
Special Article - Pegylated Interferon and Ribavirin Treatment for Hepatitis
J Hepat Res. 2015; 2(3): 1030.
Serum Anti-Interferon Alpha Antibodies in Chronic Hepatitis C Patients Treated with Pegylated Interferon Alpha Containing Therapy
Loggi E¹#, Vukotic R¹#, Cursaro C¹, Scuteri A¹, Martello Panno A¹, Grandini E¹, Margotti M¹, Lorenzini S1,2, Brillanti S¹, Bernardi M¹ and Andreone P¹*
¹Department of Medical and Surgical Sciences, University of Bologna and Azienda Ospedaliero-Universitaria Policlinico Sant’Orsola-Malpighi, Italy
²Emergency Department, Azienda ASL Ravenna, Ospedale Santa Maria delle Croci, Italy #Both authors contributed equally to this work
*Corresponding author: Andreone P, Department of Medical and Surgical Sciences - OU Medical Semiotics, Via Massarenti 9, 40138 Bologna, Italy
Received: August 27, 2015; Accepted: December 23, 2015; Published: December 31, 2015
Abstract
The development of anti-IFNα antibodies is an occurrence described in chronic hepatitis C patients during treatment with Interferonα/PEG-Interferonα. However, its relevance, especially in difficult-to treat patients, has not been defined.
We retrospectively measured the serum levels of anti-IFNα antibodies (baseline and week 12) and IFNα levels (week 12) by ELISA in 76 previous nonresponders, and in 14 naïve patients treated with Pegylated-IFNα and Ribavirin. A group of 57 Healthy Donors (HD) was also assessed as control. Positivity to anti-IFNα antibodies was established on the values of HD.
Baseline anti-IFNα antibodies were detected in 15.5% of patients and in 7% of HD, with significantly higher concentrations in patients than HD (181.5±389.9 vs 95.9±143.0 ng/mL, p=0.0023). All positive patients were IFNα-experienced. At week 12, the prevalence of positivity increased to 22.3 and 28.5% in experienced and naïve patients, respectively, and the levels of anti-IFNα antibodies did not differ between the two groups (391±792.3 vs 384.7±662.6 ng/mL, respectively). IFNα concentrations were significantly lower in antibody-positive patients than in antibody-negatives (988.2±1402 vs 3462±830.8 pg/mL, p≤0.0001) and the levels of antibodies and IFNα were inversely correlated (r=-0.405, p=0.0001). The antibody-positive population clustered in null responders (67%) and 19/21 patients (90%) did not achieve SVR.
In Conclusion, the development of anti-IFNα antibodies is a non-negligible occurrence in patients treated with PEG-IFNα, is stable over time, and has a relevant clinical impact when associated with low levels of circulating PEG-IFNα. It should be considered in patients undergoing treatments including PEG-IFNα.
Keywords: Chronic Hepatitis C; Interferon alpha antibodies; Interferon alpha; Treatment; Antiviral therapy; Non-response
Abbreviations
HCV: Hepatitis C Virus; CHC: Chronic HCV Infection; PEGIFNα: Pegylated Interferon alpha; RBV: Ribavirin; DAAs: Direct Acting Antivirals; anti-IFNα-Ab: anti-IFN alpha Antibodies; IFNα: Interferon-alpha; EVR: Early Virological Response; EOT: End of Treatment response; SVR: Sustained Virological Response; NR: Null Response; PR: Partial Response; RR: Relapser; SD: Standard Deviation; PCR: Polymerase Chain Reaction; SNPs: Single Nucleotide Polymorphisms; HD: Healthy Donor
Introduction
The primary goal of Hepatitis C Virus (HCV) treatment is to achieve a sustained virological response, defined as persisting undetectable HCV-RNA after treatment withdrawal leading to the resolution of liver disease, at least in patients without cirrhosis [1].
The treatment of hepatitis C has dramatically improved over the past decade, so much that a significant proportion of chronic patients can now be cured.
