Review Article
Austin J Genet Genomic Res. 2014;1(2): 5.
Myc: Master Regulator of Global Genomic Expression
S. Idiyasan Chanu and Surajit Sarkar*
Department of Genetics, University of Delhi South Campus, India
*Corresponding author: Surajit Sarkar, Department of Genetics, University of Delhi South Campus, Benito Juarez Road, New Delhi-110 021, India;
Received: August 14, 2014; Accepted: October 10, 2014; Published: October 28, 2014
Abstract
The proto-oncogene Myc family is one of the most comprehensively studied group of genes in the contemporary area of genomic research. Myc interacts with other small proteins such as Max, Mad/Mnt, Miz1 etc. and modulates the dynamics of global gene expression. In the recent decade, high throughput screening based on microarray gene profiling, Serial Analysis of Gene Expression (SAGE), Chromatin Immunoprecipitation (ChIP) followed by genomic array analysis have generated a great deal of information regarding genomic binding loci of Myc and its putative target genes. Intriguingly, several thousands of genomic targets have been identified and estimated that Myc regulates approximately 10-15% of the global transcriptome. In view of above, Myc has been proposed to function as a master regulator of global genome expression and in this capacity Myc regulates major cellular functions such as cell proliferation, cell adhesion, metabolism, protein biosynthesis, etc. In consequent to its broad spectrum of target genes, dysregulation of Myc expression has been implicated with development of several fatal diseases including cancer. Therefore, extensive understanding of the genomic targets of Myc is essential to delineate the enigma of the complex biological functions of this exceptionally important gene family and to develop novel therapeutic strategies.
Keywords: Myc; Transcription factor; Genome; Transactivation; CpG islands
Introduction
In the area of contemporary genomic research, the proto-oncogene Myc family has emerged as a most exhaustively studied group of genes. Decades ago, myc oncogene was discovered as a homolog of v-myc oncogene from an avian retrovirus [1,2]. It was found that translocation of myc loci from 8q24 chromosomal position to one of the immunoglobin loci is responsible for Burkitt's lymphoma [3,4]. This was the first report which suggested a potential involvement of myc loci in tumorigenesis. Subsequently, several studies have reported frequent overexpression of Myc in most of the types of human cancers including lung, ovarian, breast and prostate cancer which contribute about 70% of the all types of human tumours [5]. It was proposed that abnormal amplification due to chromosome translocation is not the only cause of Myc mediated tumour transformation, rather modulation in the expression level of Myc accounts for abnormal cellular homeostasis and tumour transformation of normal cells [6]. Therefore, anticipating the potential involvement of Myc in major cellular functions and human diseases; comprehensive analyses were performed to characterize the molecular role of Myc in normal development.
The Myc family includes three important oncogenes - c-Myc, L-Myc and N-Myc. In response to the diverse signals, Myc binds with Max protein and functions as transcription factor. Subsequently, Myc-Max complex binds to the specific DNA sequences to modulate the expression of downstream target genes [7]. Therefore, in view of a notable impact of Myc on global gene regulation; deregulation of Myc has a massive negative effect on cellular system. Supporting the role of Myc in tumorigenesis, enhanced expression of Myc has been demonstrated both in-vivo and in-vitro to provide proliferative advantages and cellular transformation [8,9]. Moreover, signifying its key role in normal development, targeted homozygous deletion of c-Myc resulted in embryonic lethality in mouse [10]. In addition, inactivation of Myc resulted in elongation of cell cycle, tumour regression and cellular re-differentiation in Myc induced tumour cells; which further supports a significant role of this protein in cell proliferation [11-13]. Therefore, in view of a fundamental role of Myc in normal cellular functioning and tumorigenesis; understanding the molecular functions of Myc have emerged as an area of global interest with the hope of developing novel therapeutic approaches against several diseases.
