biodiversity paradigm) may represent a staggering contrast with the effective speed of reform measures in society. As shown above, the scientific studies that documented the negative effects of N-emission on atmospheric aerosol formation and N-deposition have been published several decades ago. Also conferences, discussions, declarations and manifestos have been put on paper already in the previous century, but still are awaiting the desired effect. In this respect it is interesting to note a trade-off between the adaptation speed (Sadapt) and the costs of behavioral change (PΔbehavior) in the social groups concerned, resulting in the following formula for a human Resilience Strength Equation (huRSE):
huRSE=k(x,y)·Sadapt ·PΔbehavior
Although the costs of behavioral changes are difficult to estimate, we may grasp its meaning by comparing the discourses of individuals or different groups in a socio-economic context. For a distant observer, a conversational phrase (while reading the morning newspaper) such as “Oh dear dear, .” (pouring himself another cup of coffee) could become the only noticeable difference. For another, it might be another political U-turn or the end of a career. Still, for the countless who see their investments and ambitions melting down or vanish in smoke, it might become all too serious. Farmers are known to be familiar with crop rotation (on a yearly basis), but real occupational transitions of a population of farmers are mostly characterized by the time span of a generation (i.e. several decades). It is tempting to investigate the role of GDP and population size, density and demographic structure on the latter equation constant (k[x,y]), but obviously this falls outside the scope of the present paper.
Conclusions
In scientific studies, the impending risks of greenhouse gas emissions were already reported more than half a century ago. This year 2022, the Rio de Janeiro conference on biological diversity ‘celebrated’ a thirty years anniversary. But there isn’t much enthusiasm for an anniversary party. In the first decades after the publication of the Club of Rome’s report [68], the main emphasis was laid on the environmental effects of greenhouse gases, on air pollution, acid rain as well as the effects on atmospheric ozone. In the recent decades, the effects of greenhouse gases on climate warming, as well as the alarming rates of biodiversity decline (especially due to global deforestation) came to the forefront. Although the role of nitrogen emission (both the oxidized forms and the ammoniaderived forms) in countries of the moderate climate zone were also known for thirty years (or more), so far effective measures to reduce agricultural nitrogen emission have been lacking in certain countries.
In this review paper, due attention is paid to both the complexity of nitrogen emission and interaction with atmospheric aerosols and the complexity of biodiversity estimation. It seems accurate to conclude that especially the nitrogen-enrichment of the rural environment, due to intensive agricultural activities aiming at meat production, poses an unacceptable load on the viability of plant communities and biodiversity nearby the emission sources. Biodiversity impairment in natural reserves, however, may not become reversible following the replacement of one nitrogen source for another. Moreover, the necessity of farmland reforms seems at conflict with the desired speed of behavioral changes in the population envisaged.
References
- PJ Crutzen, J Fishman. Average concentrations of OH in the troposphere and the budgets of CH4, CO, H2. and CH3CCl3. Geophys res lett. 1977; 4: 321-324.
- PJ Crutzen, LE Heidt, JP Krasnec, WH Pollock, W Seiler. Biomass burning as a source of atmospheric gases CO, H2, N2O, NO, CH3Cl and COS. Nature. 1979; 282: 253-256.
- JE Lovelock. Natural halocarbons in the air and in the sea. Nature. 1975; 256: 193-194.
- J Hansen, M Sato, A Lacis, R Ruedy, I Tegen, E Matthews. Climate forcing in the industrial era. Proc Natl Acad Sci. USA (PNAS). 1998; 95: 12753-12758.
- X Li, H Maring, D Savoie, K Voss, JM Prospero. Dominance of mineral dust in aerosol light-scattering in the North Atlantic trade winds. Nature. 1996; 380: 416-419.
- IE Galbally, CR Roy. Destruction of ozone at the earth’s surface. Quarterly Journal of the Royal Meteorological Society. 1980; 106: 599-620.
- JA Logan. Tropospheric ozone: Seasonal behavior, trends, anthropogenic influence. J Geophys res. 1985; 90: 10463-10482.
