
Research Article
Austin J Infect Dis. 2017; 4(1): 1030.
Microbiological Profile and Molecular Characterization of Multidrug-Resistant Gram-Negative Bacilli Producing Catheter-Associated Urinary Tract Infections in the Internal Medicine Services of a Venezuelan University Hospital
Quijada-Martínez P1,2, Flores-Carrero A3,4, Labrador I¹, Millán Y¹ and Araque M¹*
¹Molecular Microbiology Laboratory, University of Los Andes, Venezuela
²Internal Medicine Service, Autonomous Institute University Hospital of Los Andes, Venezuela
³Institute of Social Assistance and Welfare of the Ministry of Education (IPASME), Venezuela
4Center of Electron Microscopy, University of Los Andes, Venezuela
*Corresponding author: Araque M, Molecular Microbiology Laboratory, University of Los Andes, Mérida, Venezuela
Received: October 20, 2017; Accepted: November 13, 2017; Published: November 20, 2017
Abstract
The aim of this study was to determine the epidemiological features of patients with Catheter-Associated Urinary Tract Infection (CAUTI) admitted to internal medicine services of the University Hospital of The Andes (UHTA), Mérida. Venezuela. Also, we determined microbiological profiles and molecular characteristics of multidrug-resistant Gram-negative bacilli causing this infection. A total of 73 patients with indwelling urinary catheters were hospitalized in the UHTA’s internal medicine wards during January-Jun 2015. Of these, 40 (54.8%) had CAUTI. Microbiological processing of urine samples was performed by conventional and automated methods. Extended spectrum β-lactamases (ESBLs) and carbapenemases were phenotypically detected. Determination of bla genes, phylogenetic groups, virulence factors and clonal typing were performed by molecular assays. Epidemiological variables were analyzed using standard statistical methods. We found a CAUTI rate of 5.9; old age and a prolonged catheterization were predisposing factors. Candida tropicalis and C. albicans were the main etiological agents followed by Escherichia coli and Klebsiella pneumoniae. Gram-negative bacteria showed resistance to betalactam antibiotics. The majority of ESBL-producing enterobacteria harbored blaCTX-M-15 genes associated to other ESBLs and/or carbapenemases as KPC-2 and VIM-2. All K. pneumoniae was classified into the phylogroup KpI and E. coli strains among the four major phylogenetic groups (A, B1, B2 and D). All E. coli strains harbored the fimH gene. In conclusion, the knowledge of correlations between resistance pattern, virulence factors and phylogroups are of great value for clinical diagnosis, treatment and predictive prognosis of CAUTI.
Keywords: Catheter–associated urinary tract infections; Microbiological profile; Molecular characterization; Multidrug-resistant Gram-negative bacteria; Venezuela
Introduction
Urinary tract infections attributed to the use of an indwelling urinary catheter are widely recognized as the most common healthcare-associated infection worldwide. 15%-25% of patients in general hospitals have a urethral catheter inserted at some time during their stay [1]. In the National Healthcare Safety Network (NHSN) 2012 surveillance report, 45–79% of patients in adult critical care units had an indwelling catheter, 23% in surgical wards, 17% in medical wards and 9% in rehabilitation units. Thus, indwelling urethral catheter use is exceedingly common in health care facilities, prolonging hospitalization stay, increasing costs and mortality [2,3]. The incidence of bacteriuria in catheterized patients is directly related to the duration of catheterization and the risk of Urinary Tract Infection (UTI) increases between 3 and 10% for each day of catheterization, reaching a probability of infection of 100% at 30 days of catheter permanence [1,4]. In 2009, the Centers for Disease Control and Prevention (CDC) and NHSN revised definitions concerning UTI and removed Asymptomatic Bacteriuria (ASB) from the Catheter-Associated UTI (CAUTI). Thus, CDC/NHSN indicates that the presence of symptoms, bacteriuria levels between =103 and =105 CFU/mL urine and a positive urinalysis are valid criteria for CAUTI [5].