The paradox of Myc as potent transcription factor and its ability to influence expression of hundreds of the target genes has occupied the central position in the growing areas of Myc research. It was estimated by both genomic and functional approaches that Myc alone could regulate the expression of ~15% of the genome from Drosophila to human [14,15]. Myc regulates transactivation of several key genes those are involved in cellular proliferation and growth, ribosome biogenesis, protein synthesis, metabolism, mitochondrial biogenesis and cell reprogramming, through direct or indirect mechanisms [16-18]. In addition to the above noted functions, active involvement of Myc in trans-repression of genes those are involved in cell growth arrest, cell adhesion and cell-cell communication has also been found to be essential for maintenance of cellular homeostasis [16,19,20]. In view of several reports, it appear that expression level of Myc is a key deciding factor for cells to undergoes cell division or differentiate into various cell lineages during development and subsequently, it is not surprising that cellular amplification of Myc provides some proliferative advantage to the dividing cells during tumour transformation. Therefore, oncogenic properties of Myc are the consequent result of its capability to influence the status of global transcriptome. However, in spite of a great deal of available information, a unified view on the Myc functions is still elusive due to poorly defined sets of target genes. Further analyses with comprehensive efforts would be required to dissect out the complex Myc networks with the hope to develop better understanding of these genes in normal development and disease pathogenesis. A brief overview of some of the major signalling network modulated by Myc and the subsequent consequences has been discussed below.
Myc, Max, Mad/Mnt network in regulation of the functional dynamics of Myc
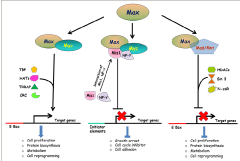
Myc, Max, Mad/Mnt network includes a group of transcription factors and distinct interactions of these factors regulate cellular functions such as cell proliferation and differentiation. This network includes members of the small basic Helix-loop-Helix Leucine Zipper (bHLH-LZ) proteins such as Myc and Mad family, Max and the Mad-related protein Mnt. Myc harbours a bHLH-LZ domain at its C-terminal and a potential transactivation domain at N-terminal [21]. In this context it is interesting to note that when Myc was demonstrated to be a nuclear protein decades ago, a probable role of this protein in transcription was postulated [22,23]. Subsequent discovery of its obligatory interacting partner, Max (a bHLH-LZ protein) has further established Myc as a transcription factor [24]. With the help of its bHLH-LZ domain, Max heterodimerises with Myc to form sequence specific DNA binding complex. Studies in yeast to mammalian system using synthetic reporter genes have demonstrated that Myc-Max heterodimer complex binds to the canonical binding sites E-box sequence (5'-CACGTG-3') as well as related non-canonical sites and accomplish transcriptional activities [25,26]. Although, Max itself has the ability to form homodimer with its own partners, however, it lacks the proficiency to initiate transcriptional activity. Moreover, recent evidences suggest that the transcriptional activities of Myc-Max heterodimers are the result of their ability to recruit several chromatin-modifying complexes such as TBP, TRRAP coactivator, histone acetyl transferases and GCN5, to the E-box sites, which facilitates histone acetylation [27- 29]. In support to the general role of Myc-Max protein complex in transactivation, it has been demonstrated that mutation in the bHLH-LZ domain of Myc which requires for interaction with Max and DNA, or mutation in the transactivation domain, makes a negative impact on Myc mediated cellular proliferation [30]. In addition, high throughput chemical screening has demonstrated that some small molecules which antagonize the dimerization process of Myc-Max complex inhibit Myc-induced transformation in chicken fibroblast cells [31]. Taken together, above observations clearly suggest a tight relationship between interaction dynamic of Myc-Max complex and biological activities of Myc.
