
Clinical Image
Austin J Gastroenterol. 2016; 3(1): 1056.
A Rare Ampullary Mass: Think Outside the Box
Klair JS¹, Girotra M1,2,3 and Rego RF³*
¹Department of Internal Medicine, University of Arkansas for Medical Sciences, USA
²Division of Gastroenterology and Hepatology, University of Arkansas for Medical Sciences, USA
³Division of Gastroenterology and Hepatology, Central Arkansas Veterans Health System, USA
*Corresponding author: Rayburn F. Rego, Division of Gastroenterology and Hepatology, Central Arkansas Veterans Health System, Little Rock, AR, 72202, USA
Received: March 16, 2016; Accepted: March 26, 2016; Published: March 28, 2016
Clinical Image
A 75-year-old lady presented with nausea/vomiting and right upper quadrant pain of 1-day duration, preceded by dizzy spells. She had prior history of coronary bypass a decade ago, and hypertension. She was hemodynamically stable and initial lab work, including complete blood count, kidney profile, amylase/lipase, and hepatic panel were unremarkable. Ultrasound suggested dilated common bile duct (CBD) and intrahepatic bile ducts. Computed tomography (CT) with pancreas protocol showed severe intra- and extra-hepatic biliary ductal dilation with a 1.1x0.5 cm mass near the ampulla, raising concerns for possible neoplastic process. She was unable to get magnetic resonance imaging (MRI) due to recent shoulder prosthesis. Endoscopic ultrasound (EUS) revealed a dilated (1.2 cm) CBD that tapered towards a bulbous ampulla, and confirmed an ampullary mass but no lymph nodes were detected (Figure A/B). During endoscopic retrograde cholangiopancreatography (ERCP), bullous ampulla was visualized and no evidence of distal stricture was found (Figure C). Immunohistochemical stains on ampullary biopsies revealed expression of pan-cytokeratin AE1/AE3 (patchy), synaptophysin (strong and diffuse), chromogranin (patchy), but negative Congo-red stain, with margins negative for malignancy, no increased mitotic activity supporting diagnosis of ampullary carcinoid (AC). The patient underwent uneventful ampullectomy.
ACs are extremely rare neuroendocrine tumors, comprising <0.05% of all gastrointestinal carcinoids and <2% of all ampullary tumors [1,2]. Common presentations include obstructive jaundice (>60%), abdominal pain (<40%), weight loss (<10%), and pancreatitis (~5%) [1-3]. The sensitivity of octreotide scintigraphy decreases with smaller size of tumor and decreased tumor Somatostatin receptor expression [2]. Endoscopic biopsy during esophagogastroduodenoscopy has limited value and high failure rate due to the sub-epithelial nature of this tumor. EUS is superior to CT and equivalent to MRI for tumor staging [3]. Unlike other midgut carcinoids, the size of the AC is a poor predictor of metastatic potential, with varying rates of metastasis at different tumor sizes [1]. Since these tumors have a high propensity of lymph node metastasis, unlike other duodenal carcinoids, local resection of AC is technically difficult and Pancreatico-duodenectomy has been suggested for complete resection of the lesion [3]. Indications of local excision include tumor size less than 2 cm in diameter, absence of nodular involvement, tumor limited to submucosa and high surgical risk patients. Complete resection with pancreaticoduodenectomy is considered if patients do not fulfill the above indications. Less radical approach with endoscopic resection should be considered as it has achieved long-term survival with local resection only [1,3]. Predicting metastases among small intestinal carcinoids using a gene expression profiling (GEP)-based mathematical model is an emerging strategy to improve detection accuracy [2]. Key to diagnosis is adequate biopsies, which are often not possible with regular EGD due to sub-mucosal nature of tumor. EUS has the best diagnostic (obtaining deep biopsies), as well as prognostic value (assessing depth, extent and nodal involvement) and is indispensable for all ampullary pathologies [3].