Until recently, the standard of care for Chronic HCV Infection (CHC) has been based on the antiviral and immunomodulatory effect of Pegylated Interferon alpha (PEG-IFNα) and Ribavirin (RBV) [2]. Current schedules take advantage of direct acting antiviral (DAAs), agents able to interfere with HCV enzymes essential for viral replication. The “new era” of HCV treatment has started with the approval of two drugs against the NS3/4A serine protease for genotype 1 infections [3-6], and since then, several DAAs with other viral targets have been approved or are in the pipeline [7]. These drugs promise to lead to viral eradication with simplified regimens, very low toxicity, and without the use of interferon. However, the “first generation” (telaprevir and boceprevir) and the most recent secondwave DAAs (simeprevir, sofosbuvir and daclatasvir) will continue to include PEG-IFNα plus RBV, even if with shorter schedules [1].
Due to the important limitations of this therapy, such as the suboptimal response rates, severe side effects and high costs, its efficacy also depends on an appropriate selection of patients. Selection should include a careful evaluation of the well-described host and viral factors associated with therapeutic failure [8].
Among these factors, the development of serum anti-IFN alpha antibodies (anti-IFNα-Ab) that able to both bind and neutralise the biologic activity of IFNα, has been proposed as a mechanism against non-response in patients treated with recombinant IFNα [9-11], while others did not arrive at the same conclusions [12,13]. More recently, the role of anti-IFNα-Ab in combination with the pegylated form of IFN has been evaluated, providing controversial results [14-17].
In addition, the “natural” production of anti-IFNα-Ab has been reported in patients with various autoimmune disorders [18-20], and auto antibodies anti-cytokines have also been detected in healthy donors, with unknown significance [21].
Thus, this phenomenon could be more complex than it appears, and its clinical impact remains elusive. In particular, its role should be addressed in difficult to treat patients, who remain the clinical category less prone to benefit from advancements in HCV therapy, and for whom there is the urgent need of optimising the costeffectiveness of new treatment schedules containing both interferon and antiviral.
On the basis of these remarks, we performed a retrospective study on stored serum samples from CHC patients with the following purposes:
- To assess the presence of anti-IFNα-Ab during PEGIFNα plus RBV treatment in CHC, both treatment-failure and naïve patients, and in a group of healthy blood donors as control;
- To assess the impact of anti-IFNα-Ab on serum levels of IFNα and virological response to treatment.
- In particular:76 patients were previously non-responders to one or more course of IFNα/PEG-IFNα plus RBV.
- 14 patients were treatment-naïve.
- Early Virological Response (EVR): HCV-RNA decrease ≥2 log10 IU/mL at week 12 of treatment;
- End of treatment response: HCV-RNA undetectable at the end of treatment;
- Sustained Virological Response (SVR): HCV-RNA undetectable at 24 week after the end of therapy;
- Null response: HCV-RNA decrease <2 log10 IU/mL at week 12 of treatment;
- Partial response: HCV-RNA decrease ≥2 log10 IU/mL at week 12 but still detectable at week 24 of treatment;
- Relapser: HCV-RNA undetectable at the end of treatment, with viral rebound after the end of treatment.
- European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014; 60: 392-420.
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009; 361: 580-593.
- Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011; 364: 2417-2428.
- Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011; 364: 1195-1206.
- Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011; 364: 1207-1217.
- Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011; 364: 2405-2416.
- Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int. 2014; 34 Suppl 1: 69-78.
- Asselah T, Estrabaud E, Bieche I, Lapalus M, De Muynck S, Vidaud M, et al. Hepatitis C: viral and host factors associated with non-response to pegylated interferon plus ribavirin. Liver Int. 2010; 30: 1259-1269.
- Leroy V, Baud M, de Traversay C, Maynard-Muet M, Lebon P, Zarski JP. Role of anti-interferon antibodies in breakthrough occurrence during alpha 2a and 2b therapy in patients with chronic hepatitis C. J Hepatol. 1998; 28: 375-381.
- Bonetti P, Diodati G, Drago C, Casarin C, Scaccabarozzi S, Realdi G, et al. Interferon antibodies in patients with chronic hepatitic C virus infection treated with recombinant interferon alpha-2 alpha. J Hepatol. 1994; 20: 416-420.
- Milella M, Antonelli G, Santantonio T, Currenti M, Monno L, Mariano N, et al. Neutralizing antibodies to recombinant alpha-interferon and response to therapy in chronic hepatitis C virus infection. Liver. 1993; 13: 146-150.