CAUTI are caused by a variety of pathogens such as Escherichia coli, Klebsiella, Proteus, Enterococci, Pseudomonas, Enterobacter, Serratia and Candida. Microorganisms may ascend into the bladder extraluminally either at the time of catheter insertion or intraluminally, when the internal lumen of the catheter is colonized either through failure of a closed drainage system or contamination. In any case, indwelling urinary catheters facilitate colonization of uropathogens by providing a surface for the attachment of host cell binding receptors recognized by bacterial adhesions, thus enhancing microbial adhesion and biofilm formation [1,3]. Biofilms are structured communities of microorganisms that produce a matrix of exopolysaccharides that cover and protect them from the action of antibiotics and the host immune response. The rate of genetic material exchange occurring within the biofilm is important, thereby allowing the potential spread of antibiotic resistance genes and others genetic characteristic [1-4].
It should be noted that catheterized patients are an important reservoir of multiresistant microorganisms, including Gram-negative bacteria that produce Extended Spectrum Beta-Lactamases (ESBL) and carbapenemases enzymes, both associated with nosocomial outbreaks [3]. Prevention of CAUTI would be an important step in reducing the reservoir of multidrug-resistant Gram-negative organisms in hospitals. Hence, the implementation of robust infection control policies and shorting the duration of catheterization are measures widely believed to be important in the control and prevention of CAUTI [6]. To our knowledge, the epidemiology and genetic characteristics of uropathogens isolated from adult patients with CAUTIs are unknown in Venezuela. So, the objective of this study was to describe the epidemiological features in patients with CAUTI admitted to internal medicine services of the University Hospital of The Andes, Mérida. Venezuela. Also, we determined the microbiological profiles and molecular characteristics of multidrugresistant Gram-negative bacilli that cause this infection.
Materials and Methods
Setting and study population
This study was conducted at the University Hospital of The Andes (UHTA), an 850-bed teaching hospital in the Andean State of Mérida in western Venezuela. This is a type IV hospital, with clinical specialties and subspecialties, teaching and research. It has an area of influence of approximately 907,938 inhabitants, corresponding to the state Mérida and of nearby provinces.
From January to Jun 2015, a total of 73 adult patients with indwelling urinary catheters were hospitalized in UHTA’s internal medicine wards. Of these patients, only 40 (54.8%), who were later selected for this research, met the criteria established by the CDC/ NHSN for the diagnosis of CAUTI [5]. Exclusion criteria included patients with ASB or symptoms of UTI prior to catheterization, urologic surgery, known underlying renal pathology or chronic renal disease, pregnant and individuals who expressed their refusal to participate in this study. Demographic and clinical data, as well as microbiological results for each patient, were collected and recorded in an adequate data sheet. The CAUTI rate was calculated using the following formula: number of CAUTI infections in a particular location divided by the number of urinary catheter days in a particular location ×1,000 [2].
The study was approved by the Committee for Medical and Health Research Ethics of Faculty of Medicine and the Council of Scientific, Humanistic, Technological and Arts (CDCHTA) of the University of The Andes, Mérida, Venezuela.
Specimen collection and microbiological processing
Urine samples were aseptically collected from each patient after a catheter change. 10 mL of urine were obtained from the distal edge of the catheter by aspiration with a sterile syringe and transferred to a sterile container that was refrigerated (4oC), transported in an ice-pack to the medical laboratory and processed within 1 hour of collection.
The collected samples were analyzed macroscopically and microscopically and by culturing for microbiological profiling. Using the calibrated loop method, urine samples (0.01mL) were used to inoculate culture medium (BBL, Becton Dickinson, Cockeysville, MD, USA) as MacConkey agar, Salad Manitol agar and Blood agar. Significant bacteriuria was defined as urine culture plates showing =103 CFU/mL of single bacterial species. All microorganism isolates were identified by the VITEK 2 Compact system (bio Mérieux, Marcy-l’Étoile, France).
