
Special Article - Esophageal Cancer
Gastrointest Cancer Res Ther. 2016; 1(2): 1011.
Immune Therapy in Esophageal Cancer: A Rationale and Current Status
Giuroiu I and Leichman L*
Perlmutter Cancer Center, New York University School of Medicine, New York, USA
*Corresponding author: Lawrence Leichman, MD, Perlmutter Cancer Center, 160E. 34th St, Room 8-941, New York, NY10016, USA
Received: October 25, 2016; Accepted: November 17, 2016; Published: November 21, 2016
Keywords
Esophageal adenocarcinoma; Esophageal squamous cell carcinoma; Immune checkpoint inhibition
Introduction
Esophageal cancer carries a poor prognosis within the United States and worldwide, with adenocarcinoma (EA) prevailing in the U.S. and the West as it is etiologically tied to rising obesity rates, associated acid reflux, and Barrett esophagus. However, the number afflicted with squamous cell carcinoma (ESCC) throughout the world is far greater than adenocarcinoma. ESCC is most prevalent in the Far East, including Japan and China, as well as in South Africa, Turkey, and Iran as it is etiologically tied to tobacco use, diets low in fresh fruit and vegetables, chemical preservatives in food, and exposure to the human papillomavirus (HPV) [1]. Surgical resection remains the gold standard of treatment for early tumors, but the addition of chemotherapy and radiation therapy has proven necessary for the control and enhanced survival of those presenting with locally advanced disease. While systemic cytotoxic chemotherapy is the chief option for the palliation of metastatic disease, immune checkpoint inhibitor therapy has emerged as a promising new therapeutic option as it has for several other malignancies. The theoretical basis for testing immune checkpoint inhibitor therapy for those with esophageal cancer rests with the tumor’s association with mutagenic toxins and its increased mutational burden. Herein we review current results and ongoing studies seeking improved outcomes in patients with esophageal cancer treated with immune therapy.
Esophageal Cancer
Esophageal cancer represents one percent of all new cancer diagnoses in the United States but carries a dismal average survival rate of less than 20 percent at 5 years [2]. An estimated 16,980 cases were diagnosed in the U.S. in 2015, with an estimated 15,590 patients expected to die from this disease [2]. Worldwide, approximately 455,800 cases are diagnosed each year, with 400,200 deaths per year representing the 6th most common cause of cancer death [3]. In spite of advances in combining chemotherapy, radiation therapy, and surgery, the 5-year overall survival rate ranges from 4.2% for those presenting with distant metastases at diagnosis to 40.4% for those presenting with locally advanced cancer at diagnosis [4].
Histologically, esophageal cancer can be divided into adenocarcinoma (EA) and squamous cell carcinoma (ESCC). These differ in etiology, precursor lesions, molecular properties, and epidemiology. While the incidence of EA has surpassed that of ESCC in the U.S., ESCCs represent 80 percent of esophageal cancer cases worldwide [5]. Inspite of these statistics, clinical trials and the treatment guidelines they helped establish have not distinguished between patients with the two different histologies. Specifically, clinical trials based in the U.S. largely reflect the performance of patients with EA, leaving ESCC an understudied disease [1].
Current Standard of Care for Esophageal Cancer
Multiple randomized controlled trials and meta-analyses have demonstrated survival benefit for patients with EA and ESCC treated with neoadjuvant chemoradiation [6]. The current standard of care for both EA and ESCC was established after publication in 2012 of the chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS), in which patients with both histologies were treated with weekly carboplatin AUC 2 and paclitaxel 50 mg/m2 administered concurrently with 41.4 Gy external-beam radiation prior to surgery versus surgery alone [7]. At initial publication, after a median follow-up of 45.4 months, the median overall survival (OS) was 49.4 months in the chemoradiotherapy-surgery group versus 24 months in the group undergoing surgery alone (P=0.003); notably, patients with ESCC had not yet reached a median OS [7]. Subsequently, at a median follow-up of 84 months, patients treated with neoadjuvant chemoradiotherapy followed by surgery still showed significant benefit in median progression-free survival (PFS) and OS as compared to those undergoing surgery alone [8]. Furthermore, among the patients treated neoadjuvantly, those with ESCC experienced near doubling of PFS and OS [8]. It is worth noting, however, that in spite of these significant gains, only 41% of the patients originally enrolled in the chemoradiotherapy plus surgery group were still alive at this median follow-up period of 7 years [8].
