
Review Article
Ann Hematol Oncol. 2015; 2(9): 1065.
Brentuximab Vedotin, Expanding Role in Therapy for Lymphomas
Sanchez A and Provencio M*
Medical Oncology Service, Hospital Universitario Puerta de Hierro-Majadahonda, Spain
*Corresponding author: Mariano Provencio, Medical Oncology Service, Hospital Universitario Puerta de Hierro-Majadahonda, Spain
Received: September 05, 2015; Accepted: November 28, 2015; Published: November 30, 2015
Abstract
The CD30 antigen has an important role as therapeutic target in patients with CD30-positive Hodgkin lymphoma and anaplastic large-cell lymphoma. Brentuximab vedotin (SGN-35) is an antibody that consists of the anti-CD30 monoclonal antibody conjugated with an antimitotic microtubule inhibitor (monomethyl auristatin E). The high response rates and prolonged survival times seen with brentuximab vedotin have changed the treatment paradigm for patients with relapsed or refractory disease which led to its approval for patients with relapsed Hodgkin lymphoma after autologous transplant or after more than two treatment regimens and who are not candidates for chemotherapy intensification and subsequent autologous transplant. It has also been approved for treating patients with anaplastic large-cell lymphoma who relapse after a line of chemotherapy treatment.
It has undergone important clinical development in recent years, and now its utility is being evaluated at different stages of these diseases: as front-line therapy in treatment-naive patients, prior to autologous stem cell transplant, as an adjunct to stem cell transplant and to facilitate a consolidative allogenic transplant. Further investigation is ongoing to assess the potential benefit in other disease settings and other lymphoid malignancies. It has an acceptable toxicity profile, with its main toxicity being accumulative peripheral neurotoxicity. Mature data on overall survival and quality of life are pending.
Keywords: Brentuximab Vedotin; CD30; Hodgkin Lymphoma; Anaplastic Large-Cell Lymphoma; Target Therapy
Abbreviations
Allo-SCT: Allogeneic Stem Cell Transplantation; ALCL: Anaplastic Large-Cell Lymphoma; ASCT: Autologous Stem-Cell Transplant; CR: Complete Response; DLBCL: Diffuse Large B-Cell Lymphoma; DFS: Disease-Free Survival; FDG-PET: 18-Fluoro- Deoxyglucose Positron Emission Tomography; FDA: Food and Drug Administration; HL: Hodgkin Lymphoma; mAb: Monoclonal Antibodies; MMAE: Monomethyl Auristatin E; OR: Objective Response; ORR: Overall Response Rate; OS: Overall Survival; PFS: Progression-Free Survival; PR: Partial Responses; PTCL-NOS: Peripheral T-Cell Lymphoma Not Otherwise Specified
Introduction
The CD30 antigen has emerged as an ideal therapeutic target in patients with CD30-positive Hodgkin Lymphoma (HL) and Anaplastic Large-Cell Lymphoma (ALCL). CD30 is highly expressed by Reed-Sternberg cells in HL and by ALCL cells, and can also be also be present in many other lymphoma cells. However, it is not expressed in most human tissue under physiologic conditions.
HL derives from the pre-apoptotic germinal center B cells and is characterized by the presence of a scant number of large bi- or multinucleated cells with prominent nucleoli (Reed-Sternberg cells) in a non-neoplastic inflammatory microenvironment. Reed-Sternberg cells stain positive for CD30 and CD15 [1] (Figure 1). For most patients with refractory or relapsed HL, high-dose therapy followed by Autologous Stem-Cell Transplant (ASCT) is the treatment of choice. ASCT can induce durable responses in approximately 50% of these patients [2,3]. Prognoses are especially poor in those patients with refractory disease or those that relapse during the year after high-dose therapy followed by ASCT [4]. At this stage of the disease there is no standard of care, and novel therapies, such as brentuximab vedotin, bendamustine, everolimus, panobinostat, lenalidomida, ipilimumab and nivolumab, are currently under investigation. Brentuximab vedotin has shown significant clinical activity with a manageable safety profile in patients with relapsed or refractory HL.
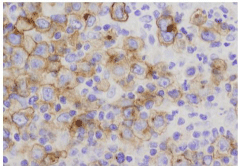
Figure 1: Classical CD30-positive Hodgkin lymphoma.
ALCL accounts for fewer than 5% of cases of non-Hodgkin lymphoma. It is a peripheral lymphoma composed of large neoplastic cells with abundant cytoplasm and pleomorphic nuclei, with paracortical and intrasinusoidal T-cell growth. It stains for CD30 [5] (Figure 2). The chromosomal translocation t (2;5) (p23;q25), which results in the formation of a fusion gene and over expression of the ALK protein (ALK+), is present in 60% of patients. ALK+ is a good prognostic factor [6]. Fifty percent of the patients will show progression or relapse to initial therapy [7]. ASCT is recommended for refractory or relapsed patients. At this stage of the disease, there is no standard of care. New treatments for ALK+ ALCL are based on the development of therapies directed against CD30 and ALK. Crizotinib, a small-molecule selective inhibitor of ALK, has durable responses in advanced, heavily pretreated ALK+ lymphoma patients [8]. Brentuximab vedotin represents an attractive treatment option for this scenario in ALCL patients.
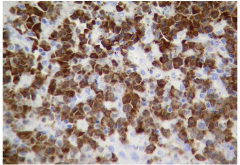
Figure 2: CD30-positive anaplastic large-cell lymphoma.
This article reviews the role of brentuximab vedotin in lymphomas and the latest investigations into its use in HL as an agent in combination with pre-existing front-line chemotherapies, as a stand-alone salvage therapy for use prior to ASCT, in high risk patients, early after ASCT, as a bridge to transplant as well as in ALCL and other lymphomas.