- Bonino F, Baldi M, Negro F, Oliveri F, Colombatto P, Bellati G, et al. Clinical relevance of anti-interferon antibodies in the serum of chronic hepatitis C patients treated with interferon-alpha. Journal of interferon & cytokine research. 1997; 17: S35-38.
- Giannelli G, Antonelli G, Fera G, Del Vecchio S, Riva E, Broccia C, et al. Biological and clinical significance of neutralizing and binding antibodies to interferon-alpha (IFN-alpha) during therapy for chronic hepatitis C. Clinical and experimental immunology. 1994; 97: 4-9.
- Matsuda F, Torii Y, Enomoto H, Kuga C, Aizawa N, Iwata Y, et al. Antiinterferon- α neutralizing antibody is associated with nonresponse to pegylated interferon-α plus ribavirin in chronic hepatitis C. J Viral Hepat. 2012; 19: 694-703.
- Scagnolari C, Trombetti S, Solda A, Milella M, Gaeta GB, Angarano G, et al. Development and specificities of anti-interferon neutralizing antibodies in patients with chronic hepatitis C treated with pegylated interferon-alpha. Clinical microbiology and infection. 2012; 18: 1033-1039.
- Halfon P, Perusat S, Bourliere M, Bronowicki JP, Trimoulet P, Benhamou Y, et al. Neutralizing antibodies to interferon-alpha and circulating interferon in patients with chronic hepatitis C non-responding to pegylated interferon plus ribavirin re-treated by pegylated interferon-alpha-2a and ribavirin (ANRS HC16 GAMMATRI substudy). Journal of medical virology. 2010; 82: 2027- 2031.
- van der Eijk AA, Vrolijk JM, Haagmans BL. Antibodies neutralizing peginterferon alfa during retreatment of hepatitis C. N Engl J Med. 2006; 354: 1323-1324.
- Ching KH, Burbelo PD, Tipton C, Wei C, Petri M, Sanz I, et al. Two major autoantibody clusters in systemic lupus erythematosus. PLoS One. 2012; 7: e32001.
- Meager A, Vincent A, Newsom-Davis J, Willcox N. Spontaneous neutralizing antibodies to interferon--alpha and interleukin-12 in thymoma-associated autoimmune disease. Lancet. 1997; 350: 1596-1597.
- Meager A. Natural autoantibodies to interferons. J Interferon Cytokine Res. 1997; 17 Suppl 1: S51-53.
- van der Meide PH, Schellekens H. Anti-cytokine autoantibodies: epiphenomenon or critical modulators of cytokine action. Biotherapy. 1997; 10: 39-48.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011; 55: 245-264.
- Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. Journal of immunological methods. 1998; 221: 35-41.
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2014. J Hepatol. 2014; 61: 373-395.
- Strayer DR, Carter WA. Recombinant and natural human interferons: analysis of the incidence and clinical impact of neutralizing antibodies. Journal of interferon & cytokine research. 2012; 32: 95-102.
- Sørensen PS, Deisenhammer F, Duda P, Hohlfeld R, Myhr KM, Palace J, et al. Guidelines on use of anti-IFN-beta antibody measurements in multiple sclerosis: report of an EFNS Task Force on IFN-beta antibodies in multiple sclerosis. Eur J Neurol. 2005; 12: 817-827.
- Schmaljohn AL. Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts. Curr HIV Res. 2013; 11: 345-353.
- Hangartner L, Zellweger RM, Giobbi M, Weber J, Eschli B, McCoy KD, et al. Nonneutralizing antibodies binding to the surface glycoprotein of lymphocytic choriomeningitis virus reduce early virus spread. The Journal of experimental medicine. 2006; 203: 2033-2042.
- Meager A, Wadhwa M. Detection of anti-cytokine antibodies and their clinical relevance. Expert Rev Clin Immunol. 2014; 10: 1029-1047.
- Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013; 368: 34-44.
Patients and Methods
The serum levels of anti-IFNα-Ab and IFNα were retrospectively measured on stored serum samples collected from 90 consecutive CHC patients who had received antiviral treatment with PEG-IFNα2a (180 μg/weekly subcutaneously) and RBV (1000-1200 mg/daily, according to body weight, <75 or ≥75 kg, respectively) in the period from 2008 to 2010 at the Outpatient Clinic for Liver Disease, Azienda Ospedaliero-Universitaria di Bologna, Policlinico Sant’Orsola- Malpighi, Bologna, Italy.
