
Review Article
Ann Hematol Oncol. 2022; 9(4): 1402.
Blood Transmission of Corona Virus Disease 2019. Are there any Threat?
Nagwa A. Sabri¹* and Eslam MS²
¹Department of Clinical Pharmacy, Faculty of Pharmacy-Ain Shams University, Cairo, Egypt
²Clinical and Bio-analysis Department, Drug Research Centre, Cairo, Egypt
*Corresponding author: Nagwa Ali Sabri, Department of Clinical Pharmacy, Faculty of Pharmacy- Ain Shams University, Cairo, Egypt
Received: August 15, 2022; Accepted: September 08, 2022; Published: September 15, 2022
Abstract
Background: Corona Virus infection is rapidly expanding and has a significant worldwide influence on the status of health and the economic process.
Aim: Performing a literature search to investigate possible impacts of transmission of COVID-19 disease through blood transfusion process in health care system, and disease prevalence.
Methods: Internet search in scientific publications and collecting data regarding different viral transmission routes, viral detection and disease diagnosis methods, what are the potential problems and risks in COVID-19 management, and COVID-19 protective measures to undertake during blood transfusion.
Discussion and Conclusion: Coronavirus spreads mostly by droplets produced by infected persons while coughing, sneezing, or speaking. Different Covid-19 diagnosis methods include Computed Tomography (CT) scan, reverse transcription-polymerase chain reaction, antibody testing (IgG, and IgM), and biochemical tests. Different disease management risks may occur including antibiotic resistance, occupational stress, immune function suppression or dysregulation, pharmacodynamic interactions like prolongation of the QT interval. Although the findings indicated that blood transfusion did not transmitted coronavirus, but protective measures should be undertaken during blood transfusion process in healthcare facilities and blood transfusion centers. This will aid in mitigating viral transmission and associated management risks that may occur.
Keywords: COVID-19; Blood transfusion; Polymerase chain reaction; C-reactive protein; Social distancing
Introduction
Infection with Corona Virus Disease 2019 is exponentially spreading and has a remarkable global impact on the state of health and the economic process [1]. Estimated patients suffering from COVID-19 as of 31 January 2021 were 102,139,771 cases, with mortality rate of 2.17 percent [2].
This disease was caused by infection with the SARS-CoV-2 virus, where, Numerous cases of pneumonia with unknown Etiology by the end of 2019 has been observed in China and is thought to be a source of infection in the seafood market in Wuhan City, China [3].
The incidence of COVID-19 severity and mortality is much greater than that of usual influenza, and as population’s age and lifestyle and dietary habits change, the prevalence of chronic diseases such as diabetes and hypertension increases. It was found that diabetes and hypertension are closely related to severity and mortality [1].
The national cumulative prevalence of COVID-19 (past and current infections relative to population size) is estimated at 31% in Peru, 27% in Mexico, 22% in Brazil, 12% in USA, 11% in the United Kingdom, 8.2% in France, 7.4% in Sweden, 4.2% in Canada, 1.8% in Germany and 0.12% in Japan [4].
The first COVID-19 symptoms are commonly known as fever, dry cough, tachypnea, and shortness of breath. Even though diarrhea was present in approximately 20–25 percent of patients with MERSCoV or SARS-CoV infection, intestinal symptoms are rarely seen in COVID-19 patients. Confusion, chest pain, vomiting, and nausea were also reported as COVID-19 symptoms [5,6].
Other symptoms include pain in the throat, sneezing, nasal congestion, production of sputum, anosmia and dyspepsia, skin rash, finger or toe discoloration, and viral conjunctivitis. Some laboratory studies have shown that cytokine storm, and sepsis has occurred in COVID-19 infected patients [7].
COVID-19 can be spread by asymptomatic people, and the average incubation time for SARS-CoV-2 infection has been observed to range between 0 and 14 days. These characteristics may be important in the probability of disease transmission in blood transfusion centers [8].
Concerns about transfusing positive donors’ blood products to unknowing recipients arose as the number of asymptomatic cases increased. As a result, China and Pakistan conducted real-time SARSCoV- 2 screening in blood products held at various blood centers [9].
According to an observational research in Zhejiang, China, the data showed that the province experienced a 67 percent decline in blood donation activities. Furthermore, 81 percent of the donor respondents’ top worry during the donation procedure was acquiring COVID-19 [10].