Targeting Cd30 with Sgn-35 or brentuximab vedotin, mechanism of action
The CD30 antigen is a single chain membrane glycoprotein which belongs to the tumor necrosis factor receptor super family. It contributes to the regulation of cellular growth and transformation as well as to tumor growth by promoting cell proliferation and survival, upregulating susceptibility to apoptotic signaling and downregulating immune response [9,10]. CD30 expression is generally restricted to activated B and T lymphocytes and NK cells in the hematopoietic tissue. It shows strong expression on the surface of malignant cells in HL (multinucleated Reed-Sternberg cells), in mononuclear Hodgkin cells and in the majority of ALCL, which makes it an ideal target for treatment with monoclonal Antibodies (mAb). It is also expressed in non-Hodgkin lymphoma and other malignancies [11,12]. Nevertheless, the pertinence of CD30 to the pathogenesis of lymphoma remains unclear, even though it has been an extensively investigated target for therapy.
The value of CD30 as a diagnostic marker for HL and ALCL, its restricted expression in normal tissue, its selectivity for malignant cells and its characteristics as an inducer of apoptosis, have led to the investigation of this antigen as a target for immunotherapy intervention, initially in relapsed and refractory HL and ALCL without effective treatment options.
SGN-30, a chimerical anti-CD30 mAb was conjugated with the cytotoxic Anti-Microtubule Agent Monomethyl Auristatin E (MMAE), an antimitotic agent that inhibits the polymerization of tubulin in dividing cells, creating the antibody-drug conjugate known as SGN-35 or brentuximab vedotin. The mechanism of action of SGN-35 consists of binding to transmembrane CD30 proteins present on the surface of tumor target cells, its internalization through Endocytosis, and the release of cytotoxic molecule MMAE in the cell through enzyme degradation of the ligand at the level of the lysosomes, where the peptide link between the antibody and the drug is broken by enzymatic activity, and the unaltered cytotoxic antitubulin drug MMAE, responsible for antitumor action, is released [13,14] (Figure 3).
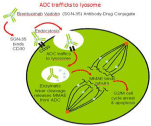
Figure 3: Mechanism of action of brentuximab vedotin. ADC (Antibody-Drug
Conjugate); MMAE (Monomethyl Auristatin E).
Brentuximab vedotin, phase I trials
The initial phase I clinical trial with brentuximab vedotin was performed with 45 relapsed or refractory CD30+ lymphoma patients, most of them with relapsed HL, with a dose of 0.1 to 3.6 mg/Kg every 3 weeks, using a habitual dose-escalation design [15]. Brentuximab vedotin was administered intravenously over 30 minutes every 3 weeks in an outpatient setting. The final dose considered that the maximum tolerated dose was 1.8 mg/kg intravenously every 3 weeks. The most commonly seen side-effects were fatigue, fever, diarrhea, nausea, neutropenia and peripheral neuropathy. Dose-limiting toxicities were grade 4 thrombocytopenia, grade 3 hyperglycemia and febrile neutropenia. Seventeen of the 45 patients with HL experienced an Objective Response (OR), including 11 Complete Responses (CR), tumor regression was observed in 36 of the 42 patients who could be evaluated (86%) and the median duration of response was at least 9.7 months.
In a second phase I dose-escalation study, brentuximab vedotin was administered intravenously on days 1, 8, and 15 of each 28-day cycle in 38 patients with HL, 5 with ALCL and 1 with peripheral T-cell lymphoma NOS [16]. Patients had received a median of 3 prior regimens and 62% had undergone ASCT. Dose-limiting toxicity was 1.2 mg/kg. The most common adverse events were peripheral sensory neuropathy, fatigue, nausea, diarrhea, arthralgia and pyrexia; and the majority of events were mild to moderate in severity. Dose-limiting toxicities were grade 3 gastrointestinal and grade 4 hyperglycemia. Tumor regression occurred in 85% of patients and Overall Response Rate (ORR) was 59% with 34% CR. With this scheme, patients suffered similar side-effects to those above and finally, the former was adopted with administration every 3 weeks Table 1.
Author, year
Reference
Study
Patients
Comments
Younes A, 2010
[15]
Phase I
HL / ALCL
Maximum tolerated dose was 1.8 mg/kg iv every 3 weeks
Fanale MA, 2012
[16]
Phase I
HL / ALCL
Dose of 1.2 mg/kg on days 1, 8, and 15 each 28-days
Table 1: Phase I clinical trials with brentuximab vedotin.
Brentuximab vedotin, pivotal phase II trial in HL
The pivotal open-label phase II clinical development trial recruited 102 relapsed or refractory HL patients after ASCT, 71% of whom had relapsed within 12 months of ASCT [17]. The median age was 31 years (15-77) and all had been treated with a median of 3.5 chemotherapy regimens. Patients received brentuximab vedotin at doses of 1.8 mg/Kg administered once every 3 weeks. Fifty-five percent of the patients developed grade 3 or higher adverse events. The most common grade 3 or higher toxicities were hematological, including neutropenia in 20%, anemia in 6% and thrombocytopenia in 8%. One of the most common non-hematological toxicities was peripheral sensorial neuropathy (all grades, 42%; grade 3 or higher in 8%), which was reversible in 50% of those affected. Nausea and fatigue were seen in 35% and 34% of patients, respectively.