Antimicrobial susceptibility tests were carried out by Minimum Inhibitory Concentration (MIC) using AST-GP-298, AST-GN-299 and AST-YS07 susceptibility cards for Gram-positive bacteria, Gram-negative bacteria and yeast, respectively (VITEK 2 Compact system) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [7,8]. ESBL expression was confirmed by the disc diffusion method on Mueller Hinton agar (BBL) using cefotaxime (30 μg) and ceftazidime (30 μg) with and without clavulanic acid (10 μg), as recommended by the CLSI [7]. E. coli ATCC 25922 and Klebsiella pnumoniae ATCC 700603 were used as quality control strains. Initially, detection of carbapenemases was carried out by the modified Hodge test (MHT) [7]. Also, we included a disk diffusion assay using ertapenem and 3-aminophenyl boronic acid (400 μg) as an additional confirmatory phenotypic test of activity of Klebsiella Pneumoniae-Carbapenemase (KPC) and the presence of metallo-β- lactamases determined by the synergism test using imipenem and EDTA disks according to CLSI [7]. K. oxytoca LMM-SA26 and P. aeruginosa 77297 were used as quality control strains, respectively.
PCR amplification and sequencing of genes encoding ESBL and carbapenemases
DNA was extracted using the X-trem preparation kit (Biotech, SL, Granada, Spain) according to the manufacturer’s instructions. blaTEM, blaSHV and group blaCTX-M genes were detected by PCR based on previously described primers and protocols [9]. Carbapenemaseencoding genes blaKPC, blaVIM, blaSPM and blaIMP were screened using a PCR as previously described [10]. All amplification products were purified (PCR-Accuprep kit Bioneer, Daejeon, Korea) and their nucleotide sequencing was performed with the 3730XL genetic analyzer (Applied Biosystems, CA, USA). Nucleotide and amino acid sequence alignments were analyzed using the Basic Local Alignment Search Tool (BLAST) suite of programs (https://www.blast.ncbi.nlm. nih.gov/Blast.cgi).
Determination phylogenetic groups
E. coli isolates were assigned to the phylogenetic groups A, B1, B2, or D using a multiplex PCR strategy with specific primers for chuA, yjaA, and TspE4.C2 determinants as previously described [11]. E. coli LMM36-ULA (chuA + and yjaA +) and E. coli LMM32- ULA (TspE4.C2 +) were used as positive controls. Identification of K. pneumoniae phylogenetic groups (KpI, KpII and KpIII) were performed using a rapid method combining gyrA PCR-Restricted Fragment Length Polymorphisms analysis (RFLP), parC-PCR and adonitol fermentation as previously described [12]. K. pneumoniae LMM27-ULA (parC+) and K. pneumoniae X1LMM-ULA (gyrA+) were used as quality controls.
Identification of virulence genes
All E. coli isolates were investigated for the following six virulenceassociated genes: fimH (type 1 fimbriae), papAH (P fimbriae), kpsMII (group 2 capsular polysaccharide), fyuA (yersiniabactin), usp (uropathogenic specific protein) and PAI (Pathogenicity Island) markers. Simple PCR reactions were realized for detection of papAH, fyuA, usp genes and presence of fimH, kpsMII and PAI were determined by multiplex PCR using primers and conditions previously described [13]. E. coli LMM/E02-ULA (fimH +, fyuA +,kpsMII + and PAI +), E. coli LMM/Sc03-ULA (papAH +) and E. coli LMM/E02-ULA (usp +) were used as positive controls.
Rep-PCR typing
Repetitive sequence PCR (Rep-PCR) using primers Rep-PCR1 IIIG CGC CGI CAT CAG GC and Rep-PCR2 ACG TCT TAT CAG GCC TAC were carried out according to previously described protocols [14]. Rep-PCR patterns were analyzed using the Treecon 1.3b software (https://www.bioinformatics.psb.ugent.be/software/ details/TREECON) and interpreted according to criteria previously described [15]. Strains showing =85% similarity were classified as genetically related and were assigned to the same clusters.
Statistical analysis
Data was analyzed using the SPSS version 21 software (IBM Corporation, NY, USA). Categorical data was compared using the Chi square test with Yates correction. Statistical significance was set at P<0.05. Hierarchical clustering was performed using Past v3.06 program. The dendrogram was generated from clinical and epidemiological characteristic analyses of patients with CAUTI using the paired Group Algorithm (UPGMA) and Gower similarity index.