According to world Health Organization (WHO), the coronavirus family is not known to be transmitted by blood and blood products. Hundreds of case reports have revealed that receivers who received blood products from positive donors did not get Covid-19 infection [9,11].
Corona Virus Disease 2019 Viral Transmission
Coronavirus spreads mainly through droplets that are generated by infected people during coughing, sneezing, or speech. While it was previously assumed that since these droplets are too heavy to remain in the air, coronavirus is not airborne. Viruses may be airborne in the form of aerosols, which are tiny pieces that can remain in the air for a sustained period of time. A recent research on aerosol and surface stability found that SARS-COV-2 was stable in its aerosol form for three hours [12].
This indicates that air transmission of the virus is probable due to its ability to stay viable as an aerosol. While aerosolized viral particles cannot travel far from the normal respiration of an infected patient, coughing and sneezing can lead to an estimated traveling distances of up to 20 feet [13].
Corona Virus Disease 2019 Testing
Reverse Transcription-Polymerase Chain Reaction (RTPCR)
The test results for COVID-19 may lead to the primary step to determine the scale of the disease outbreak. Previously, the diagnostic method of choice was reverse transcription-polymerase chain reaction (RT-PCR) tests. However, due to their long wait times of two to three hours, as well as the need for expensive laboratory equipment and trained professionals, these methods are not ideal for rapid diagnosis. For the rapid diagnosis of current infection, COVID-19 testing stations use molecular tests to determine the presence of SARS-CoV-2 relying on the genetic sequence of SARSCoV- 2 and use PCR [15].
Isothermal Nucleic Acid Amplification Technology
Currently, Abbott’s ID Now, a rapid in vitro diagnostic molecular test using isothermal nucleic acid amplification technology, has a COVID-19 test feature that takes less than 13 minutes. It may be possible to use a nasopharyngeal, throat, and nasal swab. Although the sensitivity of 90% of these molecular tests is considered, the risks and consequences of false-negative tests with these current devices remain [16].
Computed Tomography Scan
As COVID-19 is considered to be a respiratory infection, several studies focus on the use of pulmonary screening scans to track disease progression. A study in China, looked at the progression of COVID-19 in the lungs of 81 patients and used CT scans to track disease progression [17].
Abnormal chest CTs were observed in all patients with and without ‘viral pneumonia’ symptoms, specifically as bilateral, subpleural, ground-glass opacities with air bronchograms, poorly defined margins and a slight predominance in the lower right lobe. As the patient heals, the lungs of the patient show improvements in the lung lesions. It was considered that this medical screening is faster and more sensitive than the RT-PCR test. Ultrasound is a safer way to test for lung changes, as the evolution of the disease can be tracked to inform clinical decision-making. Ultrasounds still cannot detect lesions deep inside the lung, in which case a CT scan would be needed. However, lung ultrasonography may be the first step of detection because it does not use radiation and the detection would be repeatable at a lower cost [15].
CT has limited COVID-19 sensitivity and a lower specificity than testing with RT-PCR. it carries a risk that providers may be exposed to Severe Acute Respiratory Syndrome, Coronavirus 2 (SARS-CoV-2). Chest CT, particularly for patients who show symptoms, should be considered a supplementary diagnostic tool [18].
Antibody Testing (SARS-CoV-2 IgG/IgM Assay)
Abbott and LabCorp looked at mass-producing antibody tests for rapid diagnosis of COVID-19. These tests focus on the testing of immunoglobulin G (IgG) antibodies because their reactions relate to adaptive immunity and to the immunological memory of previous virus encounters. The presence of these IgG antibodies can identify the specific antigen that has a high affinity to the previous virus. The antibody test (SARS-CoV-2 IgG assay) has shown 100 percent sensitivity and 99.9 percent specificity to date [19,20,21].
Immunoglobulin’s against SARS-CoV indicated that IgM and IgG antibodies were detectable 7 days later. COVID-19 patients also demonstrated that both IgM and IgG can be detected by an anti- SARS-CoV-2 (ELISA) after 5 days of onset [22].
The ELISA-based IgM and IgG antibody tests showed more than 95% specificity as a diagnostic test for COVID-19, as antibodies are produced against viral nucleocapsid and the receptor-binding domain of the spike protein are produced. The nucleocapsid is a protein containing viral nucleic acid and the RBD-S is a protein used by the virus for attachment to the host cell; these antigens can be used individually or together to detect IgM and IgG antibodies, as well as increase overall sensitivity [23].