The main aim of the study was to find the ORR as determined by independent observers, which was 75%, with a CR rate of 34% and 41% Partial Responses (PR). Twenty-seven (77%) of the 35 patients with B symptoms showed symptom resolution. After a mean follow-up of 18.5 months, the overall disease control rate was 99%. Responses occurred quickly, with an average of 12 weeks to reach CR. The duration of CR was 20.5 months and Progression Free Survival (PFS) for all patients was 5.6 months.
The initial Overall Survival (OS) of the series was 22.4 months. After a median of 3 years, median OS and PFS were estimated at 40.5 months and 9.3 months, respectively. Improved outcomes were observed in patients who achieved a CR on brentuximab vedotin, with estimated 3-year OS and PFS rates of 73% and 58%, respectively [18]. Younger age, good performance status and lower disease burden at baseline were prognostic factors for OS.
Brentuximab vedotin, before stem-cell transplantation (as a bridge to) in HL
Several ongoing studies are examining the role of brentuximab vedotin as first-line salvage therapy prior to stem-cell transplantation (a bridge to ASCT or a bridge to Allogeneic Stem Cell Transplantation (allo-SCT)).
Brentuximab vedotin prior to ASCT: The German Hodgkin Study Group (GHSG) retrospectively analyzed brentuximab vedotin as a bridge to ASCT in 14 transplant-naive HL patients who were relapsed or refractory to conventional chemotherapy [19]. The ORR was 71% with 5 CR. Consolidating ASCT (n = 4) or allo-SCT (n = 1) was performed in 5 patients. Median PFS was 9 months (Table 2).
Author, year
Reference
Study
Patients
Comments
Sasse S, 2012
[19]
Retrospective
HL
ORR of 60% in a heavily treated population
Chen R, 2015
[20]
Phase II
HL
ORR was 68%. Not worsening of stem cell collection or post-ASCT engraftment
Moskowitz AJ, 2013
[21]
Phase II
HL
PET-adapted sequential treatment prior to ASCT
Table 2: Trials of brentuximab vedotin as a bridge to ASCT (Autologous Stem-Cell Transplant).
A prospective phase II trial [20] examined the outcomes of brentuximab vedotin prior to ASCT in 37 patients with HL relapsed or refractory after induction therapy. ORR was 68% (13 CR, 12 PR). 89% of patients proceed to ASCT. Thirteen patients in CR, 4 in PR, and 1 with stable disease (49%) received ASCT without salvage combination chemotherapy. Main toxicity was lymphopenia, neutropenia, rash, and hyperuricemia. Brentuximab vedotin did not adversely impact on stem cell collection or post-ASCT engraftment.
As pre-transplant 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET, PET) normalization is a strong predictor of outcome for ASCT, the role of brentuximab in a PET-adapted sequential treatment with augmented ICE (augICE) was analyzed in a trial [21]. This phase II trial included 32 relapsed or refractory HL patients who had failed one previous regimen. They received brentuximab vedotin 1.2 mg/Kg weekly for 3 weeks on and 1 week off. If PET was normal after 2 cycles they proceeded to ASCT; patients with a positive PET received 2 additional cycles of augICE and were then re-evaluated. Those with a normal PET proceeded to ASCT and those with persistent PET abnormalities were treated as recommended by the attending physician. After 2 cycles of single agent brentuximab, 33% of patients obtained CR, this increased to 92% after sequential brentuximab-ICE chemotherapy. Even the 2 persistently PET positive patients achieved a normal PET after radiation therapy and all 24 who completed the induction program proceeded to ASCT. At a median follow-up of 6 months post-ASCT, all but 1 (95%) remained in remission. The authors concluded that PET-adapted sequential salvage therapy with brentuximab vedotin avoids chemotherapy in 33% of patients and obtains PET normalization in >90% of the cases which supports the idea of using brentuximab as a single agent or in combination with less toxic chemotherapy prior to ASCT.
Brentuximab vedotin prior to allo-SCT: Several studies have confirmed use of brentuximab vedotin as a bridge to reduced intensity allo-SCT.
A consecutive case series of 21 HL patients, who received brentuximab vedotin prior to reduced intensity allo-SCT, was compared with a similar group without brentuximab vedotin prior to allo-SCT [22]. The 2-year PFS and OS were 59.3% and 71.1%, respectively, for surviving patients treated with brentuximab vedotin. In the comparative group, the 2-year PFS and OS were 26.1% and 56.5%, respectively (Table 3).
Author, year
Reference
Study
Patients
Comments
Chen R, 2014
[22]
Retrospective
HL
Improved survival prior to transplantation with reduced intensity allo-SCT
Garciaz S, 2014
[23]
Retrospective
HL
Brentuximab prior to transplantation and no relapses after 20 months
Gibb A
[24]
Retrospective
HL / ALCL
One quarter of refractory patients end in allo-SCT
Illidge T, 2015
[25]
Retrospective
HL / ALCL
2-year OS of 80% after allo-SCT
Table 3: Trials of brentuximab vedotin as a bridge to allo-SCT (Allogeneic Stem Cell Transplantation).
In another retrospective series, 24 patients with relapsed or refractory HL were treated with four cycles of brentuximab vedotin before allo-SCT [23]. The ORR was 66.6%, 11 patients (45.8%) obtained CR, while 5 patients (20.8%) were in PR. All of the responding patients were able to receive consolidation treatment: 3 underwent ASCT, 3 received tandems ASCT/allo-SCT, 9 received allo-SCT and one patient was treated with donor lymphocyte infusion. With a median follow-up of 20 months, none of the patients relapsed or died.
In a UK study of patients treated on a Named Patient Programme [24], 24 patients with relapsed/refractory HL and ALCL were treated with brentuximab vedotin. The ORR across all histologies was 67%, CR was 25% and median PFS was 5.1 months. Six patients proceeded to allo-SCT demonstrating that, in one quarter of refractory patients, the use of brentuximab vedotin provides a bridge to allo-SCT. As best response was seen after only four doses, allo-SCT should be considered early on.