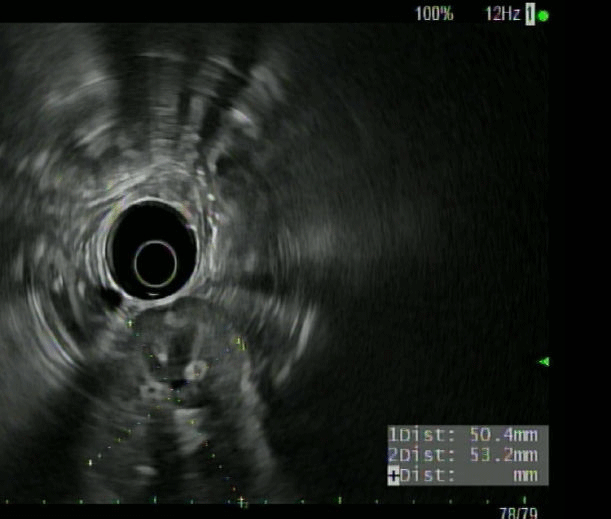
Figure A:
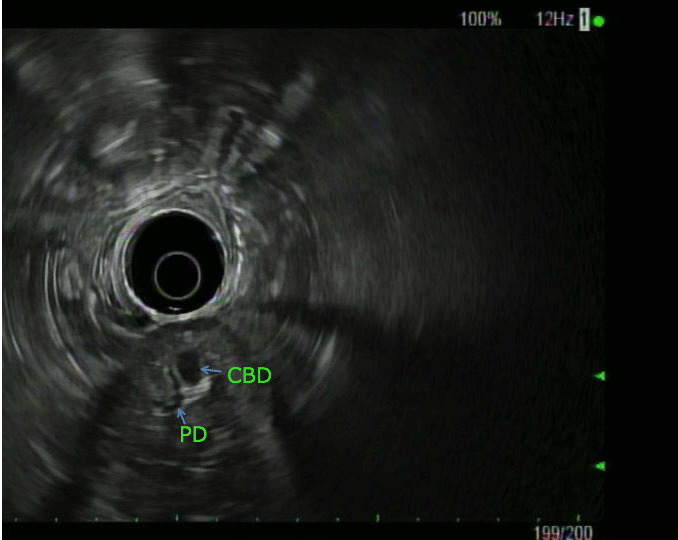
Figure B:
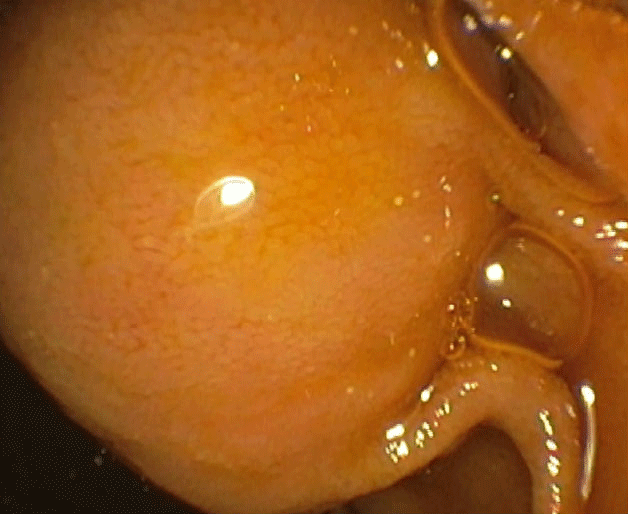
Figure C:
References
- Defrain C, Chang CY, Srikureja W, Nguyen PT, Gu M. Cytologic features and diagnostic pitfalls of primary ampullary tumors by EUS-guided FNA biopsy. Cancer. 2005; 105: 289-297.
- Pyun DK, Moon G, Han J, Kim MH, Lee SS, Seo DW et al. A carcinoid tumor of the ampullary of vater treated by endoscopic snare papillectomy. Korean J Intern Med. 2004; 19: 257-260.
- Jaoude WA, Lau C, Sugiyama G, Duncan A. Management of ampullary carcinoid tumors with pancreaticoduodenectomy. J Surg Case Rep. 2010; 2010: 4.