For the control group, serum samples from 57 healthy donors, recruited at the Hospital Blood Transfusion Service, were also frozen and stored until analysis.
The inclusion and exclusion criteria were those applied for the dual antiviral treatment eligibility [22]. More specifically, the major inclusion criteria were the following: adult age (>18), CHC confirmed by detectable serum HCV-RNA levels and histologically documented. The major exclusion criteria were the following: decompensate cirrhosis, evidence of hepatocellular carcinoma, other concomitant causes of hepatic disease, pregnancy or breastfeeding, co-infection with HIV or HBV, severe concomitant diseases and haematological values not compatible with treatment.
Virological definitions used in the text and graphics are the following:
The anti-IFNα-Ab and IFNα concentration measurement was performed at baseline and at week 12 of treatment.
The local Ethical Committee approved the study protocol and written informed consent was obtained from each patient.
Detection of anti-IFNα-Ab and IFNα
Serum concentrations of anti-IFNα-Ab and IFNα were measured by commercially available enzyme-linked immunosorbent assays (Bender Medsystems, eBiosciences, Vienna, Austria), according to manufacturer’s instruction. The level of sensitivity for the anti-IFNα- Ab test was 1.4 ng/mL. The level of sensitivity for the IFNα test was 3.2 pg/mL.
Virological assays
HCV–RNA was measured by PCR ([Cobas–Roche, Hoffmann-La Roche Ltd, Basel, Switzerland] Amplicor HCV qualitative, version 2.0, LOD 50 IU/mL; Amplicor HCV quantitative, HCV RNA Monitor, version 2.0, LOD 500–600 IU/mL).
HCV genotype was determined using a commercially available line-probe assay (INNO-LiPA®, Innogenetics, and Antwerp, Belgium).
HCV-RNA quantification was performed at baseline, at weeks 12, 24, and 48 during treatment and at 24 weeks after the end of treatment.
IL28B genotyping
IL28B genotyping was performed in patients who consented to the analysis and for whom mononuclear cell samples were available.
Genomic DNA was extracted from whole blood samples collected in EDTA-tubes or total PBMCs by paramagnetic particles using an automated platform (Maxwell 16, Promega, Milan, Italy), according to the manufacturers protocol.
The single nucleotide polymorphisms (SNPs) rs 12979860 in the gene for IL28B were determined by real time PCR using a commercially available kit (Experteam, Venice, Italy).
Statistical analysis
Parametric and non-parametric tests were used, as appropriate. In particular, quantitative variables were expressed as a mean ± Standard Deviation (SD), and categorical variables as absolute and relative frequencies. Groups of quantitative and qualitative variables were compared using the Mann-Whitney and the Fisher-exact tests, respectively. Correlations were performed using Spearman rank correlation.
A p-value <0.05 was considered to be statistically significant. Data handling and analysis were performed with Graph Pad Prism software, version 5.
Results
Anti-IFNα-Ab prevalence
The levels of serum anti-IFNα-Ab were assessed in 90 consecutive CHC patients at baseline of treatment with PEG-IFNα plus RBV, and in a group of 57 healthy donors. The baseline characteristics of CHC patients are listed in Table 1. Among the 90 treated patients, 76 were treatment-failure and 14 were treatment-naïve.
Age, median [range]
55 [28-71]
Sex, male [%]
51 [56.6]
HCV-RNA IU/mL, median [range]
1.2x106 [4.4x104- 2.7x106]
High viral load (≥8x105 IU/mL), no. [%]
56 [62]
HCV genotype 1, no. [%]
87 [96.6]
IL28B (rs12979860) genotype*, no. [%]
CC
7 [10.8]
CT
41 [63]
TT
17 [26.2]
Metavir Score, no. [%]
F0-F1
36 [40]
F2
14 [15.4]
F3
25 [28]
F4
15 [16.6]
Body Mass Index, median [range]
26 [20-41]
Number of previous treatment in experienced patients (N=76) no. [%]
1
40 [52]
2
18 [23]
3
6 [8]
4
6 [8]
5
5 [6]
6
1 [3]
*Data available on 65 patients out of *Data available on 65/90; No: absolute number.
