Abstract
Diabetes is a common disorder of carbohydrate metabolism that produces significant complications when either poorly treated or overtreated. Before 1978, the HgbA1c modality was not available to diagnose or monitor diabetes but now has become the standard of care. The results from this laboratory test are proportional to the glycosylation of hemoglobin and represents an accepted measure of glycemic control. Effects of hemoglobinopathies depend on their quaternary structure and have been shown to have impact HgbA1c results. Hgb Wayne is a rare hemoglobinopathy that occurs from a frameshift mutation in which either an asparagine (Hgb Wayne 1) or aspartic acid (Hgb Wayne 2) is located at residue 139 of the HBA2 gene. When separated by electrophoresis, this peak appears very close to that of the HgbA1c. Most HgbA1c monitoring equipment is either electrophoresis or ion exchange high-performance liquid chromatography which combine these two peaks together as one, falsely elevating the patient’s HgbA1c level. This co-elution can create an incongruity between HgbA1c levels and those of random glucose readings. Acting upon a falsely elevated HgbA1c with medication risks life-threatening hypoglycemia. In these following cases, this is explored with two related patients diagnosed independently by separate physicians in the same practice.
Keywords: Diabetes; Hypoglycemia; Testing; Hemoglobin Wayne; Hemoglobinopathies; Hgb a1c
Introduction
Diabetes is a widespread and omnipresent pathology in America, which requires an accurate method of glycemic control to evaluate, diagnose, and manage. Traditionally, this was accomplished with fingerstick glucose levels that monitored point-in-time levels [1,2]. Hemoglobin (Hgb) A1c, first commercially available in 1978, became a recommended method for monitoring long-term average glycemic control in 1994 [2]. Since 2010, it has also been recognized by the American Diabetes Association as a standard biomarker for diagnosis of diabetes, requiring two consecutive readings of 6.5+% [2-6]. Typically, an elevated Hgb A1c level is indicative of abnormal elevated serum glucose levels, however, false elevations can occur not only from decreased red blood cell turnover from asplenia and B12 or Folate deficiency, but also severe hypertriglyceridemia, severe hyperbilirubinemia and various hemoglobinopathies [3-5,7].
Hgb Wayne, a rare hemoglobin variant, is a mutation first discovered in 1976 in a 7-year-old Michigan boy with Fanconi’s anemia and subsequently in multiple generations of his family (maternal grandmother, mother, maternal aunt, and maternal uncle). Subsequently, it was seen in a 9-month old child in Georgia, who had three family members as carriers [8,9]. While, the prevalence of this hemoglobinopathy is still unknown [3,10], a study by Szuberski et al identified 62 cases in the United States over a 16 year period [11]. Like most hemoglobinopathies, Hgb Wayne is often clinically silent, not even causing anemia [5,6,12]. Its clinical importance arises from the ability to significantly and falsely elevate Hgb A1c values, thus impacting provider decision making regarding medication choice and dosage. Below are described two cases of related patients diagnosed independently by different providers within the same practice.
Case
Case 1
A 59-year-old male without significant medical history presented to discuss elevated blood pressures at home. He noted headaches but otherwise felt well. The exam was normal except for a body mass index of 32 kg/m2 and blood pressure of 140/100. An antihypertensive medication was initiated along with advisement to lose weight. Fasting lipid testing revealed elevated total cholesterol of 256 mg/dL, triglycerides of 249 mmol/L and LDL of 174 mg/dL. While his fasting sugar was 96 mg/dL, his HgbA1C was 10.7%. Both metformin and a statin were initiated, and he was referred to diabetic education, advised to check glucose readings at home, and scheduled for a short interval follow up. During his next visit, he reported tolerating metformin, losing 20# through dietary changes, and monitoring his fasting glucose reading, the highest of which was 121.
Three-months later, his weight loss totaled 38-pounds but repeat HgbA1C had only decreased to 9.1 despite normal glucose readings. Given the discrepancy between his home readings and the HgbA1C, additional labs were ordered including complete blood count, iron studies, thyroid stimulating hormone and pancreatic antibodies. All were normal, but his A1C remained high on repeat test. The patient was referred to endocrinology for further evaluation. While awaiting this evaluation, low dose long-acting insulin was contemplated but he did not start this due to consistently normal sugars.