Identification of Max interacting partner, Mad/Mnt protein family further helped in understanding the networks which regulate the dynamics of Myc functions [32,33]. In addition to Myc, Max also interacts with a small bHLH-LZ protein Mad/Mnt and binds to the E-box consensus sequences which are identical to the Myc-Max binding regions. Max-Mad complex competes with the Myc-Max complex to bind the identical genomic sites and consequently, inhibits transactivation mediated by Myc-Max heterodimer. Binding of the Max-Mad protein complex at the genomic sites leads to recruitment of chromatin remodelling complexes such as Sin3, N-CoR and several histone deacetylases; which results in close chromatin conformation and inhibition of E-box mediated transcriptional activation [34,35]. Counteracting the Myc-Max mediated cell proliferation during terminal cell differentiation, switching of Myc-Max binding to Max- Mad binding has been demonstrated in terminally differentiated mammalian cell models [36]. Similarly, induction of Mad and Mnt proteins expression in terminally differentiated cells also signifies the importance of Max-Mad mediated repression of gene expression during cellular differentiation [37]. Thus, unlike ubiquitous expression of Max, it is well evident that tight regulation of Myc and Mad/Mnt expression is essential for a cell to take critical decision during normal development. A brief overview of Myc/Max network and its implications on gene expression have been provided in figure 1. Nevertheless, despite much of these impressive advances, major challenges remain in understanding the complex Myc networks and their potential association with disease pathogenesis.
Genomic binding sites of Myc
Since the period when Myc was established as transcription factors, identification and quantification of the Myc targets have been one of the key objectives. After several decades of studies, efforts from various groups have resulted in identification of hundreds of candidates which are directly regulated by Myc. Initially, target gene screenings were based on empirical methods such as candidate based approaches, functional screening and Serial Analysis of Gene Expression (SAGE) [16]. These approaches had resulted in identification of several downstream targets of Myc [16]. Subsequently, analyses based on some contemporary techniques such as genome wide microarray had resulted in exponential increase in the list of Myc target genes, and therefore, aggravated the complexity of Myc biology. However, despite all these efforts, it is difficult to establish precise numbers of target genes which are being regulated by Myc. Moreover several efforts were remain unsuccessful in differentiating those of the direct target genes of Myc from the indirect ones [38-40].
Initial genomic binding studies by chromatin immunoprecipitation (CHIP) in different human cancer cell lines expressing high level of Myc have found that Myc binds with 44-55% of 723 individual genes which were known to comprise E-box sites [41]. Interestingly, depending upon the cellular level of Myc; these E-box sites show distinct binding patterns. This indicates discrete responsive pattern of different genomic sites to varying level of Myc and also provides a physiological significance of the high level of Myc in disease conditions. Similarly, an accompanying study has identified Myc/ Max/Mnt binding loci in Drosophila genome [14]. In this study, DNA sequences encoding for Myc/Max/Mnt were fused to a gene encoding DNA methylase and based on the genomic methylation pattern, the genomic loci being regulated by Myc response were identified [14]. Out of the total 6255 genes screened, about 15% of the Drosophila genes were found to be influenced by the complexes of Myc/Max/ Mnt network. Performing unbiased genome wide location analyses in cancer cells, another group has further attempted to determine the Myc associated molecular targets and also examined the genomic binding sites [15]. Experiments based on customised DNA microarray containing randomly selected annotated promoters of 4,839 human genes and subsequent ChIP analysis have demonstrated that 721 of the 4,839 gene promoters were occupied by Myc-Max protein complex [15]. Confirming the earlier observation [14], it was estimated that about 15% of the total human genes were bound by Myc in Burkitt's lymphoma cells [15].
High throughput sequencing of ChIP DNA (ChIP-Seq) has emerged as a remarkable approach to identify the Myc binding genomic loci. It was estimated that Myc occupies 4,296 genomic loci and majority of these sites are near proximal promoter regions which could be frequently associated with CpG islands [42]. Utilizing ChIP-Seq strategy, 668 genes were identified as direct target of Myc, among which 48 genes were found to encode for various transcription factors [42]. Interestingly, Myc was also suggested to be involved in mitochondrial metabolism as 107 of Myc target genes encode for proteins involved in mitochondrial biogenesis (including POLG, POLG2, and NRF1) [43]. In some of the recent studies, more than 7,000 genomic loci have been identified by high throughput ChIP-Seq [44-46]. Identification of novel Myc targets not only enriches our knowledge about the enormous complexity of the Myc regulatory network but also helps in designing novel experimental strategies for subsequent analysis.