Researchers using a single-lane rapid IgG/IgM lateral flow assay for SARS-CoV-2 nucleocapsid protein found that IgG sensitivity and specificity were 92.2 percent and 97.0 percent. Even so, IgM sensitivity and specificity were only 57.9 percent and 91.3 percent respectively [24].
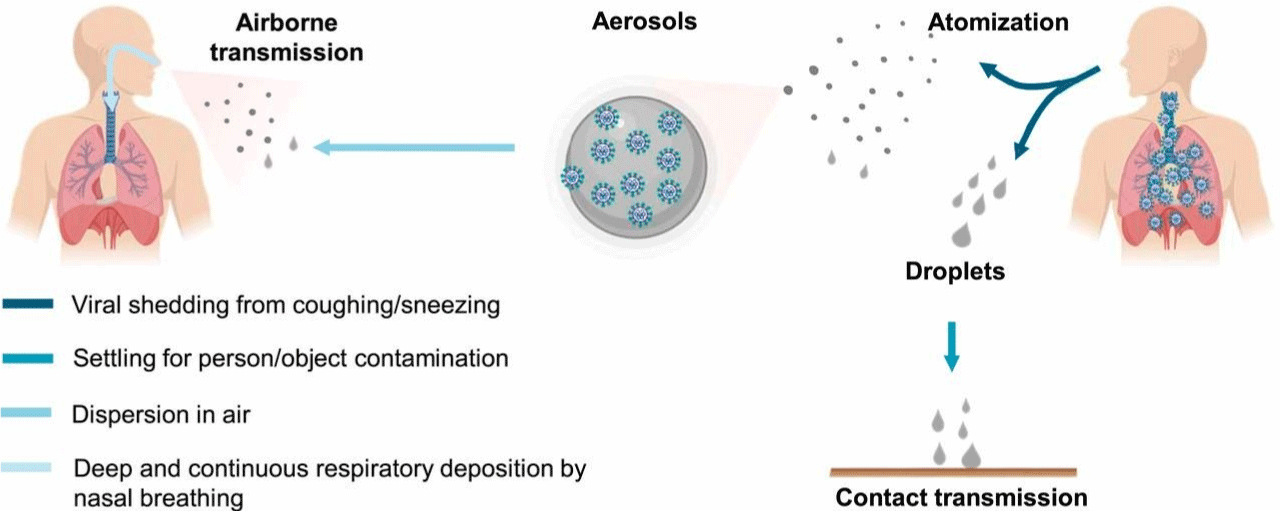
Figure 1: Transmission of COVID-19. Human atomization of viruses arises from coughing or sneezing of an infected person, producing virus-containing droplets
(>5 μm) and aerosols (<5 μm). Virus transmission from person to person occurs through direct/indirect contact and airborne aerosol/droplet routes [14].
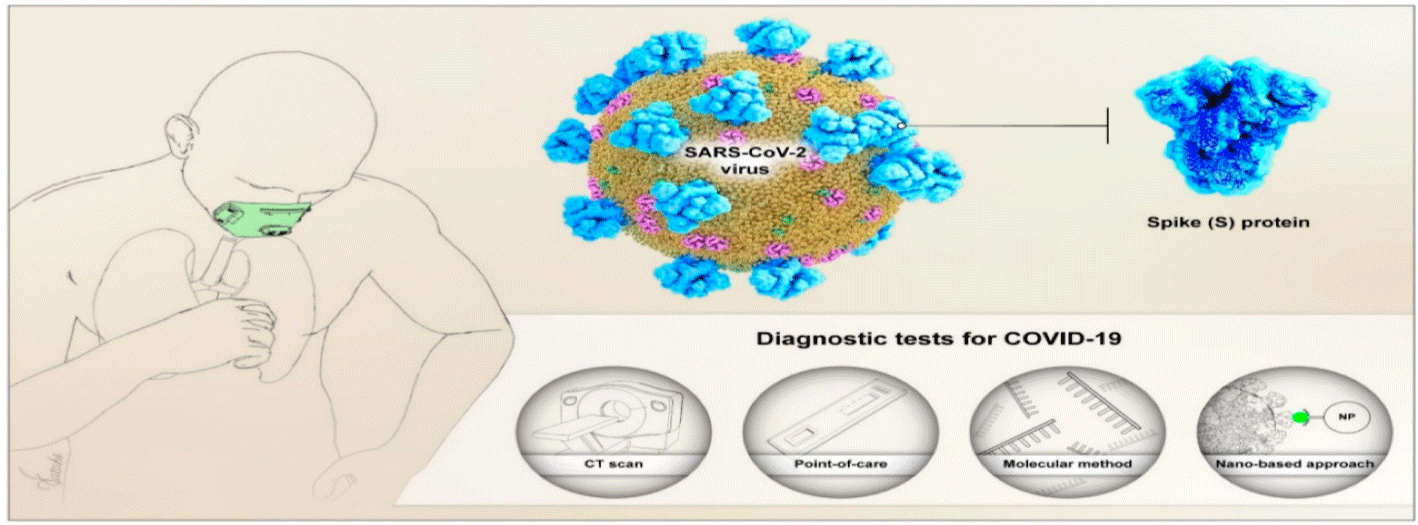
Figure 2: Schematic illustration of common Lung Computed Tomography (CT) and molecular test for diagnosis of Severe Acute Respiratory Syndrome Coronavirus
2 (SARS-CoV-2), and emerging detection methods, based on the optical-based biosensor, Point-Of-Care (POC) testing, and the optical-based nanosensor [29].
The current WHO recommendations are that these new immunodiagnostic serology point-of-care tests be used only in a research environment and are advised not to be used in a clinical decision-making environment. These simple testing kits with a sensitivity range of 34% to 80% are based on either the detection of COVID-19 virus protein in respiratory samples such as sputum, throat swab, or the detection of human antibodies produced in response to infection in blood or serum. The performance of these tests depends on a number of factors, including the time of onset of the disease, the concentration of the virus in the sample, the quality of the sample collected from the individual and how it is processed, and the accuracy of the reagent formulation in the test kits [25].
Biochemical Tests (Biomarkers Representing Inflammatory Host Response)
Reported biochemical tests in confirmed cases of COVID-19 include biomarkers representing inflammatory host response to pathogens and/or early end-organ dysfunction markers in severe cases. Common abnormalities, although non-specific, include lymphopenia, decreased albumin, elevated C - Reactive Protein (CRP) and Erythrocyte Sedimentation Rates (ESR), and elevated Lactate Dehydrogenase (LDH) levels. Additional biomarkers that may be