Endocrinology evaluation demonstrated normal continuous glucose monitoring and fructosamine levels. Ultimately, a Hgb electrophoresis showing Hgb Wayne variant. A repeat HgbA1c at an outside lab not using an ion-exchange High-Performance Liquid Chromatography (HPLC) immunoassay was 5.3%. To exclude the potential that this glycemic control had improved due to his metformin, a glucose tolerance test after stopping metformin was conducted to exclude the potential that this glycemic control had improved due to his medication. This was normal.
Case 2
A 37-year-old male presented to establish care. He reported a 70 pound weight gain in the last few years. He had started a high-intensity exercise program and a Keto diet, which resulted in a 15 pound weight loss in the previous 3-4 weeks. He denied medical issues but noted a strong family history for hypertension and hyperlipidemia. His physical exam was normal, save a blood pressure of 141/93. Monitoring home blood pressures and continuation of diet and exercise was planned. Screening labs of complete blood count, complete chemistry profile, fasting lipid profile, and thyroid stimulating hormone were normal except for a HgbA1c of 10.0% despite his fasting glucose of 74 mg/dL. When discussing his results, the patient noted that his father had recently been diagnosed by his family physician with a blood disorder causing a falsely high Hgb A1c. Medication initiation was deferred in favor of home glucose monitoring instead.
One month later, the patient was seen for follow-up. His blood pressure was 118/78 and his weight was down another 28 pounds. He denied polyuria, polydipsia, and polyphagia. Home glucose readings continued to be normal. Further investigation into his father’s condition revealed the diagnosis of Hgb Wayne. With that information, diabetes was felt to be a less likely diagnosis. A repeat HgbA1c not on an ion-exchange high-performance liquid chromatography machine revealed a normal reading of 5.1%.
Discussion
There are over 1000 variants of hemoglobin [11], whose function correlates with their quaternary structure. Specifically, simple amino acid substitutions at the c-terminal ends of either alpha and beta chains can confer unique properties [7,10]. The Hgb Wayne variant is a result of a frame shift mutation leading to an elongated alpha chain on HBA2 gene [3-12]. Two forms of this hemoglobinopathy have been accounted for in the literature, Hgb Wayne Asn (Hgb Wayne I) and Hgb Wayne Asp (Hgb Wayne II). These two mutations are characterized by an asparagine or aspartic acid located at residue 139 of the HBA2 gene [5,7,8,10,11]. It has been noted that Hgb Wayne Asn may spontaneously deaminate to Hgb Wayne Asp which may result from catabolic destruction [8]. Both forms of this mutation result in hemoglobin with increased oxygen affinity and markedly reduced Bohr effect [3,8-10], as well as some differences in carbon dioxide binding properties [9].
HgbA1c is created through the non-enzymatic attachment of glucose to the N-terminal valine within the beta globulin chain [5,12]. The rate of this glycosylation is proportional to the level of a patient’s glycemic control [9]. A measurement of HgbA1c can be separated from standard hemoglobin (HgbA0) with an immunoassay, enzymatic assays, ion exchange HLPC, or boronate affinity HPLC [5]. Electrophoresis and ion-exchange HPLC are the most common HgbA1c testing methods [4]. ‘Co-elution’ of Hgb Wayne and HgbA1c, however, can occur in ion-exchange HPLC as the proximate peaks are not read as separate from each other by ‘fast’ reading machines (Figure 1) [3,7,8,11,12]. Hgb Wayne is not the only hemoglobinopathy to impact HgbA1c, as Hgb F, Hgb Sherwood Forrest, Hgb Gratz, Hgb O Pavoda, Hgb D, Hgb S, and Hgb N-Baltimore have all been implicated in the literature [7,10,12].
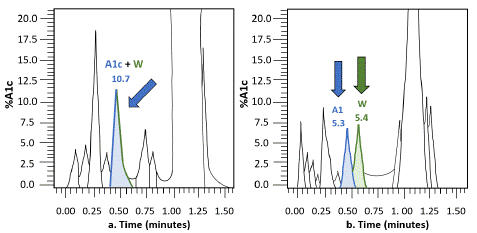
Figure 1: Representative of the difference of high performance liquid Chromatography between fast (Figure 1a) and slow (Figure 1b) techniques regarding the seperation of Hgb A1c and Hgb Wayne. Figure recreated by Michelle Peters, MAT.