From the two pivotal phase 2 studies of brentuximab vedotin in relapsed or refractory HL and ALCL, 15 of the 160 patients who participated received consolidative allo-SCT [25]. The estimated 2-year PFS was 66%, however the median PFS has not yet been reached. Eleven of the 15 patients were still alive and the estimated 2-year OS rate was 80%.
Brentuximab vedotin, after allo-SCT in HL
The role of allo-SCT in HL is controversial and it has been offered to young patients after ASCT failure. An interesting question is the role that brentuximab vedotin might play in relapse after an allo-SCT (Table 4).
Author, year
Reference
Study
Patients
Comments
Gopal AK, 2012
[28]
Phase II
HL
First study post allo-SCT
Moskowitz CH, 2015
[29]
Phase III
HL
It is the first placebo-controlled trial in HL. Improvement with early treatment after ASCT
Table 4: Trial of brentuximab vedotin after allo-SCT (Allogeneic Stem Cell Transplantation) and ASCT (Autologous Stem-Cell Transplant).
The prognosis in these patients is very poor, with low response rates to any attempted treatment [26,27].
In the first study [28] aimed at finding the efficacy and safety of brentuximab vedotin in the setting of relapse after allo-SCT, 75% of 25 patients with refractory disease immediately before treatment obtained, with a dose of 1.8 mg/kg every 3 weeks, a 50% ORR, a CR of 38% and a PFS of 7.8 months. Toxicity was high and treatment had to be suspended in 36% of patients due to adverse effects. This represents an increase of 16% in adverse effects compared to the pivotal trial and is perhaps due to the cumulative toxicity of brentuximab in the context of comorbidities from prior lines of intensive therapy. Viremia due to cytomegalovirus was detected in 5 patients, but was only clinically relevant in one of them.
Brentuximab vedotin, post-ASCT in HL
ASCT is the standard treatment for relapsed or refractory HL patients, however only 50-60% of them are considered cured with this approach and the majority of patients with multiple risk factors will progress after ASCT. The phase III AETHERA trial [29], the first placebo-controlled trial in HL, was performed to assess the role of brentuximab vedotin when administered early after ASCT (Table 4).
In this trial, 329 patients with a median age of 32 years (18-76) received best supportive care plus brentuximab vedotin 1.8 mg/ kg or placebo (n=164) every 3 weeks for up to 12 months, or until progression or relapse, after undergoing ASCT within the previous 30-45 days. Patients had at least one risk factor and were enrolled in 1 of 3 high-risk categories: refractory to front-line therapy (60%), relapse <12 months after front-line therapy (33%), and relapse ≥12 months after front-line therapy with extranodal disease (8%). Patients received a median of 15 cycles of study treatment with 49% receiving the complete 16 cycles. Treatment was discontinued due to progressive disease in 28% of patients and adverse events in 19%. Altogether, 67% of patients in the brentuximab vedotin arm experienced peripheral neuropathy of any grade versus 19% in the placebo arm, and grade 3 or higher was 13% versus 1%, respectively. Overall, 85% of patients with peripheral neuropathy improved with a median time of 23.4 weeks. After a median follow-up of 24.4 months, 65% of patients receiving brentuximab vedotin continued to experience PFS, compared with 45% of patients who received placebo (HR 0.50; 95% CI, 0.36-0.70). This 20% difference in PFS at 2 years can be considered essential due to the fact that relapses almost never occur more than 2 years after ASCT. However, no advantage in OS has been demonstrated, nevertheless the follow-up is short and, at the time of relapse in the placebo arm, 85% of the patients crossed over to treatment with brentuximab vedotin.
Brentuximab vedotin, front line treatment in HL
Brentuximab vedotin is being studied in patients with treatment- naive HL. The multicenter phase I trial [30] was designed to evaluate the safety of brentuximab vedotin combined with standard ABVD treatment or treatment modified with AVD in the front-line setting of HL (stage IIA bulky disease or stage IIB-IV disease). Patients received a dose of 0.6, 0.9 or 1.2 mg/kg, depending on the cohort, every 2 weeks up to 6 cycles. At the time of presentation, 51 patients had been recruited. Altogether, 21 (95%) of 22 patients given brentuximab vedotin and ABVD achieved CR, as did 24 (96%) of 25 patients given brentuximab vedotin and AVD. The highest grade 3 toxicity seen in the ABVD and AVD cohorts was, respectively, neutropenia (80% vs. 65%), anemia (20% vs. 12%), febrile neutropenia (20% vs. 8%) and pulmonary toxicity (24% vs. 0%). In the brentuximab vedotin and ABVD group, 11 of 25 (44%) had pulmonary toxicity, interstitial lung disease, or pneumonitis that led to discontinuation of bleomycin. Therefore, the recommendation of not including bleomycin with brentuximab vedotin due to pulmonary toxicity was confirmed.
The high rate of CR obtained in this study advocated a comparative study between AVD plus brentuximab vedotin and ABVD.
The currently ongoing phase III ECHELON-1 trial (NCT01712490) is exploring brentuximab vedotin (1.2 mg/kg) in combination with AVD versus ABVD as a front-line treatment in 1040 naïve patients with stage III/IV HL [31]. The primary endpoint of this study is modified PFS (death, progression, receipt of chemotherapy or radiotherapy by patients not in CR after completing front-line therapy) and secondary endpoints include OS and ORR. The estimated study completion date is 2020.