Results
Seventeen three patients with indwelling urinary catheters were hospitalized in the UHTA’s internal medicine wards during January - Jun 2015. Of these patients, 40 (54.8%) had CAUTI as defined in this study, corresponding to a rate per 1,000 catheter-day of 5.9. Nineteen of them (47.5%) were females and 21 (52.5%) males, with an age range of 19 – 94 years. The average age was 52.1 years (s= 18.3). (Table 1) shows clinical and epidemiological characteristics of patients with CAUTI. Regardless of gender, the group 56 – = 85 years was most affected by CAUTI with a frequency of 50%. The patients were hospitalized in two different areas of internal medicine: emergency and intermediate care services. Of these, trauma shock and the emergency observation room of adults provided the highest portion (80%) of catheterized patients (32/40). Patients with CAUTI were divided into four main groups according to the diagnosis of admission, being the most frequent vascular diseases (cerebrovascular disease/acute coronary syndrome) in male patients (22.5%), whereas the diseases of the infectious type (sepsis or septic shock) was predominant in females (17.5%). The mean duration of catheterization was 13.3 days (s=6.2), with a duration range of 5 – 30 days. Over half of patients with CAUTI (55%) had an inserted bladder catheter between 8 and 14 days. Of the 40 patients studied, 75% received at least one type of antibiotic before to CAUTI onset. Of these, 42.5% had an indicated therapy with two antibiotics. Cephalosporins (21.7%), fluoroquinolones (21.7%) and carbapenems (17.4%) were the most commonly administered antibiotics.
Characteristics
Female
19 (47,5%)
Male
21 (52,5%)
Total
40 (100%)
Age (years)
N (%)
N (%)
N (%)
£ 25
1 (2.5)
4 (10.0)
5 (12.5)
26 - = 55
7 (17.5)
7 (17.5)
14 (35.0)
56 - = 85
10 (25.0)
10 (25.0)
20 (50.0)
= 86
1
0
1 (2.5)
Area of hospitalization
Emergency of adults
Trauma shock room
8 (20.0)
12 (30.0)
20 (50.0)
Observation room
6 (15,0)
6 (15.0)
12 (30.0)
Adult hospitalization services
P4 room
0 (0)
2 (5.0)
2 (5.0)
P5 room
4 (10.0)
1 (2.5)
5 (12.5)
P6 room
1 (2.5)
0 (0)
1 (2.5)
Admission diagnosis
Diabetes mellitus
1 (2.5)
1 (2.5)
2(5.0)
Cerebrovascular disease / Acute coronary syndrome
3 (7.5)
9 (22.5)
12 (30.0)
Polytrauma / Craneal trauma
4 (10.0)
4 (10.0)
8 (20.0)
Sepsis or septic shock
7 (17.5)
4 (10.0)
11 (27.5)
Others
4 (10.0)
3 (7.5)
7 (17.5)
Duration of catheterization (days)
1 - 7
2 (5.0)
3 (7.5)
5 (12.5)
8 - 14
10 (25.0)
12 (30.0)
22 (55.0)
15 - 21
4 (10.0)
4 (10.0)
8 (20.0)
21 - 27
2 (5.0)
1 (2.5)
3 (7.5)
> 28
1 (2.5
1 (2.5)
2 (5.0)
Antibiotic therapy received prior to CAUTI onset
None
2 (5.0)
8 (20.0)
10 (25.0)
Monotherapy
6 (15.0)
7 (17.5)
13 (32.5)
Two antibiotics
11 (27.5)
6 (15.0)
17 (42.5)
Type of antibiotic received
Aminoglycosides
0 (0)
1 (2.2)
1 (2.2)
Broad spectrum cephalosporin
7 (15.2)
3 (6.5)
10 (21.7)
Carbapenems
4 (8.7)
4 (8.7)
8 (17.4)
Fluoroquinolones
7 (15.2)
3 (6.5)
10 (21.7)
Glycylcycline
1 (2.2)
1 (2.2)
2 (4.3)
Glycopeptides
1 (2.2)
2 (4.3)
3 (6.5)
Imidazole
6 (13.0)
1 (2.2)
7 (15.2)
Lincosamides
0 (0)
1 (2.2)
1 (2.2)
Monobactams
1 (2.2)
0 (0)
1 (2.2)
Polymyxin B
0 (0)
1 (2.2)
1 (2.2)
Ureidopenicillin
1 (2.2)
1 (2.2)
2 (4.3)
Table 1: Clinical and epidemiological characteristics of patients with CAUTI.
