
Research Article
Austin J Environ Toxicol. 2015;1(1): 1004.
Pre-exposure to Fine Particulate Matters may Induce Endotoxin Tolerance in a Mouse Model
Bai Y1,2, Shang Y¹, Liu Z², Lu B³ and Sun Q1,2*
1Division of Environmental Health Sciences, Ohio State University, USA
2Davis Heart and Lung Research Institute, Ohio State University, USA
3Division of Biostatistics, Ohio State University, USA
*Corresponding author: Sun Q, Division of Environmental Health Sciences, College of Public Health, Ohio State University, USA
Received: April 22, 2015; Accepted: May 03, 2015; Published: May 05, 2015
Abstract
Exposure to low or moderate doses of Lipopolysaccharides (LPS) renders the host tolerance to a subsequent lethal dose of LPS, which is termed as endotoxin tolerance. It is characterized as the decrease in production of pro-inflammatory cytokines and the increase in production of anti-inflammatory mediators in response to subsequent LPS challenge. Whether other environmental factors also trigger endotoxin tolerance remains unclear. Both epidemiologic and experimental studies have provided a link between particulate matters and human health. Here, we speculated on the effect of fine particles priming on endotoxin tolerance in a mouse model.
Keywords: Lipopolysaccharides; Fine particulate matters; Survival curves; Heat-shock response
Abbreviations
PM: Particulate Matter; TLR: Toll-Like Receptor; LPS: Lipopolysaccharides; IL: Interleukin; HSP: Heat-Shock Protein
Introduction
The inhalation of toxic ambient particles is a worldwide public health problem; both epidemiologic and experimental studies have provided compelling evidence supporting the association between Particulate Matter (PM) and human diseases, including mortality and hospital admissions [1], cardiovascular diseases [2, 3], type 2 diabetes [4,5], asthma and chronic obstructive pulmonary disease [6,7], and non-alcoholic fatty liver disease [8]. Inflammatory response has been implicated as the key mechanism of PM-mediated healthy problems. Current evidence suggests that inhaled particles trigger innate immune signals in the lung through interacting with Toll-Like Receptors (TLRs), releasing cytokines into circulation and causing systemic inflammatory response [9]; and that direct penetration of leachable components such as reactive oxygen species and stable organic compounds into circulation also contributes to systemic inflammatory response [10].
Ambient particle pollution is a mixture of microscopic solids and liquids droplets suspended in air; it consists of a number of components, including acids, organic chemicals, metals, soils or dust particles, and allergens. According to its aerodynamic diameter, PM is classified into coarse (10 to 2.5 μm; PM10), fine (<2.5 μm; PM2.5), and ultrafine (<0.1 μm; PM0.1) particulate matters. The size of particles is directly linked to their potential for causing health effects. It is believed that fine particulate matters pose the greatest health problems, because they hold the potential to get and deposit deep into the lung, and may even penetrate into the bloodstream. PM composition and size together influence its adverse effects on public health [11,12].
Endotoxin, also known as Lipopolysaccharides (LPS), is a structural component of the gram-negative outer membrane. Leukocytes recognize LPS via TLR4 in the presence of myeloid differentiation factor 2, triggering a powerful immune reaction [13]. This inflammatory response is tightly regulated and can show different forms, depending on the dose. Exposure to low or moderate doses of LPS renders the host tolerance to a subsequent lethal dose of LPS, which is termed as endotoxin tolerance. It is characterized as the decrease in production of pro-inflammatory cytokines such as tumor necrosis factor a, Interleukin (IL)-6 and IL-1β, and the increase in production of anti-inflammatory mediators such as IL- 10 in response to a second LPS challenge [14, 15]. The alteration of cytokine profile protects LPS-primed hosts against a normally lethal dose of subsequent LPS challenge. Nevertheless, whether other environmental factors also trigger endotoxin tolerance remains unclear. Here, we speculated on the effect of PM2.5 priming on endotoxin tolerance in a mouse model.
Materials and Methods
Animal care
C57BL/6 mice (6-8 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME). Animals were maintained at 21°C and exposed to a 12-h light, 12-h dark cycle with free access to water and food. The protocols and the use of animals were approved by and in accordance with the Ohio State University Animal Care and Use Committee.