The interim results of an ongoing randomized phase II trial indicates that brentuximab vedotin plus BEACOPP variants (BEACOPP escalated is detoxified by brentuximab vedotin) in firstline for advanced-stage HL is feasible [32].
Brentuximab vedotin, retreatment and extended treatment (in HL and in ALCL)
The optimal number of treatment cycles to administer brentuximab vedotin is currently unknown. To date, the recommended or maximum number is no more than 16, and its role in maintenance therapy remains unclear. However, studies have suggested that patients who stop taking the drug and then relapse will continue to respond to brentuximab vedotin when treatment is readministered.
A small phase II trial demonstrated that brentuximab vedotin was effective as a retreatment strategy in selected patients [33]. A total of 21 patients with HL and 8 patients with ALCL were re-treated. The ORR was 60% (30% CR) in HL patients and 88% (63% CR) in ALCL patients, with an estimated median duration of response of 9.5 months. Adverse events occurred in ≥25% of patients during the retreatment period and were generally similar in type and frequency to those observed in other trials. Peripheral neuropathy was more severe in this population, and it increased from a pretreatment baseline of 48% to 69% (new or worsening of baseline neuropathy).
Data from patients treated for more than 16 cycles of therapy were presented [34]. Prolonged duration of response was demonstrated without any worsening of side effects. The FDA changed the indication for duration of drug administration and eliminated the 16-cycle limit.
Brentuximab vedotin, in patients aged 60 years or older (in HL and in ALCL)
Recent data show that brentuximab vedotin can be safely administered and has a high rate of response in older patients.
A retrospective analysis assessed the safety and efficacy of brentuximab vedotin in adults ≥60 years (median age 66) with relapsed CD30+ lymphomas [35]. Higher rates of anemia (30% vs. 10%), peripheral sensory neuropathy (60% vs. 46%), fatigue (58% vs. 43%) and adverse events ≥grade 3 (70% vs. 56%) occurred in older patients compared to younger patients (<60 years, median age 32).
From a phase II trial [36] in a front-line setting, interim data were reported of 19 patients with a median age of 78 years (range, 64 to 92). Seventeen of the 45 patients with HL experienced response, with a CR of 63%, and all achieved tumor reduction. The most common treatment-emergent adverse events were grade 1 or 2 and included peripheral sensory neuropathy (47%), fatigue (32%) and diarrhea (26%).
Brentuximab vedotin, pivotal phase II trials in ALCL
A phase II trial with 58 patients was performed to assess the efficacy and safety of brentuximab vedotin in patients with relapsed or refractory systemic ALCL [37]. All patients had received at least 1 prior systemic regimen and 72% were ALK-negative. Brentuximab vedotin was administered at a dose of 1.8 mg/kg every 3 weeks, with a median of 7 cycles. Main toxicities were grade 3 or 4 neutropenia (21%), thrombocytopenia (14%), and peripheral sensory neuropathy (12%). ORR, the primary end point of the study, was reached by 86% of patients, of whom 57% achieved CR. The median duration was 12.6 months for patients with response and 13.2 months for patients in CR. ORR was not influenced by ALK status. Median PFS was 14.6 months. With a median observation time of 33.4 months from the first dose of brentuximab vedotin, 64% of patients with relapsed or refractory ALCL were alive at the time of last follow-up and the median OS has not yet been reached [38].
Data of trials of brentuximab vedotin in ALCL and other T-lymphomas are exposed in Table 5.
Author, year
Reference
Study
Patients
Comments
Pro B, 2012
[38]
Phase II
ALCL
At 3 years, 64% of patients are alive
Horwitz SM, 2014
[39]
Phase II
T-cell lymphomas
High response in angioimmunoblastic T-cell lymphoma
Fanale MA, 2014
[40]
Phase I
T-cell lymphomas
High response in first-line
Duvic M, 2013
[41]
Phase II
Cutaneous T-cell lymphoma
High response in lymphomatoid papulosis
Kim YH, 2014
[42]
Phase II
Cutaneous T-cell lymphoma
Uncertain values of CD30 expression level
Mehra T, 2015
[43]
Case report
Cutaneous T-cell lymphoma
It permits allo-SCT
Table 5: Trials of brentuximab vedotin in Anaplastic Large-Cell Lymphoma (ALCL) and other T-lymphomas.
Brentuximab vedotin, in T-cell lymphomas
The role of brentuximab vedotin as a single agent was evaluated in a phase II trial with relapsed or refractory CD30+ non-Hodgkin lymphomas [39]. There were 22 patients with peripheral T-cell lymphoma not otherwise specified and 22 with angioimmunoblastic T-cell lymphoma. Median age was 64 years. The ORR in the 34 evaluable patients was 41%, and 8 achieved CR. In the angioimmunoblastic T-cell lymphoma patients, the ORR was 54% and 5 patients achieved CR. Median PFS was 6.7 months. The main grade 3 adverse events were neutropenia (14%), peripheral sensory neuropathy (9%) and hyperkalemia (9%). The ORR is similar to that achieved with other approved agents such as pralatrexate and romidepsin.
Brentuximab vedotin, front line in T-cell lymphomas
A phase I trial evaluated the safety and activity of brentuximab vedotin, sequentially with CHOP or in combination with CHP (CHOP without vincristine), as front-line treatment in 26 patients with newly diagnosed CD30+ mature T-cell lymphomas [40]. Patients received sequential treatment (once every 3 weeks) with brentuximab vedotin 1.8 mg/kg (2 cycles) followed by CHOP (6 cycles) or brentuximab vedotin 1.8 mg/kg plus CHP for 6 cycles (once every 3 weeks). The ORR was 85% and 100%, and CR 62% and 88% in the sequential arm and in the combination arm, respectively. The main grade 3 and 4 adverse events in the combination arm were febrile neutropenia (31%), neutropenia (23%), anemia (15%) and pulmonary embolism (12%).