Interestingly, CpG islands were found to be major determinant of Myc binding to promoter regions of the target genes. CpG islands are stretches of cytosine-guanine (CpG) dinucleotide and in mammalian genome these regions could be generally associated with actively transcribing genes and DNA methylation [47]. Interestingly, high affinity E-box sites were found to be frequently associated with the CpG islands which enhance the transcriptional efficiency of the associated promoters [41,42]. Supporting the preferential binding of Myc within CpG islands, in-vitro analysis has demonstrated that methylation of the central CpG in CACGTC element prevents Myc- Max complex formation [48]. In view of above, Myc bound loci were also investigated based on the CpG islands in human acute myeloid leukaemia cells. This resulted in identification of 177 loci, out of which 107 loci overlap with previously identified genes [49].
As discussed earlier, genomic binding analyses suggest that about 10-15% of the genomic loci interact with Myc, however, this binding is always not dependent to the availability of E-box sites in promoters. Therefore, it appears that in addition to the E-boxes and CpG islands, there might be other determinants as well [41]. It has been proposed that status of chromatin structure and availability of additional DNA binding proteins could facilitate Myc binding to the genomic loci and assist in regulating the downstream target genes [41]. In this context it is interesting to note that in contrary to the known function of Myc in recruiting histone acetyltransferases; chromatin around Myc bound loci are highly pre-acetylated even before binding of Myc [41]. Subsequent analyses have revealed that stretches of chromatin bearing highly acetylated and methylated (especially H3-K4/K79) histones exhibit increased affinity to Myc [50]. Therefore, probability of preferential Myc binding is more toward the chromatin regions which is already poised for transcription. Significant overlap between genomic binding site of Myc and TFIID (basal transcription complex) further strengthen the above hypothesis [15].
Impact of Myc on global gene expression
Besides the fact that Myc could regulate about 10-15% of all the genes from flies to human, its operating mechanism is debatable. Several mechanisms including recruitment of histone acetyltransferases, chromatin modulating proteins, DNA methyltransferases and other transcription factors were suggested to explain the Myc mediated modulation in genomic expression. A number of studies have demonstrated that Myc interacts with Max and binds to the E-box consensus sequences which result in recruitment of multiple co-activator complexes such as TBP, TRRAP and histone acetylases [27-29]. Histone acetylases open up the compact chromatin structure and make the DNA easily approachable to basal transcriptional machinery in order to initiate transactivation network [51]. It has been found that Myc-Max heterodimer influences transactivation of several genes involved in cellular growth, proliferation, protein biosynthesis and metabolism [18]. Moreover, those of the identified Myc targets such as Cyclins and Cyclin Dependent Kinases (CDKs) are already known to be involved in regulation and progression of cell cycle [14,18]. It was suggested that Myc influence cell growth possibly through transactivating the genes encode for ribosomal RNAs and ribosome biogenesis proteins. Interestingly, in order to compensate the increased demand of protein synthesis due to Myc mediated induced cell proliferation; protein synthesis machinery is constantly regulated at multiple levels. Identification of sub-groups of Myc target genes which encodes for tRNA and rRNA further supports the role of Myc in regulation of protein biosynthesis [52]. In addition, Myc was found to regulates several genes involved in mitochondrial functioning along with the genes encoding key enzymes to facilitate glucose metabolism such as glucose transporter, hexokinase II, lactate dehydrogenase A, enolase A etc. [53,54]. Moreover, role of Myc in DNA metabolism is well evident as it activates the genes encoding for enzymes such as ornithine decarboxylase (ODC), carbamoyl phosphate synthase, aspartate transcarbamylase etc. [55].