Even before the establishment of neoadjuvant chemoradiotherapy and surgery as the new standard of care for patients with esophageal cancer, studies explored the benefit of surgery in patients who achieved a complete pathologic response to neoadjuvant chemo radiotherapy. RTOG 85-01, a randomized trial comparing chemoradiotherapy to radiotherapy alone, without subsequent surgery, showed a 21% 5-year OS in patients with ESCC and a 27% 5-year combined OS for EAs and ESCCs [9,10]. The degree of pathologic response to neoadjuvant chemoradiotherapy was then shown to correlate with survival [11]. Subsequently, two randomized trials compared definitive chemoradiation to chemoradiation followed by surgery in patients with ESCC [12,13].
Neither trial showed an overall survival advantage for the addition of surgery to chemoradiation, although surgery was associated with a lower rate of local progression at 2 years [12]. Ultimately, these studies support the currently accepted practice of treating ESCC patients with definitive chemoradiation and foregoing surgery for patients who demonstrate a clinical complete response (cCR) by endoscopic clearance and 18F-fluorodeoxyglucose (FDG)-PET scan resolution of all FDG-avid areas.
Taken together, the above data underscore the need for novel local and systemic treatment modalities to improve upon the progress achieved with current chemoradiation for those with locally advanced esophageal cancer. The overarching goal, as with most neoadjuvant therapy, is to achieve a complete clinical response that can be translated into a complete pathologic response with attendant improved survival.
Immune Checkpoint Blockade and PD-L1 Expression in Esophageal Cancer
By evolving from a series of mutations arising in healthy cells, often as a result of extrinsic toxic insults, some cancers can be characterized as being “foreign” to their hosts. More specifically, they escape immune editing, a process by which tumors find a way to overcome their host’s natural immune defenses [14-16]. Most of these tumors have been shown to carry a relatively high mutational burden, thus presenting a larger variety of novel antigens to the immune system [15]. The classic example of a tumor with a high mutational burden that has demonstrated the property of overcoming immune editing is melanoma. Cancers that do not appear “foreign” to their host use immune editing to bypass the host’s immune defenses, and tumor growth proceeds largely unimpeded as a result of immune tolerance toward cancer cells [16].
The immune system’s attack against foreign antigens is highly regulated by stimulatory and inhibitory mechanisms that evolved to prevent overly destructive immune responses to pathogen invasion. Cancer cells exploit immune checkpoints to avoid recognition [17,18]. To date, two inhibitory receptors, programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4), have been successfully employed in the clinic for therapeutic inhibition that releases the breaks on immune attack against tumor cells. PD-1 and CTLA-4 are expressed on tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment, while PD-L1 and PD-L2 are expressed on the surface of tumor cells [19-21]. Monoclonal antibodies against PD-1(nivolumab and pembrolizumab) and its ligand, PD-L1 (atezolizumab), as well as against CTLA-4 (ipilimumab) are currently selectively approved in the treatment of melanoma, small cell and non-small cell lung cancer, renal cell carcinoma, urothelial carcinoma, Hodgkin lymphoma, and head and neck squamous cell carcinoma. These tumors generally feature high numbers of somatic mutations and neoantigens, which may be recognized as “foreign” to their host (Figure 1). In general, these are the tumors that have been associated with better clinical outcomes following PD-1 blockade [15,22]. Therefore, we can hypothesize that esophageal cancer and ESCC in particular, ranking high among mutation-bearing tumors, would also present promising targets for immune checkpoint inhibitors [15,23].
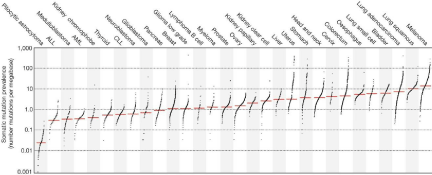
Figure 1: Somatic mutation burden in human cancers Used with permission: LB Alexandrov et al. Nature 500, 415-421 (2013) doi: 10.1038/nature12477.
However, a clear biomarker for response to immune checkpoint blockade remains elusive. Many of the tumors that bear a high somatic mutation burden can be recognized by pathologists as having a high tumor lymphocytic infiltration, which also correlates with better clinical outcomes [24,25]. Absolute numbers and relative proportions of different lymphocyte subtypes affect prognostic value [26].