- JA Logan. Trends in the vertical distribution of ozone: An analysis of ozonesonde data. J Geophys res. 1994; 99: 25553-25585.
- G Likens, F Borman, M Johnson. Acid rain. Environment. 1974; 14: 33-40.
- A Tremblay, A Leighton. A three-dimensional cloud chemistry model. J Clim appl Meteorol. 1986; 25: 652-671.
- M Tiedtke. A comprehensive mass flux scheme of cumulus parameterization in large-scale models. Monthly Weather Review. 1989; 117: 1779-1800.
- C Li, S Frolking, T Frolking. A model of nitrous oxide evolution from soil driven by rainfall events: 1. Model structure and sensitivity. J Geophys res. 1992; 97: 9759-9776.
- H Levy II, WJ Moxim, PS Kasibhatla. A global three-dimensional timedependent lightning source of tropospheric NOx. J Geophys res. 1996; 101: 22911-22922.
- SM Kreidenweis, Y Zhang, GR Taylor. The effects of clouds on aerosol and chemical species production and distribution. 2. Chemistry model description and sensitivity analysis. J Geophys res. 1997; 102: 23867-23882.
- GP Brasseur, RG Prinn, AAP Pszenny (2003, Eds.). Atmospheric Chemistry in a Changing World. Berlin, Heidelberg: Springer Verlag.
- O Hertel, CA Skjøth, P Løfstrøm, C Geels, LM Frohn, et al. Modelling Nitrogen Deposition on a Local Scale – A Review of the Current State of the Art. Environ Chem. 2006; 3: 317-337.
- SA Morman, GS Plumlee. The role of airborne mineral dusts in human disease. Aeolian Research. 2013; 9: 203-212.
- SV Krupa. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: a review. Environmental Pollution. 2003; 124: 179-221.
- KE Altieri, SE Fawcett, AJ Peters, DM Sigman, MG Hastings. Marine biogenic source of atmospheric organic nitrogen in the subtropical North Atlantic. Proc Natl Acad Sci USA. 2016.
- SA Penkett, KS Law, T Cox, P Kasibhatla, et al. Atmospheric Photooxidants. In: G.P. Brasseur, R.G. Prinn and A.A.P. Pszenny (Eds.). Atmospheric Chemistry in a Changing World. Berlin, Heidelberg: Springer Verlag. 2003; 73-124.
- Kenniscentrum InfoMil. AERIUS-2021 beschikbaar vanaf 20 januari (2022). (Source:www.aerius.nl) (Accessed: July 2022).
- F. Pilotto, I. Kühn, R. Adrian, R. Alber, et al. Meta-analysis of multidecadal biodiversity trends in Europe. Nature Communications. 2020; 11: 3486.
- W Allaerts. Estimating biodiversity and the fractal nature of ecosystems. International Journal of Bioinformatics and Computational Biology. 2020; 5: 15-24.
- W Allaerts. A comment on the Unified Neutral Theory of Biodiversity and Biogeography.Journal of Earth Science and Climatic Change, JESCC-108. 2021.
- E Codorniu-Hernandez, KW Hall, AD Boese, D Ziemianowicz, S Carpendale, PG Kusalik. Mechanism of O(3P) formation from a hydroxyl radical pair in aqueous solution. Journal of Chemical Theory and Computation. 2015; 4740- 4748.
- RL Mauldin, S Madronich, SJ Flocke, FL Eisele, GJ Frost, ASH Prevot. New insights on OH: Measurements around and in clouds. Geophys Res lett. 1997; 24: 3033-3036.
- RL Mauldin III, GJ Frost, G Chen, DJ Tanner, ASH Prevot. OH measurements during the First Aerosol Characterization Experiment(ACE-1): Observations and model comparisons. J Geophys Res. 1998; 103: 16713-16729.
- M Scheffer, J Bascompte, WA Brock, V Brovkin, SR Carpenter, et al. Earlywarning signals for critical transitions. Nature. 2009; 461: 53-59.
- PC Singer, WB Zilli. Ozonation of ammonia in wastewater. Water Research. 1975; 9: 127-134.