seen with disease progression include leukocytosis and/or leukopenia, elevated ferritin, elevated Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), or elevated Creatinine Kinase (CK). Emerging data suggest that infection with SARS-CoV-2 may result in cardiovascular injury and an elevated troponin detected in isolation without clinical or electrocardiographic features of an Acute Coronary Syndrome (ACS) [26,27,28].
Corona Virus Disease 2019 Management
The treatment is symptomatic, and the first step in addressing respiratory impairment is oxygen therapy. Non-Invasive (NIV) and Invasive Mechanical Ventilation (IMV) may be necessary in cases of respiratory failure refractory with oxygen therapy [30].
Although systemic corticosteroids for the treatment of viral pneumonia or Acute Respiratory Distress Syndrome (ARDS) have not been recommended for other therapeutic strategies, these drugs are usually used in severe COVID-19-induced ARDS (e.g. methylprednisolone 1 mg/Kg/day) [31]. However no antiviral therapy has been approved, several approaches have been suggested, such as lopinavir/ritonavir (400/100 mg every 12 hours orally) [32].
As an immunomodulatory therapy, chloroquine (500 mg every 12 hours) and hydroxychloroquine (200 mg every 12 hours) were proposed. A Trial showed that Hydroxychloroquine was significantly associated with a reduction in viral load until viral disappearance and this effect was augmented by azithromycin [33].
Acalabrutinib is a selective Bruton tyrosine kinase inhibitor that regulates the signaling and activation of macrophages. A clinical study proved that Acalabrutinib improved oxygenation in a majority of COVID-19 patients, ameliorating measures of inflammation such as C-reactive protein and IL-6 [34].
As regards the management protocols used and direct antivirals included in these protocols, it was evident that different direct antivirals had several drug interactions with other therapeutics used for the treatment of certain co-morbid conditions such as diabetes compared to the Ledipasvir/Sofosbuvir combination. This combination has demonstrated efficacy, safety and tolerability in a number of cases and can therefore be superior to other direct antiviral agents reported in COVID19 management [35].