Usually, blood glucose and HgbA1c are directly correlated enough that this test can be used to confirm diagnosis and guide treatment. However, in these cases of this father and son with Hgb Wayne hemoglobinopathy, the HgbA1c is drastically and discordantly elevated. Had the providers aggressively treated their patients based solely on the Hgb a1c, potentially dangerous hypoglycemia could have occurred. The caution exercised, despite the desire to meet the quality measure of controlling the HgbA1c, in combination with intellectual curiosity to explore the divergent readings prevented potential harm to the patients. Once the HgbA1c was measured with immunoassay or boronated affinity HPLC, there was identification of a proper HgbA1c measurement. Furthermore, our literature search revealed two other, but unrelated individuals within our region with a similar pathology [5]. Therefore, it also possible that Hgb Wayne’s genetic component may lend itself to pockets of higher penetrance within geographic locations.
For primary care physicians, this poses questions of appropriate testing scenarios for hemoglobinopathies. At what point should testing for these rare hemoglobinopathies occur? A delay of diagnosis in the face of discordant fingerstick glucose and HgbA1c readings may save the patient the risk for hypoglycemic episodes while attempting to medically treat a false diabetes. Should Hgb Wayne testing occur for any patient above a certain Hgb A1c threshold? If one family member has this mutation, is testing recommended for the entire family? These questions, unconsidered prior to the advent of Hgb a1c, as well as others, should be discussed among primary care providers moving forward.
Conclusion
Diabetes is encountered daily by front-line primary care providers. Decisions regarding diagnosis, monitoring, and treatment are generally made from a combination of patient home glucose readings, point-of-care office monitoring and the long-term information provided by HgbA1c testing. Relying exclusively on HgbA1c measurements in a patient with Hgb Wayne can result in over diagnosis of diabetes and potentially life-threatening treatments from oral hypoglycemic medications and insulin. None of these modalities should be used independent of the others when making clinical decisions, as discrepancies between can indicate underlying pathology. This begs the question, ‘when should a provider consider testing for hemoglobinopathies?’ The rarity of Hgb Wayne would answer, that hemoglobin electrophoresis should be reserved for situations when reliable fingerstick glucose testing is incompatible with Hgb a1c measurements, or when a hemoglobinopathy is identified within a family.
Author Statements
Acknowledgements
The authors would like to acknowledge Michelle Peters for her work in formatting and image development.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Oliver Schnell, J Benjamin Crocker, Jianping Weng. Impact of HbA1c Testing at Point of Care on Diabetes Management - J Diabetes Sci Technol. 2017; 11: 611-7.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standard of Medical Care in Diabetes – 2020. Diabetes Care. 2020; 43: S14-S31.
- Led Astray by Hemoglobin A1c: A case of misdiagnosis by falsely elevated hemoglobin A1c (Chen, Diesburg Stanwood, Bodor, Rasouli).
- Hemoglobin Wayne: A Rare Variant That can cause falsely elevated hemoglobin A1c (Ao, Ganta, Choe, Patel, Turro, Cheriyath).
- Hemoglobin Wayne Variant Interfering with Hemoglobin A1c Measurement (Milhem, July, Elhamdani, BenHamed).
- Alarming Increase in HbA1c and near misdiagnosis of diabetes mellitus resulting from a clinical laboratory instrument upgrade and hemoglobin variant (Chessler, Lee).
- A Patient with Discordant Hemoglobin A1c Results (Sterling, Griffith, Shajani-Yi).
- Hemoglobin Wayne: A frameshift mutation detected in human hemoglobin alpha chains (Seid-Akhavan, Winter, Abramson, Rucknagel).
- Hemoglobin Wayne in a British Family: Identification by Electrospray Ionization/Mass Spectrometry (Reynolds, Harvey, Green, Smith, Hartland).
- Hemoglobin Wayne Trait with Incidental Polycythemia (Ambelil, Nguyen, Dasgupta, Risin, Wahed).
- Rodriquez-Capote – Identification of Hgb Wayne and its effects on Hgb a1c measurement by 5 methods.
- Hemoglobin Wayne Causing a Falsely Elevated Hemoglobin A1c (Bejeck, Wenkert).