Additionally, several tumor types have been analyzed to determine the reliability of PD-L1 as a predictive biomarker for response to anti-PD-1 therapy [27,28]. Among these, studies in gastric and GEJ adenocarcinoma, as well as one study of patients with EA yielded a mixed set of outcomes, mostly associating PD-L1 expression with a poor prognosis [29-38]. Furthermore, EA was found to express PDL1 at lower rates than gastric adenocarcinoma and to preferentially express PD-L2 over PD-L1 [31,38]. Over-expression of PD-L1 and associated poor prognosis may also support the pursuit of checkpoint blockade in esophageal cancer [27]. While high expression of PD-L1 has been used as a criterion for treatment with the PD-1 inhibitor pembrolizumab, its role as a predictive biomarker for all tumors has yet to be established [27,39].
Immune Checkpoint Inhibitor Therapy in Esophageal Cancer
Although reports testing ipilimumab and tremelimumab, both CTL4-A inhibitors, for patients with gastroesophageal cancers were disappointing, more recent trials testing the PD-L1 inhibitor pembrolizumab and the PD-1 inhibitor nivolumab have shown encouraging results [40-42].
An abstract presented at the 2016 ASCO Gastrointestinal Cancers Symposium described results of a Japanese phase II trial that evaluated treatment with nivolumab alone in 65 patients with ESCC who had progressed on up to 4 previous lines of therapy [43]. The investigators reported 1 complete response and 10 partial responses for a total response rate of 17.2% in addition to a 25% rate of stable disease [43]. Median OS was 12.1 months [43].
KEYNOTE-028, a multicohort phase Ib trial assessing the safety and efficacy of anti-PD-1 therapy with pembrolizumab in patients with PD-L1-positive advanced solid tumors included patients with advanced EA (26%) and ESCC (74%) [44]. Results for this cohort, also presented at the 2016 ASCO Gastrointestinal Cancers Symposium, showed an overall response rate (ORR) of 30.4%, with 13% of patients showing stable disease, 30.4% PFS at 6 months, 21.7% PFS at 12 months, and a median duration of response of 40 weeks [44].
KEYNOTE-012, another phase Ib trial assessing the effect of pembrolizumab in patients with PD-L1-positive recurrent or metastatic adenocarcinoma of the stomach or GEJ suggested activity in this group of patients: 22% showed a partial response and 14% showed stable disease [42]. Tumor site (gastric versus GEJ) was not specified for the patients who responded [42].
Preliminary results of the CheckMate-032 trial assessing the effect of anti-PD-1 therapy with nivolumab alone or in combination with anti-CTLA-4 treatment with ipilimumab in patients with advanced or metastatic gastric, esophageal, or GEJ cancer who had progressed on chemotherapy showed a 16% overall response rate, including 2 patients with a CR [45]. We anticipate further breakdown of the data by tumor type in the forthcoming full report from this trial [45].
Several studies are ongoing for immune checkpoint therapy alone or in combination with immunotherapy in patients with GEJ or esophageal cancer (Table 1) [42].
Trail Name
ClinicalTrails.gov
Identifier
Description
Status
Immune Checkpoint Inhibitors
KEYNOTE-062
NCT 02494583
A randomized phase III trial of pembrolizumab alone and in combination with cisplatin and fluorouracil versus cisplatin and fluorouracil alone for treatment –naïve patients with advanced gastric and GEJ adenocarcinoma
Recruiting
KEYNOTE-061
NCT 02370498
A phase III trial of pembrolizumab versus paclitaxel for patients with advanced gastric and GEJ adenocarcinoma who progressed after platinum and fluoropyrimidine-based chemotherapy
Active
Not recruiting
KEYNOTE-059
NCT 02335411
A phase III trial of pembrolizumab alone and in combination with cisplatin+5- fluorouracil in patients with recurrent or metastatic gastric or GEJ adenocarcinoma
Active
Not recruiting
-
NCT 02642809
A pilot study combining pembrolizumab with locally delivered radiation therapy for the initial treatment of metastatic esophageal cancers
Recruiting
Adoptive T-Cell Therapy
-
NCT 02457650
A phase I trial of T-cell receptor transduced T-cells targeting NY-ESO-1 in metastatic malignancies, including esophageal cancer, that express NY-ESO-1
Recruiting
Table 1: Active Clinical Trials for Immune Therapy in Gastric and Gastroesophageal Junction Adenocarcinoma and Esophageal Adenocarcinoma and Squamous Cell Carcinoma.