Intranasal exposure to PM2.5
Mice were exposed to PM2.5 by intranasal instillation, which is an effective and noninvasive technique in toxicity studies [16,17]. This instillation technique consists in deliver drop-wise the particle suspension or the vehicle to the nares using a micropipette, while the mouse is in a supine position. Animals were lightly anesthetized with 2% isoflurane and intranasally instilled with 20 μl of free-particle saline or PM2.5 (0.5 μg/μl) saline, three times per week for eight weeks.
Survival study
Endotoxic shock was induced by peritoneal injection of LPS (20 μg/g; Escherichia coli serotype 055.B5; Sigma-Aldrich) and mice (n = 10) were monitored up to 84 hours. Survival curves were compared using Kaplan–Meyer log-rank test. All tests were conducted at the two-sided 5% significant level.
Results
All mice treated with saline without LPS injection survived; one mouse exposed to PM2.5 without LPS injection died (p > 0.05 vs. saline). LPS injection induced a significant decrease in survival rate (p < 0.01 vs. saline); pre-exposure to PM2.5 induced tolerance to death from a subsequent lethal LPS dose, however, these two survival curves were not significantly different (p > 0.05 vs. LPS) (Figure 1).
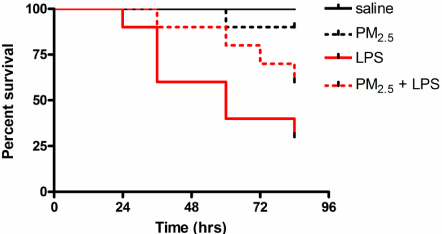
Figure 1: PM2.5 priming attenuates LPS-induced mortality in wild-type mice.
Mice (n = 10) were exposed to 20 μl of free-particle saline or PM2.5 (0.5 μg/
μl) saline, three times per week for eight weeks by intranasal instillation. After
exposure, endotoxic shock was induced by peritoneal injection of LPS (20
μg/g), and mice were monitored up to 84 hours for survival study. Survival
curves were compared using Kaplan–Meyer log-rank test. All tests were
conducted at the two-sided 5% significant level. LPS: Lipopolysaccharides;
PM2.5: Fine particulate matters.
Hypothesis and evaluation
Our preliminary data showed an evident trend of survival curves between PM2.5-exposure and PM2.5 priming plus LPS treatment, suggesting that PM2.5 priming may cause endotoxin tolerance in mice. To verify this hypothesis, a study with larger sample size is needed. Sample sizes are determined using a two-sided, 0.05-significance level log rank test with 80% statistical power and equal allocation. The log-rank test is a popular nonparametric test to compare the survival distributions of two groups. Let i =1, i be the distinct times of observed events. For each time i, let N1i and N2i be the numbers of subjects at risk at the start of time i in the two groups. Define Ni = N1i + N2i. Let em>O1i and em>O2i be the observed numbers of events in the groups respectively at time i, and define Oi =O1i + O2i. Under the null hypothesis that the two groups have the same survival function, the distribution of the number of events in the first group has expectedand variance
The log-rank statistic compares each observed event count to its expectation and is defined as
Under the null hypothesis, it follows approximately a standard normal distribution. Based on our preliminary study, 60% of mice survived after 84 hours in PM2.5 priming plus LPS treatment group, while only 30% of mice survived in LPS treatment group. Assuming a constant hazard model and using a log rank test, we would need 40 mice in each group to detect the difference in survival curve. The sample size calculation is conducted using SAS proc power (SAS 9.2, SAS Institute Inc. Cary, NC).
To verify our findings and mimic the effects of “real world” air pollution, we will repeat this experiment using Versatile Aerosol Concentrator and Enrichment System, which is used to generate ambient air pollution murine models for in vivo toxicological studies. This system has been well introduced by Chen Lab at New York University [18-20]. The system in our lab is quite similar. Mice (n = 40) will be exposed to either filtered air or concentrated ambient PM2.5 for 6 hours/day, 5 days/week for 8 weeks. The exposure protocol and PM2.5 compositions have been reported in our previous studies [4, 21-23]. After exposure, endotoxin shock will be induced as previously described.