Table 1: Demographic and Disease Characteristics of CHC Patients.
The baseline serum levels of anti-IFNα-Ab were significantly higher in CHC patients than in healthy donors (mean±SD: 181.5±389.9 vs 95.9±143.0 ng/mL, respectively, p=0.0023, Figure 1A).
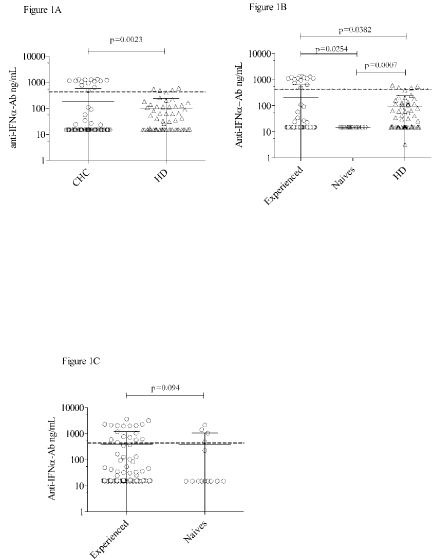
Figure 1: Baseline anti-IFNα-Ab (ng/mL) in chronic HCV patients (CHC) and healthy donors (HD); Figure 1B: Baseline anti-IFNα-Ab in CHC subdivided into
experienced, naïve and HD; Figure 1C: anti-IFNα-Ab in experienced and naives at week 12 of treatment. The Y-axis is a logarithmic scale. The grey dashed line
represents the threshold of positivity (430 ng/mL). Line and bars: Mean ± SD.
On the basis of values obtained in the control group, a conservative value of 430 ng/mL, assessed as 3 times the mean plus SD [23], was assumed as the threshold for positivity, so that CHC patients and Healthy Donors (HD) were categorised into anti-IFNα-Ab positive or anti-IFNα-Ab negative groups. Using this threshold, 14/90 (15.5%) in the entire CHC group were anti-IFNα-Ab positive, compared to 4/57 (7.0%) in the HD group (p=0.196). When the CHC patients were stratified into experienced and naïve, the highest levels of anti- IFNα-Ab were measured in experienced patients, while the levels of anti-IFNα-Ab were undetectable in naïve patients. Thus, anti-IFNα- Ab levels of treatment-naïve patients were significantly lower when compared to those of the other two groups (Figure 1B).
When the CHC patients were evaluated at week 12 of treatment, the prevalence rates of anti-IFNα-Ab positivity increased in both experienced and naïve patients (17/76, 22.3% vs 4/14, 28.5%, respectively), without significant differences between the two groups (p=0.732). Similarly, the anti-IFNα-Ab concentrations were not different between experienced and naïve patients (391±792.3 vs 384.7±662.6 ng/mL, respectively, p=0.094, Figure 1C).
Overall, 21 patients out of 90 CHC patients (23.3%) tested anti- IFNα-Ab positive at week 12.
Among the patients positive at baseline, two tested negative at week 12, while the remaining maintained the positive status. An additional nine patients developed antibodies positivity during treatment. In general, there was a trend of anti-IFNα-Ab increase from baseline to week 12, while a decrease was observed in only two patients. The increase was highly variable, ranging from 1.5 to 140- fold the basal value. Interestingly, the positivity of anti-IFNα-Ab at baseline was independent from the wash out time from the previous treatment course, which ranged from 6 to 106 months.
Anti-IFNα-Ab and IFNα concentrations
To address the therapeutic impact of the anti-IFNα-Ab, we measured the serum levels of IFNα at week 12, that resulted significantly lower in the anti-IFNα-Ab positive group compared to the negative group (mean±SD: 988.2±1402 vs 3462±830.8 pg/mL, respectively, p<0.0001, Figure 2A).