Radiation Therapy and Immune Checkpoint Blockade
The abscopal effect describes, in patients treated locally with radiation therapy, the subsequent response of unirradiated tumor sites [46,47]. Although the abscopal effect has been appreciated for decades, its mechanism has only recently been elucidated. By releasing tumor-associated antigens, radiation has been described as inducing a vaccine-like effect by priming the adaptive immune system [46,47]. An abscopal response was observed in a patient advanced melanoma previously treated with ipilimumab, who subsequently received palliative radiation to a painful metastatic site [48]. This patient’s tumor expressed NY-ESO-1, an antigen expressed in normal testicular germ cells and placenta, as well as in selected malignant tissues, including advanced melanomas and ESCC [48-50]. Significant regression was observed in tumors outside the radiation field [48]. Immune analyses revealed an increase in NY-ESO-1- specific antibody responses and in the proportion of NY-ESO-1- specific CD4+ T cells that expressed inducible co-stimulator (ICOS), a marker of T cell activation [48]. A second patient with melanoma treated with ipilimumab also experienced a similar abscopal response following radiation, with a corresponding increase in the antibody response against the cancer-testis antigen MAGE-A3 [51]. These studies offer rationale for exploring the effects of combining immune therapy with standard of care chemoradiotherapy for patients with esophageal cancer.
Ongoing Trials and Possibilities for the Future
Adoptive T-Cell therapy
An alternate form of immunotherapy, primarily developed in melanoma, isolates and expands antigen-specific T-cells from among tumor-infiltrating lymphocytes, subsequently transferring the T-cells back to lymphodepleted patients [52]. Adoptive T-cell therapy has proven effective in patients with metastatic melanoma and synovial sarcoma, particularly with T-cells specific to cancer testis antigen NYESO- 1 [53-55]. The expansion of adoptive T-cell therapy to esophageal cancer among other malignancies is under active investigation. NCT02457650 is a Phase I trial currently recruiting patients with a number of different malignancies expressing NY-ESO-1, including EA and ESCC, to therapy with T-cell receptor-transduced T cells targeting NY-ESO-1 (Table 1).
Combining immune checkpoint inhibition with radiation therapy in esophageal cancer
To our knowledge, a single active trial is available at this time for combined immune therapy and radiation therapy. NCT02642809 is a pilot study for the combination of anti-PD-1 therapy with pembrolizumab with locally delivered radiation therapy in the form of brachytherapy in patients with treatment-naïve metastatic esophageal cancers (Table 1).
Most patients with esophageal cancer present with locally advanced tumors without clinical evidence of metastatic disease. Therefore, most patients are treated with chemotherapy and radiation prior to surgery with the goal of significant down staging prior to surgery or chemotherapy and radiation as definitive therapy, especially for those with ESCC. Our group has developed a protocol to test the safety of the PD-1 inhibitor nivolumab, given initially alone and then with radiation and carboplatin and paclitaxel for patients with ESCC. Our goal is to move PD-1 therapy from the palliative setting into the curative setting for patients with ESCC.
Conclusion
Monotherapy with immune checkpoint inhibitors has shown promising response rates in early clinical trials for patients with esophageal, as well as gastric and gastroesophageal junction tumors. However, larger randomized trials for patients with esophageal adenocarcinoma and squamous cell carcinoma are needed to corroborate current results. Additionally, we hypothesize that multimodality therapy combining current standard chemoradiotherapy and immune therapy may help improve upon the positive results seen thus far.
References
- Leichman L, Thomas CR. Squamous Cell Cancer of the Esophagus: The Forgotten One. Gastrointest Can Res. 2011; 4: 22-23.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2015. CA Cancer J Clin. 2015; 65: 5-29.
- Torre LA, B.F, Siegel RL, et al. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015; 65: 87-108.
- Available from: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969-2013
, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016. Underlying mortality data provided by NCHS (www.cdc.gov/nchs). - Shridhar R, A.K, Meredith KL, et al. Radiation Therapy and Esophageal Cancer. Cancer Control. 2013; 20: 97-110.
- Huang TC, H.C, LCC, Tu YK. Systematic review and network meta-analysis: neoadjuvant chemoradiotherapy for locoregional esophageal cancer. Jpn J Clin Oncol. 2015; 45: 1023-1028.
- Van Hagen P, H.M, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med. 2012; 366: 2074-2084.
- Shapiro J, v.L.J, Hulshof MC, C.M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for esophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015; 16: 1090-1098.