How may PM2.5 priming lead to endotoxin tolerance in mice? Based on our knowledge of this compound, we speculate that at several mechanisms PM2.5 may actively regulate endotoxin tolerance (Figure 2). On the one hand, PM2.5 is a mixture of various chemical and biological constituents, including a low dose of LPS [24]. In our experiment, pre-exposure to PM2.5 may prime animals with lose dose of LPS, preventing death from a subsequent lethal dose. On the other hand, PM2.5 may induce endotoxin tolerance through regulation of heat-shock response. It has been shown that PM2.5 exposure significantly increases the expression of Heat-Shock Protein (HSP) 70 [25,26]. HSP70, a prominent chaperone protein, functions individually or as part of larger heterocomplexes to maintain protein homeostasis in response to various stress stimuli [27]. In addition, HSP70 exhibits anti-inflammatory effect and inhibits the release of pro-inf?lammatory cytokines, e.g. IL-6, IL-1β and tumor necrosis factor a, through interaction with nuclear factor-κB complex [28-30].
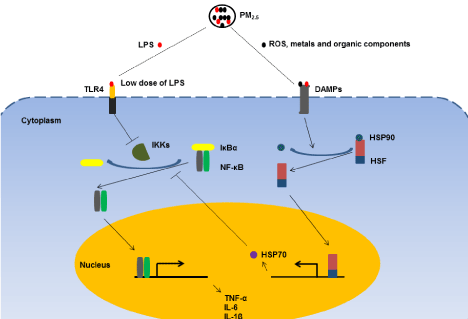
Figure 2: Proposed mechanisms of PM2.5-mediated endotoxin tolerance.
PM2.5 consists of a number of components, including acids, organic chemicals,
metals, ROS and low dose of LPS. On the one hand, pre-exposure to PM2.5
may prime animals with possible low dose of LPS and contribute to endotoxin
tolerance via inhibition of IκBa degradation. On the other, LPS and other
components such as ROS, metals and organic chemicals may activate
heat-shock response via binding to DAMPs, up regulating the expression
of HSP70. It has been shown that HSP70 interrupts NF-κB signaling and
inhibits pro-inflammatory cytokine release by stabilizing the complex between
NF-κB and its inhibitor IκBa. These signaling pathways may contribute to
particle-mediated endotoxin tolerance. PM2.5: Fine Particulate Matters; LPS:
Lipopolysaccharides; ROS: Reactive Oxygen Species; TLR4: Toll-Like
Receptor 4; DAMPs: Damage-Associated Molecular Patterns; IKKs: IκB
kinases; IκBa: κB inhibitor a; NF-κB: Nuclear Factor-κB; HSP: Heat-Shock
Protein; HSF: Heat-Shock Factor; TNF-a: Tumor Necrosis Factor-a; and IL:
Interleukin.
Potential implications
The adverse effects of PM air pollution have robustly been investigated since global air quality becomes worse, particularly in the developing countries [31,32]. Although PM-induced inflammation is implicated as one of potential mechanisms, the modulated effects of PM on immune system, particularly in response to subsequent infection, could be more complicated than we thought. If our hypothesis is to be confirmed, it may refresh our knowledge about this compound and its inflammatory effects.
Additionally, we want to highlight the use of log-rank test as a suitable tool to determine the sample size and evaluate the data fidelity for survival study. The most considerable advantage of this test is that it does not require the knowledge about the shape of the survival curve or the distribution of survival times in advance [33].
Acknowledgement
This work was supported by National Institutes of Health Grant No. ES018900. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014; 69: 660-665.
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010; 121: 2331-2378.
- Pedersen M, Stayner L, Slama R, Sorensen M, Figueras F, Nieuwenhuijsen MJ, et al. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension. 2014; 64: 494-500.
- Liu C, Fonken LK, Wang A, Maiseyeu A, Bai Y, Wang TY, et al. Central IKKbeta inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part Fibre Toxicol. 2014; 11: 53.
- Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. Eur J Endocrinol. 2014; 171: R173-R182.
- Kariisa M, Foraker R, Pennell M, Buckley T, Diaz P, Criner GJ, et al. Short- and long-term effects of ambient ozone and fine particulate matter on the respiratory health of chronic obstructive pulmonary disease subjects. Archives of environmental & occupational health. 2015; 70: 56-62.