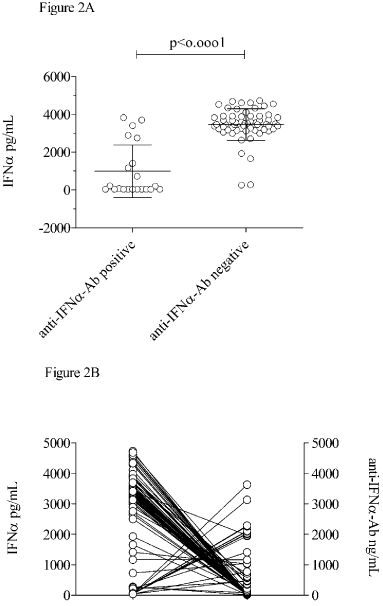
Figure 2: IFNα levels (pg/mL) in anti-IFNα-Ab positive and anti-IFNα-Ab
negative patients at week 12 of treatment. Line and bars: Mean ± SD.
Figure 2B: IFNα levels (left Y-axis and white dot plots) and anti-IFNα-Ab
levels (right Y-axis and grey dot plots) in CHC patients (n=90) at week 12.
More specifically, in the anti-IFNα-Ab negative group, just two out of 69 patients showed remarkably lower serum concentrations of IFNα (Figure 2A). The reason for this inconsistency is difficult to ascertain; however, an incomplete adherence to treatment or a defect in drug absorption cannot be excluded. Instead, in the anti-IFNα-Ab positive population, two different patterns can be observed. In the first, despite the presence of antibodies, the concentration of IFNα was comparable to that of patients without antibodies. In the second, the presence of anti-IFNα-Ab abrogates, to a different extent, the concentration of IFNα, resulting in extremely low or even under the test detection limits in twelve patients. Despite these two different conditions, the concentrations of anti-IFNα-Ab and IFNα were inversely correlated, considering both the anti-IFNα-Ab positive patients (n=21; r= -0.6233, p= 0.0025) and the entire group (n=90; r= -0.405, p=0.0001, Figure 2B). In particular, the patients with the highest concentrations of anti-IFNα-Ab (>2000 ng/mL, n=8) displayed IFNα levels under the test detection limit.
Anti-IFNα-Ab and response to treatment
19/21 (90.5%) patient’s anti-IFNα-Ab positive at week 12 did not achieve SVR at the present treatment course. Overall, the SVR was achieved only in 13/90 (14.4%) patients as it should be considered that this study was performed in a prevalent population of “very” difficult- to treat patients. Although the SVR rates were not significantly different between anti-IFNα-Ab positive and negative patients, nevertheless they were lower in anti-IFNα-Ab positive patients than in negative patients (9 vs 16%, respectively, p=0.19).
The presence of anti-IFNα-Ab at week 12 was associated with failure to achieve EVR, as 14/21 (66.7%) positive patients did not experience a ≥ 2 log decrease in HCV-RNA from baseline, compared to 23/69 negative patients (33.3%, p=0.0105). Moreover, considering the whole study population, the levels of anti-IFNα-Ab were higher in the group of null responders compared to other groups (Figure 3A). Conversely, the levels of IFNα were significantly lower in null responders (Figure 3B). In particular, all 8 patients, in whom high concentrations of anti-IFNα-Ab were associated with undetectable levels of IFNα, clustered in null responders.
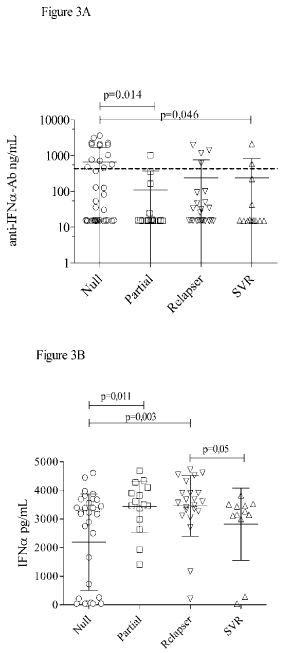
Figure 3: Serum levels of anti-IFNα antibodies (ng/mL, Figure 3A) and IFNα
(pg/mL Figure 3B) according to virological responses. Line and bars: Mean
± SD.
Additional clinical features of antibodies-positive patients
We tried to characterise in detail the clinical or genetic parameters characterising the anti-IFNα-Ab positive patients.
As for genetic factors, the IL28B genotype polymorphism was evaluated in 65/ 90 patients.