Intriguingly, in addition to the transactivation, role of Myc in transcriptional repression is equally critical for cellular functioning. It has been found that Myc represses multiple classes of genes which encode negative regulators of cell proliferation (c/EBPα, c/EBPδ), growth arrest (Gadd45, Gadd153 etc), cell cycle inhibitors (p15INK4B, p181INK4C, p21 and p27 etc.), cell adhesion (integrin β1) etc. [20]. Myc binds to core promoter of the genes to repress them directly; however, unlike the Myc mediated transactivation, it does not require the canonical binding sites. Distinct promoter sequences like initiator elements (INR) were found to be a major deciding factor [56]. One of the proposed mechanisms postulates that Myc-Max complex directly interacts with transcription factors such as Miz1, NF-Y etc. to inhibit the expression of the target genes (Figure 1) [20,57]. For instance, interaction of Myc to Miz-1 represses expression of the CDK inhibitors p15INK4B and p21, which are transactivated by Miz- 1 [58,59]. Moreover, interaction of Myc with NF-Y (a CAAT box binding protein) affects the expression of PDGFR-β and some of the collagen genes [57]. All these observations suggest that in order to promote cell proliferation, Myc inhibits expression of the genes which are otherwise involved in negative regulation of cell proliferation. Moreover, equilibrium between Myc mediated transactivation and repression function as a critical and decisive factor to mediate normal development. However, unlike transcriptional activation, the molecular mechanism(s) operating Myc mediated gene repression is still enigmatic.
Figure 1 : An overview of the Myc/Max/Mad network and its impact on global gene expression (please refer the main text for details). [TBP- TATA binding protein; HATs- Histone acetyltransferases; CRC- Chromatin remodelling complex; HDACs- Histone deacetylases; TRRAP- Transformation/ transcription domain associated protein subunit].
Conclusion
As discussed earlier, Myc oncogene has emerged as central regulator for several distinct cellular programmes and influences a broad spectrum of target genes. Over the last several years, intense interests from various research groups have resulted in generation of vast amount of information to decipher biological functions and genomic targets of Myc. Despite of all these extensive analyses, it is difficult to establish a linear correlation between Myc bound loci and expression of target genes. It appears that in spite of harbouring Myc responsive element, only a fraction of such genes are actually responsive to Myc binding [14,15,41,60]. Therefore, Myc binding to a given loci may not be always correlated well with the transcriptional status of the target gene. Depending on certain cellular environment and developmental period, Myc bound loci may function differentially, and therefore, subsets of genes regulated by Myc might have tissue specific responses. It also appears that Myc binding in the genome could be non-tissue specific, whereas the genes which are actually regulated by Myc binding are tissue specific. Moreover, varying dosage of Myc may act differentially in various cell types and disease conditions, and therefore, could be utilized as a potential target to develop novel therapeutic strategies [61]. Taken together, further extensive analyses on Myc bound loci and its expression data would be worth in defining Myc targets and delineating the complex network of Myc biology.
Acknowledgements
Research programmes in laboratory is supported by grants from the Department of Biotechnology (DBT), Government of India, New Delhi; Department of Science and Technology (DST), Government of India, New Delhi, and Delhi University R & D fund to SS. SIC is supported by the Senior Research Fellowship (SRF) from the University Grant Commission (UGC), New Delhi.
References
- Sheiness D, Bishop JM. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979; 31: 514-521.
- Vennstrom B, Sheiness D, Zabielski J, Bishop JM. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J Virol. 1982; 42: 773-779.
- Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982; 79: 7824-7827.
- Taub R, Kirsch I, Morton C, Lenoir G, Swan D, Tronick S, et al. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982; 79: 7837-7841.
- Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999; 18: 3004-3016.
- Erisman MD, Rothberg PG, Diehl RE, Morse CC, Spandorfer JM, Astrin SM. Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol Cell Biol. 1985; 5: 1969-1976.
- Amati B, Brooks MW, Levy N, Littlewood TD, Evan GI, Land H. Oncogenic activity of the c-Myc protein requires dimerization with Max. Cell. 1993; 72: 233-245.
- Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983; 304: 596-602.
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985; 318: 533-538.
- Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993; 7: 671-682.
- Mateyak MK, Obaya AJ, Adachi S, Sedivy JM. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 1997; 8: 1039-1048.
- Karlsson A, Giuriato S, Tang F, Fung-Weier J, Levan G, Felsher DW. Genomically complex lymphomas undergo sustained tumor regression upon MYC inactivation unless they acquire novel chromosomal translocations. Blood. 2003; 101: 2797-2803.
- Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004; 431: 1112-1117.
- Orian A, van Steensel B, Delrow J, Bussemaker HJ, Li L, Sawado T, et al. Genomic binding by the Drosophila Myc, Max, Mad/Mnt transcription factor network. Genes Dev. 2003; 17: 1101-1114.