Based on these results, the use of brentuximab vedotin in combination with chemotherapy as front-line treatment is being investigated in the ECHELON-2 phase III trial (NCT01777152) to compare brentuximab vedotin plus CHP (CHOP without vincristine) versus CHOP in untreated patients with CD30+ expressing mature T-cell lymphomas. The estimated study completion date is December 2019.
Brentuximab vedotin, in cutaneous T-cell lymphoma
The role of brentuximab vedotin was evaluated in a Phase II open-label trial of 48 patients with primary cutaneous CD30+ lymphoproliferative disorders including lymphomatoid papulosis and primary cutaneous ALCL or CD30+ mycosis fungoides [41]. The ORR was 71% and CR was 35%. In mycosis fungoides, the RR was 50%. In lymphomatoid papulosis and primary cutaneous ALCL, the ORR was 100%, median PFS was 9.7 years from diagnosis and 1.68 years from first dose.
In another phase II study [42], 32 patients with relapsed or refractory mycosis fungoides were treated with brentuximab vedotin with an ORR of 70%.
In a clinical report of 4 patients (3 mycosis fungoides and one Sézary Syndrome) after brentuximab vedotin, 2 cases achieved a remission enabling subsequent allo-SCT [43].
Brentuximab vedotin, in B-lymphomas
CD30 is also expressed by several types of non-Hodgkin lymphoma, including Diffuse Large B-Cell Lymphoma (DLBCL), and it seems to be a favorable prognostic factor in both the germinal center B-cell and activated B-cell subtypes. In a phase II trial, brentuximab vedotin was administered to relapsed or refractory CD30+ NHL patients [44], 49 with DLBCL and 19 with other B-cell NHLs. The ORR was 44% for DLBCL, with a CR of 17%, and a median duration of 16.6 months.
Brentuximab vedotin, safety issues
Brentuximab vedotin is considered to have an acceptable toxicity profile, but it is associated with several side effects, although, in pivotal trials, adverse events leading to withdrawal of treatment occurred in 20% of patients.
The most frequent adverse event reported in brentuximab vedotin trials is infection, which occurred in 61% of patients, however, it was only associated to the drug in 16%. Severe infections included pneumonia, staphylococcal bacteremia, sepsis and herpes zoster.
The most common drug-related toxicity is peripheral sensory neuropathy (44%), followed by fatigue (42%). Other common adverse effects include neutropenia, nausea, diarrhea, constipation, thrombocytopenia, pyrexia, rash, pruritus, myalgia, arthralgia, alopecia, peripheral motor neuropathy, hyperglycemia, demyelinating polyneuropathy, tumor lysis syndrome and Stevens- Johnson syndrome. The concomitant use of brentuximab vedotin with bleomycin is contraindicated due to increased pulmonary toxicity.
Peripheral sensory neuropathy is reported in 47% of patients with ALCL and 45% of patients with HL; grade 3 neuropathy was seen in 8% of HL and 12% of ALCL patients. Neuropathy appears to be cumulative and can be reversed through dose discontinuation, modification, or interruptions. Severe neuropathy occurs after 7 to 9 cycles of treatment, thereby suggesting a cumulative effect. Fifty percent of patients recover after dose reduction or treatment discontinuation.
A rare but serious and potentially fatal toxicity related to brentuximab vedotin is pancreatitis, as described in 8 patients [45].
Consideration should be given to the report of a few cases of progressive multifocal leukoencephalopathy caused by reactivation of the JC virus [46].
Brentuximab vedotin, approval
FDA approved brentuximab vedotin in 2011 for HL patients that relapsed after failure of two chemotherapy lines with multiple drug regimens and who were not candidates for transplant, or after failure of a previous ASCT. It is also approved in patients with systemic ALCL who have progressed despite at least 1 treatment regimen. The European Medicines Agency gave marketing authorization in 2012. It is currently the only registered anti-CD30 therapeutic agent available.
Conclusion
Antibody-drug conjugates are a new way to delivery chemotherapy. The CD30 antigen, a very selective marker, emerged as an important therapeutic target in patients with CD30+ HL and ALCL, and now that interest is being translated to other lymphomas, such as B-cell lymphomas and cutaneous lymphomas. In the latter, the correlation between CD30 expression and antitumor activity is still unclear.
In HL and ALCL, due to high response rates and prolonged survival, brentuximab vedotin is changing the treatment paradigm, especially for patients with relapsed and refractory disease (Figure 4).
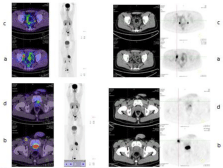
Figure 4: 20 year-old male patient diagnosed of ALCL CD30+ and ALK+,
stage IIIB, treated with CHOP x 6 cycles and achieved CR. He progressed
2 years later, and received ICE x 3 cycles followed by ASCT. A new relapse
16 months after ASCT was documented in the right external iliac (a) and
inguinal lymph (b) nodes and the biopsy confirmed ALCL CD30+, ALK+. He
was treated with brentuximab Vedotin 1.8 mg/kg every 3 weeks for 5 cycles,
with CR shown by PET (right external iliac (c) and inguinal lymph (d) nodes
post-treatment), followed by haploidentical allo-SCT. Two years after allo-
STC he is free of disease.
It is hard to predict what the exact role of brentuximab vedotin is in lymphomas. Numerous recently published studies concerning its use in various lymphoma stages and scenarios make it difficult to postulate the best moment to use this drug during the evolution of the disease.