The relationship of the culture type and distribution of the isolated microorganisms according to the age of the patients and duration of catheterization are shown in (Table 2). Monomicrobial culture was observed in 82.5% of patients with CAUTI. However, cultures with more than one microorganism were reported in patients over 26 years of age who had catheterizations longer than 8 days. A total of 47 microorganisms were identified and 41 were isolated from patients belonging to the age groups 26-55 years (19/47) and 56-86 years (22/47) with catheterizations between 8 and 21 days. Yeasts were the main etiological agents isolated (21/47, 44.7%), being Candida tropicalis (9/47) and C. albicans (7/47) the most commonly found species, followed by Gram-negative bacteria with 40.4% (19/47).In this group, E. coli (8/47) and K. pneumoniae (5/47) stood out as main representatives, while E. faecalis was the most frequent pathogen among the group of Gram-positive bacteria (3/47). In all mixed or polymicrobial cultures (17.5%; 7/40), Candida species were associated with different Gram-negative or Gram-positive bacteria. No statistically significant relationships were observed between isolated microorganisms, age groups and duration of catheterization.
Age (years)
Duration of catheterization (days)
£ 25
26 - 55
56 - 85
= 86
1 - 7
8 - 14
15 - 21
22 - 27
> 28
Type of culture (N= 40)
Monomicrobial (34)
5
11
17
1
4
18
7
3
2
Association of two microorganisms (6)
0
3
3
0
1
4
1
0
0
Polymicrobial (1)
0
1
0
0
0
1
0
0
0
Microorganisms (N= 47)
5
19
22
1
6
27
9
3
2
Yeast (21)
2
9
9
1
1
10
6
3
1
Candida tropicalis (9)
1
4
4
0
1
3
3
2
0
Candida albicans (7)
1
3
2
1
0
3
3
1
0
Candida glabrata (2)
0
0
2
0
0
2
0
0
0
Candida parapsilosis(1)
0
1
0
0
0
1
0
0
0
Candida famata (1)
0
1
0
0
0
1
0
0
0
Crytococcus laurentii (1)
0
0
1
0
0
0
0
0
1
P value
0.690
0.09
Gram-negative bacteria (19)
3
7
9
0
3
13
3
0
0
Escherichia coli (8)
1
4
3
0
1
4
3
0
0
Klebsiella pneumoniae (5)
1
1
3
0
1
4
0
0
0
Enterobacter cloacae complex (1)
0
0
1
0
0
1
0
0
0
Pseudomonas aeruginosa (3)
0
2
1
0
1
2
0
0
0
Acinetobacter baumannii complex (1)
1
0
0
0
0
1
0
0
0
Stenotrophomonas maltophilia (1)
0
0
1
0
0
1
0
0
0
P value
0.664
0.345
Gram-positive bacteria (7)
0
3
4
0
2
4
0
0
1
Enterococcus faecalis (3)
0
1
2
0
0
2
0
0
1
Enterococcus faecium (2)
0
2
0
0
1
1
0
0
0
Staphylococcus epidermidis (1)
0
0
1
0
0
1
0
0
0
Staphylococcus aureus (1)
0
0
1
0
1
0
0
0
0
P value
0.741
0.227
Table 2: Type of culture and distribution of microorganisms isolated from 40 patients with CAUTI according to age and duration of catheterization.
All yeasts were sensitive to enchant fungal tested; only one C. tropicalis strain showed resistance to flucytosine (data not shown). Several multiresistant profiles were observed in all bacteria isolated (Table 3). E. coli was the enterobacteria with the highest number of resistance markers, which included broad-spectrum cephalosporins and carbapenems. A similar pattern was observed in P. aeruginosa and Acinetobacter baumannii. All Gram-negative strains had resistance associated to quinolones and at least to one of the amino glycosides group. In Gram-positive bacteria, resistance to β-lactams, linezolid, gentamicin and fluoroquinolones was a common feature. In most cases, this phenotype was associated with poor susceptibility to macrolides and lincosamines. Resistance to quinuspritin/dalfopristin was observed in one E. faecium strain and another Staphylococcus epidermidis strain. All Gram-negative strains remained fully susceptible to tigecycline and colistin. Besides tigecycline, Grampositive bacteria were susceptible to glycopeptides and tetracyclines (data not shown).