Regarding COVID-19 second wave expected to be characterized, clinically, by a higher incidence of coagulation mainly in the intestine, lung, muscles and other parts of the body, as one of the most serious clinical presentations in patients, the use of rivaroxaban as an anticoagulant could be one of the successful candidates for the management and prophylaxis of COVID-19 complications [36].
Potential Problems and Risks in Corona Virus Disease 2019 Management
Antibiotic resistance in the highly anticipated second wave of COVID-19 may increase the incidence of mortality in patients due to multidrug-resistant secondary infections. Excessive use of antibiotics, in addition to overcrowded healthcare services and lack of social awareness of the prevention of infection, all contribute to increased antibiotic resistance. Proper and well-organized professional, highquality training for healthcare workers and the implementation of strict preventative measures are therefore highly recommended. In addition, increased awareness is a basic requirement for aid to avoid exacerbating antibacterial resistance and increasing mortality rates [37].
Patients with severe mental illness and depressive disorders are more vulnerable to the devastating infection of COVID-19, which may contribute to several factors such as cognitive impairment followed by poor awareness and failure to comply with infection prevention practices. In addition, food craving is also one of the factors contributing to an obese depressed patient with insulin resistance and type 2 diabetes mellitus contributing to an increase in the risk of the incidence and severity of COVID-19 infection [38].
Occupational stress may result in immune function suppression or dysregulation. Factors contributing to increased stress include fatigue, acute stress due to sleep deprivation during night shifts, chronic stress due to continuous work/sleep schedule disturbances, shortage of personal protective equipment, exposure to patients, negative psychological stress, critical situational decisions, social stigma and abuse due to public fears of infection [39].
HLA-DQA1 may induce the production of anti-drug antibody against anti-TNF drugs such as infliximab, adalimumab; that are used as options for the management of COVID-19 and may therefore lead to treatment failure. In addition, HLA-B*46:01 carrier people are more susceptible to COVID-19 [40].
Pharmacodynamic interactions like prolongation of the QT interval may be of specific importance. Hydroxychloroquine, broadly prescribed as an anticoronavirus medication, or azithromycin, are two drugs known to prolong the QT interval. Concomitant use of anticancer drugs that prolong QT-interval could give rise to torsade de Points and be fatal. In this case, caution should be observed and electrocardiographic monitoring should be performed to monitor the duration of the QT interval during Combination treatment. Likewise, anticancer drugs could potentiate antiviral treatment nephrotoxicity and hepatotoxicity [41].
Due to advanced age, comorbidity and immune dysfunction, Chronic Lymphocytic Leukemia (CLL) patients may be at a particularly high risk of infection and poor coronavirus-related outcomes. Data indicate that there is a high risk of death in the Chronic Lymphocytic Leukemia (CLL) patients admitted with COVID-19 regardless of disease phase or treatment status [42].
COVID-19 Protective Measures to Undertake During Blood Transfusion
In China, 38 blood centers initiated a number of protective activities against Covid-19. These included monitoring blood donor temperature, expanding space, employing air disinfectant devices that sterilize using UV irradiation systems, and offering online appointments through popular social media sites. They’ve also begun investigating donors’ travel histories, following up on their donors’ health conditions, and issuing donation appeals [9].
Preventative measure procedures are advised, such as avoiding blood donation for 21 days after any probable exposure to individuals with confirmed Covid-19 infection, and people recovering fromCovid-19 should avoid donating blood for at least 28 days after their symptoms have resolved [43].
Clinical study findings in USA showed a reduced consumption of blood products in patients hospitalized on Covid-19 rooms during the pandemic. Thirteen percent of Covid-19 hospitalized patients required transfusions, accounting for just 4% of total inpatient blood transfusions over the study period. Hospitalized Covid-19 patients had substantially lower RBCS, PLTs, and plasma transfusion rates than concurrently hospitalized patients without coronavirus disease. Furthermore, Covid-19 ICU patients had greater rates of RBC and plasma transfusion than Covid-19 patients who were not in ICUs, although overall blood utilization remained low in both categories [44].