- JW Erisman, AWM Vermetten, WAH Asman, A Waijers-IJpelaan, J Slanina. Vertical distribution of gases and aerosols: The behaviour of ammonia and related components in the lower atmosphere. Atmospheric Environment. 1988; 22: 1153-1160.
- J Heintzenberg, F Raes, SE Schwartz, et al. Tropospheric Aerosols. In: G.P. Brasseur, R.G. Prinn and A.A.P. Pszenny (Eds.). Atmospheric Chemistry in a Changing World. Berlin, Heidelberg: Springer Verlag. 2003; 125-156.
- V. Ramaswamy, O. Boucher, J. Haigh, D. Hauglustaine, et al. Radiative forcing of climate change. In: J.T. Houghton, Y. Ding, D. Griggs, et al. [Eds.], Climate change 2001: The scientific basis. Contribution of the Working group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. 2001; 350-416.
- R Hestrin, EC Hammer, CW Mueller, J Lehmann. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Communications Biology. 2019; 2: 233.
- PJ Lea, BJ Miflin. An alternative route for nitrogen assimilation in higher plants. Nature. 1974; 251: 614-616.
- HS Srivastava, RP Singh. Role and regulation of L-glutamate dehydrogenase activity in higher plants. Phytochemistry. 1987; 26: 597-610.
- HFG van Dijk, M van der Gaag, PJM Perik, JGM Roelofs. Nutrient availability in Corsican pine stands in the Netherlands and the occurrence of Sphaeropsis sapinea: a field study. Canadian Journal of Botany. 1992; 70: 870-875.
- HF van Dobben, CJF ter Braak. Effect of atmospheric NH3 on epiphytic lichens in the Netherlands: the pitfalls of biological monitoring. Atmospheric Environment. 1998; 32: 551-557.
- DW Johnson, H Van Miegroet, SE Lindberg, RB Harrison, DE Todd. Nutrient cycling in red spruce forests of the Great Smoky Mountains. Canadian Journal of Forest Research. 1991; 21: 769-787.
- DW Johnson. Nitrogen retention in forest soils. Environmental Quality. 1992; 21: 1-12.
- CJ Koopmans, A Tietema, AW Boxman. The fate of 15 Nenriched throughfall in two coniferous forest stands at different nitrogen deposition levels. Biogeochemistry. 1996; 34: 19-44.
- ED Schulze, W de Vries, M Hauhs, K Rosén, L Rasmussen, CO Tamm, J Nilsson. Critical loads for nitrogen deposition on forest ecosystems. Water, Air and Soil Pollution. 1989; 48: 451-456.
- Bobbink, D Boxman, E Fremstadt, G Heil, A Houdijk, et al. Critical loads for nitrogen eutrophication of terrestrial and wetland ecosystems based upon changes in vegetation and fauna. In: P. Grennfelt, and E. Thörnelöf (Eds.), Critical Loads for Nitrogen – A Workshop Report. Organized by the Nordic Council of Ministers in collaboration with The Convention on Long-Range Transboundary Air Pollution. 1992; 111-160.
- Compendium voor de Leefomgeving (CLO). Landbouw en milieu: Stikstofdepositie, 1990-2020 [in Dutch]. Rijksoverheid Nederland. 2022.
- World Wildlife Fund (WWF). Living Planet Report 2022.
- United Nations. Convention on biological diversity. In: United Nations conference on environment and development at Rio de Janeiro, UN: Washington DC. 1992.
- Conference of the Parties of the Convention of Biological Diversity. Johannesburg Declaration on Sustainable Development. A/Conf. 199/20, Chapter I, Resolution 1. 2002.
- T Hoogeveen. De geur van hooi [in Dutch]( Historische roman). Amsterdam: Uitg. Thomas Rap. 2020.
- W Allaerts, TW Chang. Skewed exposure to environmental antigens complements Hygiene Hypothesis in explaining the rise of allergy. Acta Biotheoretica. 2017; 65: 117-134.