Bacteria
Resistance profiles
n
Gram-negative bacteria
Escherichia coli (n= 8)
AMP, PTZ, SAM, CTX, CTZ, AZT, ERT, IMP, MER, AMK, GTM, NAL, CIP
5
AMP, PTZ, SAM, CTX, CTZ, AZT, GTM, NAL, CIP
1
AMK, GTM, NAL, CIP
2
Klebsiella pneumoniae (n= 5)
PTZ, SAM, CTX, CTZ, AZT, ERT, IMP, MER, GTM, NAL, CIP
3
PTZ, SAM, CTX, CTZ, AZT, AMK, GTM, NAL, CIP.
2
Enterobacter cloacae complex (n= 1)
PTZ, SAM, CTX, CTZ, AZT, AMK, NAL, CIP.
1
Pseudomonas aeruginosa (n= 3)
CTX, CFZ, ERT, IMP, MER, AMK, GTM, CIP, LVX
2
PIP, ERT, IMP, MER, GTM, CIP, LVX
1
Acinetobacter baumannii complex (n= 1)
CTX, CFZ, ERT, IMP, MER, AMK, GTM, CIP, LVX
1
Stenotrophomonas maltophilia (n= 1)
CIP, LVX, STX
1
Gram-positive bacteria
Enterococcus faecalis (n= 3)
AMP, ERY, CLN, LNZ, CIP, LVX
3
Enterococcus faecium (n= 2)
AMP, ERY, CLN, GTM, QNP/DLF, LNZ, CIP, LVX
1
AMP, ERY, CLN, GTM, LNZ, CIP, LVX
1
Staphylococcus epidermidis (n= 1)
OXA, ERY, CLN, GTM, QNP/DLF, LNZ, CIP, LVX
1
Staphylococcus aureus (n= 1)
OXA, GTM, LNZ, CIP, LVX
1
AMP: Ampicillin; OXA: Oxacillin; ERY: Erythromycin; CLN: Clindamycin; SAM: ampicillin/sulbactam; PTZ: Piperacillin/Tazobactam; CTZ: Ceftazidime; CTX: Cefotaxime; AZT: Aztreonam: IMP: Imipenem; ERT: Ertapenem; MEM: Meropenem; AMK: Amikacin; GEM: Gentamicin; NAL: Nalidixic acid; CIP: Ciprofloxacin; LVX: Levofloxacin; MXF: Maxifloxacin; QDA: Quinupristin/dalfopristin; LNZ: Linezolid; SXT: Trimethoprim/Ssulfamethoxazole.
Table 3: Resistance profile of bacteria isolated from patients with CAUTI .
All Gram-negative bacteria that showed resistance to beta-lactam antibiotics were subjected to genetic analyses summarized in (Table 4). Of the 8 strains of E. coli isolated from patients with CAUTI, 6 were resistant to beta-lactam antibiotics. Of this group, 2 strains were included in the phylogroup A and 2 in the B2. The remaining strains were classified into phylogroups B1 and D. Six virulence factors were studied in the E. coli strains, but only 4 genes were detected. The predominant virulence genes were in order of frequency as follows: fimH (6), fyuA (3), usp (3) and KpsMTII (1). Four strains carried the association of at least 2 virulence genes. All K. pneumoniae strains were classified into the phylogenetic group KpI. Altogether, enterobacteria isolates produced extended-spectrum-beta-lactamases (ESBL), being CTX-M-15 the most frequent enzyme associated with other BLEEs (TEM-1, SHV-2a, SHV-2a, SHV-12, CTX-M-1, CTX-M-2, CTX-M-8 and CTX-M-14). Eight of these strains co-produced carbapenemases. Three E. coli and one K. pneumoniae were positive for KPC and in the remaining four; one metallo-β-lactamase (blaTEM-1) was detected. P. aeruginosa and A. baumannii only produced metallo-beta-lactamases type VIM-2.