- Li Z, Van Calcar S, Qu C, Cavenee WK, Zhang MQ, Ren B. A global transcriptional regulatory role for c-Myc in Burkitt's lymphoma cells. Proc Natl Acad Sci U S A. 2003; 100: 8164-8169.
- Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999; 19: 1-11.
- Araki R, Hoki Y, Uda M, Nakamura M, Jincho Y, Tamura C, et al. Crucial role of c-Myc in the generation of induced pluripotent stem cells. Stem Cells. 2011; 29: 1362-1370.
- Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013; 3.
- Claassen GF, Hann SR. Myc-mediated transformation: the repression connection. Oncogene. 1999; 18: 2925-2933.
- Herkert B, Eilers M. Transcriptional repression: the dark side of myc. Genes Cancer. 2010; 1: 580-586.
- Nair SK, Burley SK. X-ray structures of Myc-Max and Mad-Max recognizing DNA. Molecular bases of regulation by proto-oncogenic transcription factors. Cell. 2003; 112: 193-205.
- Hann SR, Eisenman RN. Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol. 1984; 4: 2486-2497.
- Eisenman RN, Tachibana CY, Abrams HD, Hann SR. V-myc- and c-myc-encoded proteins are associated with the nuclear matrix. Mol Cell Biol. 1985; 5: 114-126.
- Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991; 251: 1211-1217.
- Amati B, Dalton S, Brooks MW, Littlewood TD, Evan GI, Land H. Transcriptional activation by the human c-Myc oncoprotein in yeast requires interaction with Max. Nature. 1992; 359: 423-426.
- Kretzner L, Blackwood EM, Eisenman RN. Myc and Max proteins possess distinct transcriptional activities. Nature. 1992; 359: 426-429.
- McMahon SB, Wood MA, Cole MD. The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc. Mol Cell Biol. 2000; 20: 556-562.
- Frank SR, Schroeder M, Fernandez P, Taubert S, Amati B. Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev. 2001; 15: 2069-2082.
- Barrett JF, Lee LA, Dang CV. Stimulation of Myc transactivation by the TATA binding protein in promoter-reporter assays. BMC Biochem. 2005; 6: 7.
- Eisenman RN. Deconstructing myc. Genes Dev. 2001; 15: 2023-2030.
- Berg T, Cohen SB, Desharnais J, Sonderegger C, Maslyar DJ, Goldberg J, et al. Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 2002; 99: 3830-3835.
- Bourbon HM, Gonzy-Treboul G, Peronnet F, Alin MF, Ardourel C, Benassayag C, et al. A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech Dev. 2002; 110: 71-83.
- Loo LW, Secombe J, Little JT, Carlos LS, Yost C, Cheng PF, et al. The transcriptional repressor dMnt is a regulator of growth in Drosophila melanogaster. Mol Cell Biol. 2005; 25: 7078-7091.
- Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997; 89: 349-356.
- Alland L, Muhle R, Hou H Jr, Potes J, Chin L, Schreiber-Agus N, et al. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997; 387: 49-55.
- Ayer DE, Eisenman RN. A switch from Myc:Max to Mad:Max heterocomplexes accompanies monocyte/macrophage differentiation. Genes Dev. 1993; 7: 2110-2119.
- Västrik I, Kaipainen A, Penttilä TL, Lymboussakis A, Alitalo R, Parvinen M, et al. Expression of the mad gene during cell differentiation in vivo and its inhibition of cell growth in vitro. J Cell Biol. 1995; 128: 1197-1208.
- Guo QM, Malek RL, Kim S, Chiao C, He M, Ruffy M, et al. Identification of c-myc responsive genes using rat cDNA microarray. Cancer Res. 2000; 60: 5922-5928.
- Schuhmacher M, Kohlhuber F, Hölzel M, Kaiser C, Burtscher H, Jarsch M, et al. The transcriptional program of a human B cell line in response to Myc. Nucleic Acids Res. 2001; 29: 397-406.