Conclusion
Although coronavirus can be transmitted through different routes, like air droplets, and surfaces, but literature findings indicated that it did not transmitted via blood products or blood transfusion. According to case reports, recipients who received blood products from positive donors did not get infected with Covid-19.Preventative measures are recommended to avoid viral transmission in healthcare and blood donation centers. Furthermore, such protective measures will aid in overcoming potential Covid-19 management risks.
References
- Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and metaanalysis. Journal of Clinical Virology. 2020; 127: 104371.
- WHO. Coronavirus disease (COVID-19) Weekly Epidemiological Update. Data as received by WHO from national authorities, as of 31 January 2021, 10 am CET. https://www.who.int/ publications/m/item/weekly-epidemiologicalupdate--- 2-february-2021.
- Lotfi M, Hamblin MR, Rezaei N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2020; 508: 254-266.
- Stilianos Louca, COVID-19 prevalence in 161 countries and over time. medRxiv. 2020.
- Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – The latest 2019 novel coronavirus outbreak in Wuhan, China. International Journal of Infectious Diseases. 2020; 91: 264-266.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England). 2020; 395: 507-513.
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19). Statpearls. StatPearls Publishing. 2020.
- Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: What we know. International Journal of Infectious Diseases. 2020; 94: 44-48.
- Chiem C, Alghamdi K, Nguyen T, Han JH, Huo H, Jackson D. The Impact of COVID-19 on Blood Transfusion Services: A Systematic Review and Meta- Analysis. Transfusion Medicine and Hemotherapy. 2021; 49: 107-118.
- Wang Y, Han W, Pan L, Wang C, Liu Y, Hu W, et al. Impact of COVID-19 on blood centres in Zhejiang province China. Vox Sanguinis. 2020; 115: 502- 506.
- Organization WHO. Guidance on maintaining a safe and adequate blood supply during the coronavirus disease 2019 (COVID-19) pandemic and on the collection of COVID-19 convalescent plasma: World Health Organization; 2020.
- Doremalen NV, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. The New England Journal of Medicine. 2020; 382: 1564-1567.
- COVID-19 and The Problem with Dental Aerosols. [(accessed on 12 May 2020)]; Available online: https://www.perioimplantadvisory.com/periodontics/ oral-medicine-anesthetics-and-oral-systemic-connection/article/14173521/ covid19-and-the-problem-with-dental-aerosols.
- Renyi Zhang, Yixin Li, Annie L Zhang, Yuan, Mario J Molina. Identifying airborne transmission as the dominant route for the spread of COVID-19. PNAS. 2020; 117: 14857-14863.
- Allam M, Cai S, Ganesh S, Venkatesan M, Doodhwala S, Song Z, et al. COVID-19 Diagnostics, Tools, and Prevention. Diagnostics. 2020; 10: 409.
- Abbott Launches Molecular Point-of-Care Test to Detect Novel Coronavirus in as Little as Five Minutes- 27 March 2020.
- Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. The Lancet. Infectious Diseases. 2020; 20: 425-434.
- Waller JV, Kaur P, Tucker A, Lin KK, Diaz MJ, Henry TS, et al. Diagnostic Tools for Coronavirus Disease (COVID-19): Comparing CT and RT-PCR Viral Nucleic Acid Testing. AJR. American journal of roentgenology. 2020; 215: 834-838.
- LabCorp, Quest Expand COVID-19 Antibody Testing Nationwide.
- Corrales-Aguilar E, Trilling M, Reinhard H, Falcone V, Zimmermann A, Adams O, et al. Highly individual patterns of virus-immune IgG effector responses in humans. Medical Microbiology and Immunology. 2016; 205: 409-424.
- Research from University of Washington Demonstrates High Performance of Abbott’s SARS-CoV-2 Antibody Blood Test-8 May 2020..
- Pan Y, Lia X, Yang G, Fan J, Tang Y, et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Inf Secur. 2020. 81: e28-e32.
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARSCoV- 2. JAMA. 2020; 323: 2249.
- Sanyaolu A, Okorie C, Marinkovic A, Ayodele O, Abbasi AF, et al. Navigating the Diagnostics of COVID-19. SN Compr Clin Med. 2020; 2: 1393-1400.
- WHO. Advice on the use of point-of-care immunodiagnostic tests for COVID-19. World Health Organization. 2020.