All variants (CC, CT and TT) were homogenously present in the naïve population, while, among experienced patients, only three were CC. Thus, the majority of patients carried the non-favourable allele (CT or TT), so that an association between the presence of antibodies and the IL28B genotype cannot be addressed. Moreover, when the anti-IFNα-Ab positive and negative patients were subdivided based on fibrosis stage, the percentage of patients with F0-F2 or F3-F4 METAVIR score in antibody-positive and in antibody-negative groups (F0-F2: 42.8 vs 59.4; F3-F4: 57.2 vs 40.6, respectively) indicated a slightly higher prevalence of positive patients with more advanced liver disease, but the difference was not statistically significant (p=0.215).
Finally, the majority of patients enrolled in this study continued to be followed at our centre, and 29 of them were retreated afterwards with a regimen containing DAAs. Among the anti-IFNα-Ab positive patients, 8 underwent a subsequent course of antiviral treatment with IFN-based (n=4) or IFN-free regimens (n=4). Interestingly, all patients treated with an IFN-free regimen achieved SVR while, among those retreated with a triple regimen containing PEG-IFNα, only one reached SVR (Figure 4). Of note, despite the presence of anti-IFNα-Ab, the patient who reached SVR maintained high serum levels of IFNα, while in the remaining three patients the levels were undetectable. Among the anti-IFNα-Ab negative patients, 21 were retreated with schedules containing PEG-IFNα, except for 1 patient, and half of them achieved SVR (Figure 4).

Figure 4: Subsequent antiviral treatments and relative outcomes in anti-IFNα-Ab positive and anti-IFNα-Ab negative patients.
Discussion
The results of our study demonstrate that the development of anti- IFNα-Ab in CHC patients undergoing antiviral treatments, including PEG-IFNα, is an occurrence to be considered, especially, but not only, in those previously exposed to IFNα. We decided to evaluate this phenomenon in difficult-to-treat patients because they are the most prevalent population in the real clinical practice, at greater risk of disease progression, the main candidates to receive new treatment regimens and those with higher probability of treatment failure. Furthermore, although IFN-free regimens are rapidly approaching, the most recent European recommendations for CHC treatment with DAAs still include treatment options containing PEG-IFNα [24]. In addition, while more recent DAAs promise to cure the infection in a large proportion of patients, and to radically change viral epidemiology, the daily clinical practice is already facing the problem of high drug costs, thereby limiting their access, not only in developing countries. So, treatments including PEG-IFNα will likely continue to represent a consistent part of CHC therapy, at least in the near future [1,24].
The development of anti-IFNα-Ab is a wider phenomenon that has been already described in other settings, such as the treatment of some neurological diseases [25]. In this setting, the problem has received great attention, so that the European Federation of Neurological Societies Task Force recommended the measurement of anti-IFNβ antibodies during treatment in patients with multiple sclerosis, suggesting modifications of the treatment strategy according to [26].
The data available in the literature reported that pegylated preparations have lower immunogenicity compared to standard preparations of IFNα [14,15], and accordingly with this observation, the prevalence of anti-IFNα-Ab positivity in the present study is almost consistent with that reported in a previous study performed on PEG-IFNα non-responder subjects [16] but is higher than those from other recent studies [14,15].
The different experimental approaches to detect and quantify the anti-IFNα-Ab used in these studies may explain these discrepancies, as other antiviral neutralisation tests, instead of the binding ELISA assay performed in the this study, were used. We are aware that ELISA allows the detection of different antibodies specificities, some of them binding but not neutralising, and that the latter could have a negligible impact on the antiviral mechanism of interferon. However, the biological role of non-neutralising antibodies is still unclear, but indirect mechanisms of interference with the target could be significant [27,28]. In addition, the ELISA method has the advantage of being very simple, standardised and non-demanding on particular equipment, so it is more likely to be widely introduced in clinical diagnostic setting. On the other hand, however specific expression of IFN-induced signal molecules have not been evaluated in the present study, testing the IFNα concentrations together with the anti-IFNα- Ab may help in providing an indirect measure of the neutralising activity of antibodies.