In first-line HL, the results of the phase I trial are clearly exciting and confirm the robust benefit in RR and disease control observed in relapsed patients. Still to be defined is the role of brentuximab vedotin in earlier LH treatment lines, a disease in which there is great concern to avoid intensive and toxic regimens with long-term side effects in patients that, due to the high rate of cure after front-line treatment, would not really need them.
Some clear data can be drawn from this study: we must to be careful about toxicities (brentuximab vedotin should not be given with bleomycin), nearly all patients achieve complete remission and with only a moderate increase in toxicity. Nevertheless, many questions have arisen, and we do not know if we could combine brentuximab vedotin with AVD to reduce the number of chemotherapy cycles needed for the effective treatment of newly diagnosed HL, or reduce the need for radiotherapy to avoid long-term side effects that include heart problems and other types of cancer. Given that approximately 75% of advanced-stage HL patients obtain CR with initial treatment, it would be of great interest to identify the main group of HL patients that do not require treatment in addition to initial ABVD and who could therefore be excluded from more intensive regimens, or even to select high-risk patients and use brentuximab vedotin as consolidation treatment after ABVD.
The results from the ECHELON-1 trial in 2020 will be crucial for deciding the front-line therapy to be used in advanced HL patients.
Brentuximab vedotin has also shown benefit as a bridge to stemcell transplantation, with positive data prior to ASCT and allo-SCT. It has therefore been postulated that brentuximab vedotin could be used instead of ICE as a single agent prior to ASCT. There are also positive data with respect to its use soon after ASCT in patients with a high risk of residual disease. It helps to reduce tumor burden, helps to achieve a negative PET scan and facilitates transplantation with no negative effect on engraftment. One question to be answered concerns what happens with those patients who reach response and for whom an allo-SCT is an option [47]. In the pivotal study, 8 patients underwent this procedure and were still living at the time of publication.
For older patients with comorbidities who are not candidates for chemotherapy, alternative approaches, such as brentuximab vedotin, should be considered. It can be administered safely and has a potential role in patients who are ineligible for chemotherapy; furthermore, trials have demonstrated response rates reaching 80%.
In conclusion, brentuximab vedotin is a very effective treatment for HL and ALCL patients, especially in refractory and relapsed patients with limited treatment options and no definite standard treatment. However, trials were mainly phase I and II, with no comparators and no direct evidence with which to demonstrate OS benefit compared to standard of care. Quality of life was not included in the majority of trials and therefore no improvement has been demonstrated. Adverse effects can be severe and lead to treatment discontinuation in a quarter of patients and there are no long-term safety data. Data from ongoing and emerging studies with brentuximab vedotin will help shed light on ways of maximizing treatment benefit while minimizing treatment-related side effects in patients with hematologic malignancies.
References
- von Wasielewski R, Mengel M, Fischer R, Hansmann ML, Hübner K, Franklin J, et al. Classical Hodgkin's disease. Clinical impact of the immunophenotype. Am J Pathol. 1997; 151: 1123-1130.
- Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993; 341: 1051-1054.
- Colpo A, Hochberg E, Chen YB. Current status of autologous stem cell transplantation in relapsed and refractory Hodgkin's lymphoma. Oncologist. 2012; 17: 80-90.
- Majhail NS, Weisdorf DJ, Defor TE, Miller JS, McGlave PB, Slungaard A, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006; 12: 1065-1072.
- Stein H, Foss HD, Dürkop H, Marafioti T, Delsol G, Pulford K, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000; 96: 3681-3695.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008; 26: 4124-4130.
- Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood. 2014; 123: 2636-2644.
- Gambacorti Passerini C, Farina F, Stasia A, Redaelli S, Ceccon M, Mologni L, et al. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J Natl Cancer Inst. 2014; 106: djt378.
- Granados S, Hwang ST. Roles for CD30 in the biology and treatment of CD30 lymphoproliferative diseases. J Invest Dermatol. 2004; 122: 1345-1347.
- Wahl AF, Klussman K, Thompson JD, Chen JH, Francisco LV, Risdon G, et al. The anti-CD30 mAb SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin’s disease. Cancer Res. 2002; 62: 3736-3742.
- Falini B, Pileri S, Pizzolo G, Dürkop H, Flenghi L, Stirpe F, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995; 85: 1-14.
- Gerber H-P. Emerging immunotherapies targeting CD30 in Hodgkin’s lymphoma. Biochem Pharmacol. 2010; 79: 1544-1552.
- Doronina SO, Bovee TD, Meyer DW, Miyamoto JB, Anderson ME, Morris-Tilden CA, et al. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bio conjug Chem. 2008; 19: 1960-1963.
- Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003; 102: 1458-1465.
- Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010; 363: 1812-1821.
- Fanale MA, Forero-Torres A, Rosenblatt JD, Advani RH, Franklin AR, Kennedy DA, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/ refractory CD30-positive hematologic malignancies. Clin Cancer Res. 2012; 18: 248-255.
- Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012; 30: 2183-2189.
- Gopal AK, Chen R, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015; 125: 1236-1243.
- Sasse S, Rothe A, Goergen H, Eichenauer DA, Lohri A, Kreher S, et al. Brentuximab vedotin (SGN-35) in patients with transplant-naive relapsed/refractory Hodgkin lymphoma. Leuk Lymphoma. 2013; 54: 2144-2148.
- Chen R, Palmer JM, Martin P, Tsai N, Kim Y, Chen BT, et al. Results of a Multicenter Phase II Trial of Brentuximab Vedotin as Second-Line Therapy before Autologous Transplantation in Relapsed/Refractory Hodgkin Lymphoma. Biol Blood Marrow Transplant. 2015; 21: 2136-2140.