- Montoya-Estrada A, Torres-Ramos YD, Flores-Pliego A, Ramirez-Venegas A, Ceballos-Reyes GM, Guzman-Grenfell AM, et al. Urban PM2.5 activates GAPDH and induces RBC damage in COPD patients. Front Biosci (Schol Ed). 2013; 5: 638-649.
- Tarantino G, Capone D, Finelli C. Exposure to ambient air particulate matter and non-alcoholic fatty liver disease. World journal of gastroenterology. 2013; 19: 3951-3956.
- Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011; 108: 716-726.
- Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012; 61: 3037-3045.
- Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). The lancet oncology. 2013; 14: 813-822.
- Chung Y, Dominici F, Wang Y, Coull BA, Bell ML. Associations between Long-Term Exposure to Chemical Constituents of Fine Particulate Matter (PM) and Mortality in Medicare Enrollees in the Eastern United States. Environ Health Perspect. 2015.
- Ren W, Wang Z, Hua F, Zhu L. Plasminogen Activator Inhibitor-1 Regulates LPS-Induced TLR4/MD-2 Pathway Activation and Inflammation in Alveolar Macrophages. Inflammation. 2014.
- Xiong Y, Medvedev AE. Induction of endotoxin tolerance in vivo inhibits activation of IRAK4 and increases negative regulators IRAK-M, SHIP-1, and A20. Journal of leukocyte biology. 2011; 90: 1141-8.
- Salkowski CA, Detore G, Franks A, Falk MC, Vogel SN. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infection and immunity. 1998; 66: 3569-3578.
- Schaffer BE, Park KS, Yiu G, Conklin JF, Lin C, Burkhart DL, et al. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer research. 2010; 70: 3877-3883.
- Leong BK, Coombs JK, Sabaitis CP, Rop DA, Aaron CS. Quantitative morphometric analysis of pulmonary deposition of aerosol particles inhaled via intratracheal nebulization, intratracheal instillation or nose-only inhalation in rats. Journal of applied toxicology. 1998; 18: 149-160.
- Lippmann M, Gordon T, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. I. Introduction, objectives, and experimental plan. Inhalation toxicology. 2005; 17: 177-187.
- Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhalation toxicology. 2005; 17: 189-197.
- Hwang JS, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. III. Acute and chronic effects of CAPs on heart rate, heart-rate fluctuation, and body temperature. Inhalation toxicology. 2005; 17: 199-207.
- Liu C, Bai Y, Xu X, Sun L, Wang A, Wang TY, et al. Exaggerated effects of particulate matter air pollution in genetic type II diabetes mellitus. Part Fibre Toxicol. 2014; 11: 27.
- Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, et al. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect. 2014; 122: 79-86.
- Liu C, Xu X, Bai Y, Wang TY, Rao X, Wang A, et al. Air pollution-mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environ Health Perspect. 2014; 122: 17-26.
- Yang L, Liu G, Lin Z, Wang Y, He H, Liu T, et al. Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environmental toxicology. 2014.
- Farina F, Sancini G, Mantecca P, Gallinotti D, Camatini M, Palestini P. The acute toxic effects of particulate matter in mouse lung are related to size and season of collection. Toxicol Lett. 2011; 202: 209-217.
- Sancini G, Farina F, Battaglia C, Cifola I, Mangano E, Mantecca P, et al. Health risk assessment for air pollutants: alterations in lung and cardiac gene expression in mice exposed to Milano winter fine particulate matter (PM2.5). PLoS ONE. 2014; 9: e109685.
- Neef DW, Jaeger AM, Thiele DJ. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nature reviews Drug discovery. 2011; 10: 930-944.
- Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008; 28: 53-63.
- Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell stress & chaperones. 1997; 2: 132-139.
- Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, et al. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004; 18: 1466-1481.
- Liang Y, Fang L, Pan H, Zhang K, Kan H, Brook JR, et al. PM2.5 in Beijing - temporal pattern and its association with influenza. Environ Health. 2014; 13: 102.
- Wang K, Dickinson RE, Liang S. Clear sky visibility has decreased over land globally from 1973 to 2007. Science. 2009; 323: 1468-1470.
- Bland JM, Altman DG. The logrank test. BMJ. 2004; 328: 1073.