- Al-Sarraf M, M.K, Herskovic A, et al. Progress Report of Combined Chemoradiotherapy Versus Radiotherapy Alone in Patients with Esophageal Cancer: An Intergroup Study. J Clin Oncol. 1997; 15: 277-284.
- Cooper JS, G.M, Herskovic A, Macdonald JS, et al. Chemoradiotherapy of Locally Advanced Esophageal Cancer: Long-term Follow-up of a Prospective Randomized Trial (RTOG 85-01). JAMA. 1999; 281: 1623-1627.
- Swisher SG, H.W, Wu TT, et al. Proposed Revision of the Esophageal Cancer Staging System to Accomodate Pathologic Response (pP) Following Preoperative Chemoradiation (CRT). Ann Surg. 2005; 241: 810-820.
- Stahl M, S.M, Lehmann N, et al. Chemoradiation With and Without Surgery in Patients with Locally Advanced Squamous Cell Carcinoma of the Esophagus. J Clin Oncol. 2015. 23: 2310-2317.
- Bedenne L, M.P, Bouche O, et al. Chemoradiation Followed by Surgery Compared with Chemoradiation Alone in Squamous Cell Cancer of the Esophagus: FFCD 9102. J Clin Oncol. 2007; 25: 1160-1168.
- Stratton MR, C.P, Futreal PA. The cancer genome. Nature. 2009; 458: 719-724.
- Alexandrov LB, N.-Z.S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013; 500: 415-421.
- Vonderheide RH, B.L. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol. 2013; 25: 200-205.
- Iwai Y, I.M, Tanaka Y, Okazaki T, Honjo T and N. Minato. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences. 2002; 99: 12293-12297.
- LEACH DR, K.M, ALLISON JP. ENHANCEMENT OF ANTITUMOR IMMUNITY BY CTLA-4 BLOCKADE. SCIENCE. 1996; 271: 1734-1736.
- McDermott DF, A.M. PD-1 as a potential target in cancer therapy. Cancer Med. 2013; 2: 662-673.
- Ahmadzadeh M, J.L, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired Blood. 2009; 114: 1537-1544.
- KEIR ME, B.M., FREEMAN GJ, ET AL. PD-1 AND ITS LIGANDS IN TOLERANCE AND IMMUNITY. ANNU REV IMMUNOL. 2008; 26: 677-704.
- Rizvi NA, H.M, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015; 348: 128-128.
- Sasaki Y, T.M, Koyama R, et al. Genomic characterization of esophageal squamous cell carcinoma: Insights from next-generation sequencing. World J Gastroenterol. 2016; 22: 2284-2293.
- Pages F, G.J, Dieu-Nosjean M-C, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2009; 29: 1093-1102.
- Fridman W-H, D.-N.M.-C, Pages F, et al. The Immune Microenvironment of Human Tumors: General Significance and Clinical Impact. Cancer Microenv. 2013; 6: 117-122.
- Gooden MJM, d.B.G, Leffers N, et al. The prognostic influence of tumor-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011; 105: 93-103.
- Wu P, W.D, Li L, Chai Y, Huang J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS ONE. 2015; 10: 1-15.
- Jin Z, Y.H. The promise of PD-1 inhibitors in gastro-esopheal cancers: microsatellite instability vs. PD-L1. J Gastrointest Oncol. 2016; 7: 771-788.
- Boger C, B.H, Mathiak M, et al. PD-L1 is an independent prognostic predictor in gastric cancer in Western patients. Oncotarget. 2016; 7: 24269-24283.
- Geng R, D.C, Wong A, et al. Prognostic significance of tumor infiltrating immunecells and PD-L1 expression in gastric carcinoma in Chinese patients. J Clin Oncol. 2015; 33: abstr 4042.
- Derks S, N.K, Liao X, et al. Epithelial PD-L2 Expression Marks Barrett's Esophagus and Esophageal Adenocarcinoma. Cancer Immunol Res. 2015; 3: 1123-1129.
- Tanaka H, T.T, Kimura K, et al. Association of the immune checkpoint molecule expression with neutrophil-lymphocyte ratio in patients with gastric cancer: Aretrospective study. J Clin Oncol. 2016; 34: abstr 48.
- Schloesser HA, D.U, Thelen M, et al. Comprehensive characterization of PDL-1 and CTLA-4 in gastric cancer. J Clin Oncol. 2015; 33: abstr 4056.
- Hou J, Y.Z, Xiang R, et al. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol. 2014; 96: 284-291.