- Moskowitz AJ, Schöder H, Yahalom J, McCall SJ, Fox SY, Gerecitano J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015; 16: 284-292.
- Chen R, Palmer JM, Tsai NC, Thomas SH, Siddiqi T, Popplewell L, et al. Brentuximab vedotin is associated with improved progression-free survival after allogeneic transplantation for Hodgkin lymphoma. Biol Blood Marrow Transplant. 2014; 20: 1864-1868.
- Garciaz S, Coso D, Peyrade F, Fürst S, Duran S, Chetaille B, et al. Brentuximab vedotin followed by allogeneic transplantation as salvage regimen in patients with relapsed and/or refractory Hodgkin's lymphoma. Hematol Oncol. 2014; 32: 187-191.
- Gibb A, Jones C, Bloor A, Kulkarni S, Illidge T, Linton K, et al. Brentuximab vedotin in refractory CD30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. Haematologica. 2013; 98: 611-614.
- Illidge T, Bouabdallah R, Chen R, Gopal AK, Moskowitz CH, Ramchandren R, et al. Allogeneic transplant following brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Leuk Lymphoma. 2015; 56: 703-710.
- Ram R, Gooley TA, Maloney DG, Press OW, Pagel JM, Petersdorf SH, et al. Histology and time to progression predict survival for lymphoma recurring after reduced-intensity conditioning and allogenic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011; 17: 1537-1545.
- Wudhikarn K, Brunstein CG, Bachanova V, Burns LJ, Cao Q, Weisdorf DJ. Relapse of lymphoma after allogeneic hematopoietic cell transplantation: management strategies and outcome. Biol Blood Marrow Transplant. 2011; 17: 1497-1504.
- Gopal AK, Ramchandren R, O'Connor OA, Berryman RB, Advani RH, Chen R, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012; 120: 560-568.
- Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015; 385: 1853-1862.
- Younes A, Connors JM, Park SI, Fanale M, O'Meara MM, Hunder NN, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013; 14:1348-1356.
- Ansell SM, Younes A, Connors JM, Gallamini A, Kim WS, Friedberg JW, et al. Phase 3 study of brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) versus doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as front-line treatment for advanced classical Hodgkin lymphoma (HL): Echelon-1 study. J Clin Oncol. 2014; (ASCO Annual Meeting) 32:5s.
- Borchmann P, Eichenauer DA, Plütschow A, Kreissl S, Fuchs M, Soekler M, et al. Targeted Beacopp Variants In Patients With Newly Diagnosed Advanced Stage Classical Hodgkin Lymphoma: Interim Results Of a Randomized Phase II Study. Blood. 2013; 122: 4344.
- Bartlett NL, Chen R, Fanale MA, Brice P, Gopal A, Smith SE, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014; 7: 24.
- Forero-Torres A, Bartlett NL, Berryman RB, Chen R, Matous JV, Fanale MA, et al. Extended treatment with brentuximab vedotin in patients with relapsed or refractory CD30-positive hematological malignancies. Leuk Lymphoma. 2015; 56: 1151-1153.
- Gopal AK, Bartlett NL, Forero-Torres A, Younes A, Chen R, Friedberg JW, et al. Brentuximab vedotin in patients aged 60 years or older with relapsed or refractory CD30-positive lymphomas: a retrospective evaluation of safety and efficacy. Leuk Lymphoma. 2014; 55: 2328-2334.
- Yasenchak CA, Chen R, Sharman JP, Boccia RV, Holkova B, Rosen PJ, et al. 4389 A Phase 2 Study of Single-Agent Brentuximab Vedotin for Frontline Therapy of Hodgkin Lymphoma in Patients Age 60 Years and Above: Interim Results. Blood. 2013; (ASH Annual Meeting Abstracts).
- Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012; 30: 2190-2196.
- Pro B, Advani RH, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. 1809 Three-year survival results from an ongoing phase 2 study of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2013; (ASH Annual Meeting Abstracts).
- Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O'Connor OA, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014; 123: 3095-3100.
- Fanale MA, Horwitz SM, Forero-Torres A, Bartlett NL, Advani RH, Pro B, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014; 32: 3137-3143.
- Duvic M, Tetzlaff M, Clos AL, Gangar P, Talpur R. Phase II Trial Of Brentuximab Vedotin For CD30+ Cutaneous T-Cell Lymphomas and Lymphoproliferative Disorders. Blood. 2013; 122: 367 (ASH Annual Meeting Abstracts).
- Kim YH, Tavallaee M, Rozati S, Sundram U, Salva K, Wood GS, et al. 804 Phase II Investigator-Initiated Study of Brentuximab Vedotin in Mycosis Fungoides or Sezary Syndrome: Final Results Show Significant Clinical Activity and Suggest Correlation with CD30 Expression. Blood. 2014; (ASH Annual Meeting Abstracts).
- Mehra T, Ikenberg K, Moos RM, Benz R, Nair G, Schanz U, et al. Brentuximab as a Treatment for CD30+ Mycosis Fungoides and Sézary Syndrome. JAMA Dermatol. 2015; 151: 73-77.
- Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 2015; 125: 1394-1402.
- Gandhi MD, Evens AM, Fenske TS, Hamlin P, Coiffier B, Engert A, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014; 123: 2895-2897.
- US Food and Drug Administration. FDA drug safety communication: new boxed warning and contraindication for Adcetris (brentuximab vedotin), January 13, 2012. Accessed September 2, 2015.
- Sarina B, Castagna L, Farina L, Patriarca F, Benedetti F, Carella AM, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood. 2010; 115: 3671-367.