Strain
N° code
Phylogenetic
group
Virulence
genes
bla
genes
Clonal
pattern
Rep-PCR
E. coli
LMM-1147
B2
fimH
blaTEM-1, blaSHV-5, blaCTX-M-15,blaVIM-2
A-1
LMM-1195
A
fimH
blaTEM-1, blaSHV-5, blaCTX-M-15, blaCTX-M-8,blaVIM-2
A-1
LMM-1199
D
fimH, fyuA, usp
blaTEM-1, blaCTX-M-1, blaCTX-M-8
A-1
LMM-15131.1
B2
fimH,fyuA, usp
blaTEM-1, blaSHV-2a, blaCTX-M-15, blaCTX-M-8,blaKPC-2
A-2
LMM-15131.2
A
fimH, usp
blaTEM-1, blaSHV-2a, blaCTX-M-15, blaCTX-M-8,blaKPC-2
A-2
LMM-1194.3
B1
fimH, fyuA, KpsMTII
blaTEM-1, blaSHV-5, blaCTX-M-15, blaCTX-M-8, blaCTX-M-14,blaKPC-2
B
K. pneumoniae
LMM-141060
KpI
ND
blaTEM-1, blaCTX-M-1,blaVIM-2
I-A
LMM-1194.2
KpI
ND
blaTEM-1, blaCTX-M-1,
I-A
LMM-14195
KpI
ND
blaTEM-1, blaCTX-M-15,blaVIM-2
I-A
LMM-524.1
KpI
ND
blaTEM-1, blaCTX-M-15, blaCTX-M-2
II-A
LMM-14524.2
KpI
ND
blaTEM-1, blaCTX-M-15, blaKPC-2
II-A
E. cloacae
LMM-1064
ND
ND
blaTEM-1, blaSHV-12, blaCTX-M-8
ND
P. aeruginosa
LMM-1194.3
ND
ND
blaVIM-2
ND
LMM-1195.2
ND
ND
blaVIM-2
ND
A. baumannii
LMM-496
ND
ND
blaVIM-2
ND
ND: Not Determined
Table 4: Genetic characteristics of β-lactamases-producing Gram-negative strains isolated from patients with CAUTI.
Two clonal clusters (A and B) were observed in E. coli strains. Cluster A concentrated most of the strains (5/6). This was subdivided into A1, with 3 strains showing a genetic relationship of 90% and the A2, composed of 2 E. coli strains with an approximately similarity of 95%. The LMM-1194.3 strain constituted cluster B, with less than 20% relation to groups A1 and A2. K. pneumoniae was distributed into two well-defined groups (I-A and II-A), consisting of strains with 100% similarity (Table 4).
The distribution of patients with CAUTI according to global relationships of clinical-epidemiological variables as well as phenotypic and genetic characteristics of uropathogens isolates, is shown in (Figure 1). Patients were divided into two principal groups with a similarity relation of approximately 75%. The larger group was composed of 36 (90%) patients and the other by 4 (10%). No particular population distribution was observed in relation to clinical-epidemiological variables and phenotypic or molecular characteristics of pathogens isolates, except a subgroup consisting of 2 female patients, in the larger group, which presented very similar epidemiological characteristics (98%) and also had as a common characteristic the presence of BLEE-producing E. coli from the pattern Rep-PCR A-2.
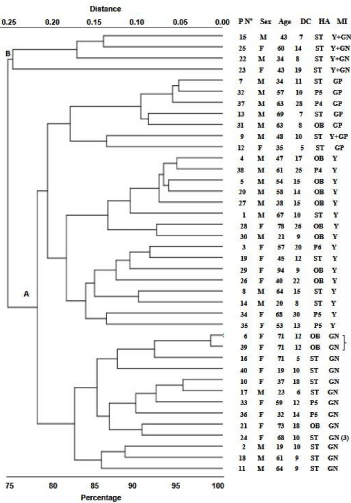
Figure 1: Hierarchical clustering dendogram. Analysis based on
epidemiological and molecular characteristics identifies two distinct clusters
of adult patients with CAUTI. Note the presence of a subgroup in the larger
cluster A (bracket) two female patients with similarity relationships of 98%.
Patients with a lower degree of similarity (=85%) were observed in the minor
cluster (B).