- Wu C, Z.Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006. 108: 284-291.
- Kelly RJ, T.E, Zahurak M, et al. Adaptive immune resistance in gastro-esophageal cancer: Correlating tumoral/stromal PDL1 expression with CD8+ cell count. J Clin Oncol. 2015; 33: abstr 4031.
- Nishina T, S.K, Iwasa S, et al. Safety, PD-L1 expression, and clinical activity of avelumab (MSB0010718C), an anti-PD-L1 antibody, in Japanese patients with advanced gastric or gastroesophageal junction cancer. J Clin Oncol. 2016; 34: abstr 168.
- Ohigashi Y, S.M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005; 11: 2947-2953.
- Long GV, L.J, Ascierto PA, et al. PD-L1 expression as a biomarker for nivolumab (NIVO) plus ipilimumab (IPI) and NIVO alone in advanced melanoma (MEL): A pooled analysis in ESMO. 2016. Ann Oncol.
- Ralph C, E.E, Burt DJ, et al. Modulation of Lymphocyte Regulation for Cancer Therapy: A Phase II Trial of Tremelimumab in Advanced Gastric and Esophageal Adenocarcinoma. Clin Cancer Res. 2010; 16: 1662-1672.
- Moehler MH, CJ, Kim YH, et al. A randomized, open-label, two-arm phase II trial comparing the efficacy of sequential ipilimumab (ipi) versus best supportive care (BSC) following first-line (1L) chemotherapy in patients with unresectable, locally-advanced/metastatic (A/M) gastric or gastro-esophageal junction (G/GEJ) cancer. in ASCO Annual Meeting 2016. J Clin Oncol.
- Muro K, CH, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive adavnced gastric cancer (KEYNOTE-012): a multicentre, open-label phase 1b trial. Lancet Oncol. 2016; 17: 717-726.
- Kojima T, HH, Yamaguchi K, et al. Phase II study of nivolumab (ONO-4538/BMS-936558) in patients with esophageal cancer: Preliminary report of overall survival. J Clin Oncol. 2016; 34: Abstr TPS175.
- Doi T, P-PS, Jalal S, et al. Updated results for the advanced esophageal carcinoma cohort of the phase Ib KENOTE-028 study of pembrolizumab. in 2016 Gastrointestinal Cancers Symposium. 2016. J Clin Oncol.
- Janjigian YY, BJ, Calvo E, et al. CheckMate-032: Phase I/II, open-label study of safety and activity of nivolumab (nivo) alone or with ipilimumab (ipi) in advanced and metastatic (A/M) gastric cancer (GC). in 2016 ASCO Annual Meeting. 2016. JClin Oncol.
- Formenti SC, DS. Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift. J Natl Cancer Inst. 2013; 105: 256-265.
- Seyedin SN, SJ, Lee DA, et al. Strategies for combining immunotherapy with radiation for anticancer therapy. Immunotherapy. 2015; 7: 967-980.
- Postow MA, CM, Barker CA, Yamada, Yoshiya, et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. The New England Journal of Medicine. 2012; 366: 925-931.
- Fujita S, WH, Jungbluth AA, et al. NY-ESO-1 Expression and Immunogenicity in Esophageal Cancer. Clin Cancer Res. 2004; 10: 6551-6558.
- Oshima Y, SH, Yahima S, et al. NY-ESO-1 autoantibody as a tumor-specific biomarker for esophageal cancer: screening in 1969 patients with various cancers. J Gastroenterol. 2016; 51: 30-34.
- Stamell EF, WJ, Gnjatic S, NY Lee, and I Brownell. The Abscopal Effect Associated With a Systemic Anti-melanoma Immune Response. International Journal of Radiation Oncology*Biology*Physics. 2013; 85: 293-295.
- Rosenberg SA, RN, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008; 8: 299-308.
- Rosenberg SA, YJ, Sherry RM, et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clin Cancer Res. 2011; 17: 4550-4557.
- Robbins PF, MR, Feldman SA, et al. Tumor Regression in Patients with Metastatic Synovial Cell Sarcoma and Melanoma Using Genetically Engineered Lymphocytes Reactive with NY-ESO-1. J Clin Oncol. 2011; 29: 917-924.
- Hunder NN, WH, Cao J, et al. Treatment of Metastatic Melanoma with Autologous CD4+ T Cells against NY-ESO-1. N Engl J Med. 2008; 358: 2698-2703.