- Neiman PE, Ruddell A, Jasoni C, Loring G, Thomas SJ, Brandvold KA, et al. Analysis of gene expression during myc oncogene-induced lymphomagenesis in the bursa of Fabricius. Proc Natl Acad Sci U S A. 2001; 98: 6378-6383.
- Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J, et al. Genomic targets of the human c-Myc protein. Genes Dev. 2003; 17: 1115-1129.
- Zeller KI, Zhao X, Lee CW, Chiu KP, Yao F, Yustein JT, et al. Global mapping of c-Myc binding sites and target gene networks in human B cells. Proc Natl Acad Sci U S A. 2006; 103: 17834-17839.
- Kim J, Lee JH, Iyer VR. Global identification of Myc target genes reveals its direct role in mitochondrial biogenesis and its E-box usage in vivo. PLoS One. 2008; 3: e1798.
- Seitz V, Butzhammer P, Hirsch B, Hecht J, Gütgemann I, Ehlers A, et al. Deep sequencing of MYC DNA-binding sites in Burkitt lymphoma. PLoS One. 2011; 6: e26837.
- Perna D, Fagá G, Verrecchia A, Gorski MM, Barozzi I, Narang V, et al. Genome-wide mapping of Myc binding and gene regulation in serum-stimulated fibroblasts. Oncogene. 2012; 31: 1695-1709.
- Krepelova A, Neri F, Maldotti M, Rapelli S, Oliviero S. Myc and max genome-wide binding sites analysis links the Myc regulatory network with the polycomb and the core pluripotency networks in mouse embryonic stem cells. PLoS One. 2014; 9: e88933.
- Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011; 25: 1010-1022.
- Prendergast GC, Lawe D, Ziff EB. Association of Myn, the murine homolog of max, with c-Myc stimulates methylation-sensitive DNA binding and ras cotransformation. Cell. 1991; 65: 395-407.
- Mao DY, Watson JD, Yan PS, Barsyte-Lovejoy D, Khosravi F, Wong WW, et al. Analysis of Myc bound loci identified by CpG island arrays shows that Max is essential for Myc-dependent repression. Curr Biol. 2003; 13: 882-886.
- Guccione E, Martinato F, Finocchiaro G, Luzi L, Tizzoni L, Dall' Olio V, et al. Myc-binding-site recognition in the human genome is determined by chromatin context. Nat Cell Biol. 2006; 8: 764-770.
- Martinato F, Cesaroni M, Amati B, Guccione E. Analysis of Myc-induced histone modifications on target chromatin. PLoS One. 2008; 3: e3650.
- Ruggero D. The role of Myc-induced protein synthesis in cancer. Cancer Res. 2009; 69: 8839-8843.
- Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O'Donnell KA, et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol. 2005; 25: 6225-6234.
- Miller DM, Thomas SD, Islam A, Muench D, Sedoris K. c-Myc and cancer metabolism. Clin Cancer Res. 2012; 18: 5546-5553.
- Dang CV, O'Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006; 16: 253-264.
- Lee LA, Dolde C, Barrett J, Wu CS, Dang CV. A link between c-Myc-mediated transcriptional repression and neoplastic transformation. J Clin Invest. 1996; 97: 1687-1695.
- Izumi H, Molander C, Penn LZ, Ishisaki A, Kohno K, Funa K. Mechanism for the transcriptional repression by c-Myc on PDGF beta-receptor. J Cell Sci. 2001; 114: 1533-1544.
- Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, et al. Repression of p15INK4b expression by Myc through association with Miz-1. Nat Cell Biol. 2001; 3: 392-399.
- Wu S, Cetinkaya C, Munoz-Alonso MJ, von der Lehr N, Bahram F, Beuger V. Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene. 2003; 22: 351-360.
- Zeller KI, Jegga AG, Aronow BJ, O'Donnell KA, Dang CV. An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome Biol. 2003; 4: R69.
- Singh MD, Raj K, Sarkar S. Drosophila Myc, a novel modifier suppresses the poly(Q) toxicity by modulating the level of CREB binding protein and histone acetylation. Neurobiol Dis. 2014; 63: 48-61.