- ACP. COVID-19: An ACP physician’s guide and resources. American College of Physicians. April 24, 2020. [Accessed April 24, 2020, https:// assets.acponline.org/coronavirus/scormcontent/ ?_ga=2.229126044.14846 31089.158376785245448268.1580931839&_gac=1.12324352.1584127450. EAIaIQobChMI04D_iPTk2gIVSFqGCh11lwA1EAAYASAAEgKoB_D_ BwE&utm_campaign=FY19-20_MD_ MEDED_EML_ COVID19_MD92941 _MAR19&utm_medium= email&utm_source=Eloqua#/].
- Cheng MP, Papenburg J, Desjardins M, Kanjilal S, Quach C, Libman M, et al. Diagnostic Testing for Severe Acute Respiratory Syndrome–Related Coronavirus-2. Annals of Internal Medicine. 2020; 172: 726-734.
- Zheng Y, Ma Y, Zhang J, Xie X. COVID-19 and the cardiovascular system. Nature Reviews. Cardiology. 2020; 17: 259-260.
- Rabiee N, Bagherzadeh M, Ghasemi A, Zare H, Ahmadi S, Fatahi Y, et al. Point-of-Use Rapid Detection of SARS-CoV-2: Nanotechnology-Enabled Solutions for the COVID-19 Pandemic. International Journal of Molecular Sciences. 2020; 21: 5126.
- Cascella M, Rajnik M, Cuomo A, et al. Features, Evaluation, and Treatment of Coronavirus. [Updated 2020 Oct 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020.
- Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020; 582: 469-469.
- Bimonte S, Crispo A, Amore A, Celentano E, Cuomo A, Cascella M. Potential Antiviral Drugs for SARS-Cov-2 Treatment: Preclinical Findings and Ongoing Clinical Research. In vivo. 2020; 34: 1597-1602.
- Gautret P, Lagier J, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents. 2020; 56: 105949.
- Roschewski M, Lionakis MS, Sharman JP, Roswarski J, Goy A, Monticelli MA, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Science Immunology. 2020; 5.
- Sara A. Raslan, Eslam M. Shehata, Mohamed Raslan, Nagwa A. Sabri, Management of Second Wave of COVID 19 with Ledipasvir / Sofosbuvir Combination. Will it Work? A Review Article, Saudi Journal of Medical and Pharmaceutical Sciences. 2020; 6: 654-657.
- Nagwa A Sabri, Sara AR, Eslam MS, Raslan M. Rivaroxaban and Therapeutic Monitoring Application: Will it be a Significant Tool for Management of COVID-19?. Acta Scientific Pharmaceutical Sciences. 2020; 4: 97-105.
- Raslan M, Eslam MS, Sara AR, Nagwa AS. Antimicrobial Resistance and Second Wave of COVID-19: Will It Impose Management Protocols Deviation?. Ann Pharmacol Toxicol. 2020; 1(1): 1001.
- Nagwa Ali Sabri, Mohamed Ahmed Raslan, Eslam Mansour Shehata, Sara Ahmed Raslan. Depressive Disorders and Incidence of COVID-19: Is There a Correlation and Management Interference? Psychological Disorders and Research. 2020; 3: 2-7.
- Mohamed AR, Eslam MS, Sara AR, and Nagwa AS, Association of Occupational Stress for Health Care Professionals and Incidence of COVID-19. Is there an Increased Risk? Acta Scientific Pharmaceutical Sciences. 2020; 4: 35-39.
- Raslan MA, Alshahawey M, Shehata EM, Sabri NA. Does human leukocyte antigen gene polymorphism affect management of COVID-19 Patients? A review article. Scientific J Genet Gene Ther. 2020; 6: 001-003.
- Gougis P, Fenioux C, Funck-Brentano C, Veyri M, Gligorov J, Solas C, et al. Anticancer drugs and COVID-19 antiviral treatments in patients with cancer: What can we safely use?. European Journal of Cancer. 2020; 136: 1-3.
- Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020; 136: 1134-1143.
- Langhi DM, Souza RCD, Barros M, Santis GCD, Kashima SH, Bordin JO. SARS-COV-2: is it a risk for blood transfusion?. Hematology, Transfusion and Cell Therapy. 2021; 44: 100-103.
- Barriteau CM, Bochey P, Lindholm PF, Hartman K, Sumugod R, Ramsey G. Blood transfusion utilization in hospitalized COVID-19 patients. Transfusion. 2020.