By choosing a conservative threshold to discriminate between anti-IFNα-Ab positive and negative patients, we show that the immunogenicity of IFNα develops early after exposure, as naïve patients resulted positive at the same rate of multiple-experienced ones, and with similar amounts. In addition, we found an unexpectedly high prevalence of antibodies also in uninfected, healthy donors, although at significantly lower levels. Detection of antibodies against several cytokines has been repeatedly reported in healthy donors [21,29], and a regulatory role in modulating a potential cytokinederived pathology has been proposed. However, their clinical relevance has not been yet defined, and requires additional studies. From this observation, it is more difficult to explain the absence of anti-IFNα-Ab in naïve patients at baseline, even if the low patient number in this group likely plays a key role.
In our study, two different profiles of antibodies positivity have been observed. In some patients, despite high levels of anti-IFNα- Ab, the serum levels of IFNα were not abrogated, while in the others the presence of antibodies is associated with low or absent sera concentrations. In particular, the amount of antibodies appears to be important, as the patients with the highest levels of antibodies have undetectable levels of IFNα.
The reasons for these two conditions are difficult to ascertain, but it is possible to speculate that it most likely reflects different capabilities of mounting the antibodies response, in terms of specificities, neutralising/non neutralising proportions, affinity and avidity.
Interestingly, the development of antibodies seems to be a particularly stable phenomenon, as the positivity of anti-IFNα-Ab at baseline was independent from the time of wash out from previous course of therapy. This point should be kept in mind, particularly when patients already exposed to IFNα need to be retreated. As far treatment response is considered, the overall response rate of this population was particularly low, compared to the standard 40-50% of SVR reported for genotype 1 naïve patients treated with PEG-IFNα and RBV [2]. In addition, the majority of anti-IFNα-Ab positive patients clustered in the null responders group. Thus, these data demonstrate that the development of antibodies is most likely responsible for at least part of the non-response rates in a population of difficult to treat patients, being a non-necessary but sufficient condition of treatment inefficacy. The development of anti-IFNα-Ab per se does not constitute a non-response motif, but it becomes an essential parameter when associated with low or undetectable IFN levels. This association between null response and presence of anti- IFNα-Ab could be also considered an indirect proof of their impact on antiviral activity.
Moreover, among the well-established parameters known to influence the IFN-response [8], the group of anti-IFN-Ab positive patients was not significantly different from antibody negative ones in terms of age, sex, IL-28B. Concerning liver fibrosis, a trend towards a more advanced liver disease can be observed in anti IFN-Ab positive patients, although this difference did not reach the statistical significance.
Finally, our data suggest that anti-IFNα-Ab positive patients retreated with an adequate IFN-free regimen have the chance to clear the infection, while if they undergo an additional course of therapy containing PEG-IFNα, these chances may be significantly reduced. Although this evaluation was possible in very few patients, this may be a very useful clinical remark, taking into account that the majority of the CHC patients who will need an effective antiviral treatment in the near future will be the non-responders to previous treatment courses. In particular, the triple therapy with PEG-IFNα/ RBV and sofosbuvir (or simeprevir or daclatasvir)-based schedule should be prescribed after excluding the presence of anti-IFNα-Ab or determining the IFNα concentrations after a 4-week lead-in phase because the anti-IFNα-Ab presence positivity would hamper the final outcome of treatment increasing the cost-effectiveness of the therapy. In these patients, an alternative IFN-free regimen should be carefully set up, also considering that the data in genotype 1 experienced patients treated with only sofosbuvir/RBV are currently lacking and the available data are not encouraging [30].
In conclusion, the present study indicates that the development of anti-IFNα-Ab is a phenomenon that should be considered when patients undergo treatment with IFN-containing regimens, also if associated with a potent DAA. To measure anti-IFNα-Ab and IFNα levels after a lead-in phase with PEG-IFNα/RBV is a very fast and simple test that may allow the option to tailor and optimise the treatment for CHC.
Acknowledgement
The authors thank Dr Elena Marasco and Dr Vilma Mantovani (Centro Unificato di Ricerca Biomedica Applicata, Sant’Orsola- Malpighi Hospital, Bologna) for genetic determinations. The study was partially supported by Alfa Wassermann (Bologna, Italy), and Education, University and Research Italian Ministry through RFO (ex-60%) Fondi Ricerca Istituzionale.
References