- R Evens, GJ Conway, IG Henderson, B Cresswell, F Jiguet, et al. Migratory pathways, stopover zones and wintering destinations of Western European Nightjars Caprimulgus europaeus. IBIS, International Journal of Avian Science. 2017; 159: 680-686.
- R Evens, N Beenaerts, N Witters, T Artois. Repeated migration of a juvenile European Nightjar Caprimulgus europaeus. J. Ornithology. 2017; 158: 881- 886.
- W Allaerts. Deconstructing self-similarity and the four dis-similitudes of biodiversity. Philosophy International Journal. 2021; 4: 000180.
- W Allaerts. Why is biodiversity of cardinal importance for public health? International Journal of Environment & Agricultural Science. 2018; 2: 013.
- T Hatton. Air pollution in Victorian-era Britain – its effects on health now revealed. The Conversation. 2017.
- J Martinez. Great Smog of London. Encyclopedia Britannica. 2021. (https:// www.britannica.com/event/Great-Smog-of-London).
- Y Zhu, WC Hinds, S Kim, S Shen, C Sioutas. Study of ultrafine particles near a major highway with heavy-duty diesel traffic. Atmospheric Environment. 2002; 36: 4323-4335.
- M Zheng, LG Salmon, JJ Schauer, L Zeng, Kiang CS, et al. Seasonal trends in PM 2.5 source contributions in Beijing, China. Atmospheric Environment. 2005; 39: 3967-3976.
- FJ Kelly, JC Fussell. Air pollution and airway disease. Clin Exp Allergy. 2011; 41: 1059-1071.
- A Karakatsani, A Analitis, D Perifanou, JG Ayres, Harrison RM, et al. Particulate matter air pollution and respiratory systems in individuals having either asthma or chronic obstructive pulmonary disease. Environ Health. 2012; 11: 75.
- P Vineis, G Hoek, M Krzyzanowski, F Vigna-Taglianti, Vegila F, et al. Air pollution and risk of lung cancer in a prospective study in Europe. Int J Cancer. 2006; 119: 169-174.
- SN Sax, K Zu, JE Goodman. Air pollution and lung cancer in Europe. Lancet Oncol. 2013; 14: e439-e440.
- W Allaerts. How could this happen? Narrowing down the contagion of COVID-19 and preventing Acute Respiratory Distress Syndrome (ARDS). Acta Biotheoretica. 2020; 68: 441-452.
- European Environment Agency (EEA). Greenhouse gas emissions from agriculture in Europe. (+ Data from Eurostat & CBS, the Netherlands) (Source: https://www.eea.europa.eu/ims/greenhouse-gas-emissions-fromagriculture ) 2022.
- Compendium voor de Leefomgeving (CLO) (2022). Stroomschema stikstof. Centraal Bureau voor Statistiek (CBS), Rijksoverheid Nederland (Source: https://www.clo.nl/nl009421) 2022.
- Institute for Contemporary History - CVCE. Discours de Sicco Mansholt. 1952. (https://www.cvce.eu/obj/discours_de_sicco_mansholt_paris_25_ mars_1952-fr-)c757933-0117-4493-8b1a=ad26aa3243ba.html )
- J von Liebig. Es ist ja dies die Spitze meines Lebens: Naturgesetze im Landbau. Stiftung Ökologie & Landbau, SÖL-Sonderausgabe, nr 22. 1861. (Dutch transl., De zoektocht naar de kringlooplandbouw.) (source: Historiek geschiedenisboeken, see: www.historiek.net/geschiedenis-van-kunstmestvan- vogelpoep-tot-ammoniaksynthese/134851/).
- NOS Nieuws. Wat heeft Nederland aan al dat gas verdiend en wat willen partijen nu?. NOS Nieuws. 2017.
- W Boersema. Gronings goud (in Dutch). Amsterdam: Ambo/Anthos uitgevers. 2021.
- DH Meadows, DL Meadows, J Randers, WW Behrens III (1972-1974). The Limits to Growth: A Report for the Club of Rome’s Project on the Predicament of Mankind. New York: Universe Books(Dutch transl. De Club van Rome [1972]: De grenzen aan de groei. Utrecht, Antwerpen: Het Spectrum).