PN°: Patient N°; M: Male; F: Female; DC: Duration of Catheterization (day);
HA: Hospitalization Area; ST; Shock Trauma room; OB: Observation Room;
P4: P4 room; P5: P5 room; P6: P6 room: MI; Microorganisms Isolated: Y:
Yeast; GN: Gram-Negative bacteria; GP: Gram-Positive bacteria; (3): Patient
with 3 Gram-negative isolated in urine.
Discussion
The true incidence of CAUTI is difficult to discern due to potential over-diagnosis from current definitions. However, all organizations agree that the incidence of CAUTI is on the rise and prevention is important [6]. In this study, a CAUTI rate of 5.9 found in the internal medicine services of UHTA is lower than the pooled, mean rate of 8.99 reported for six academic teaching hospitals of Iran during 2011 - 2012 [16] but comparable with data reported by Salgado Yepez, et al. in Ecuador (5.7) [17]. However, it is significantly higher than the rate of 1.3 published in 2013 by CDC/NHSN [18] or the value found in a multicenter prospective study on device-associated infection rates recently carried out in intensive care units of Venezuela (3.9) [19].
In our study, regardless of gender, age of patients (older than 56) and a prolonged catheterization (= 21 days), were predisposing factors for development of CAUTI. In addition, it was shown that the percentage of positive cultures increased significantly from 2.7% in patients catheterized for less than 5 days to 100% after 25 days. Previous studies have shown that between 21% and 55.7% of indications for indwelling urinary catheters in hospitalized patients are unclear [1,3,6]. At present, the UHTA lacks regulations to timely and adequately prescribe the use of bladder catheters, as well as protocols for the care of catheterized patients. Thus, it is not surprising to see a significant increase in the frequency of CAUTI in this hospital. In the current study, more of 82% of patients with CAUTI had monomicrobial urine cultures. As previously reported by Deorukhkar et al. [20] and Xie et al. [21], we also found a predominance of Candida species (44.7%), being C. tropicalis the most frequent cause of candiduria, leaving enterobacteria in the second place with 29.8%. In this regard, Pigrau [4] indicates that the candiduria is especially frequent in elderly patients, diabetic patients and those who underwent broad-spectrum antibiotic therapy. In this study, 75% of the patients were treated with antibiotics before the onset of CAUTI and 39.1% of these patients had therapies that included cephalosporins or carbapenems. Although all strains of Candida isolated in this study were susceptible to the antifungal agents tested, in the last decade the genus Candida has shown different resistance profiles according to the species [22,23]. This justifies the need to accurately identify isolated yeasts and to evaluate in vitro susceptibility prior to a therapeutic decision.
Despite bacteria studied in this work were not the main agents causing CAUTI, they showed a resistance pattern that required attention. E. coli, K. pneumoniae and E. cloacae, besides having resistance to quinolones and aminoglycosides, presented a phenotypic profile compatible with the production of ESBL. Previous studies indicated that the most common globally disseminated ESBL associated with uropathogenic Enterobacteriaceae is the CTX M-type ESBL [24]. In this study, molecular characterization of blaESBLs genes revealed that the majority of ESBL-producing enterobacteria harbored blaCTX-M-15 genes associated to other ESBLs such as blaTEM-1, blaSHV-2a, blaSHV-5, blaSHV-12, blaCTX-M-1, blaCTX-M-2, blaCTX-M-8, or blaCTX-M-14. On the other hand, we observed a high prevalence of carbapenemasesproducing strains. Three E. coli and 1 K. pneumoniae strains were co- producing KPC-2 while 4 strains (2, E. coli and 2, K. pneumoniae) co-produced metallo-beta-lactamases type VIM-2. Several studies indicate that carbapenem resistance tends to emerge in areas where ESBLs prevalence is high, driven by selection pressure from carbapenem use and that can subsequently disseminate in the hospital environment [25]. In fact, the analysis of the genetic relationships of the strains of E. coli and K. pneumoniae revealed that in both bacterial genera, two main clonal groups with a high degree of similarity were observed. In addition, these multiresistant clones circulated mostly in the trauma shock unit of the adult emergency. Similarly, VIM-2 producing P. aeruginosa and A. baumannii were isolated in patients hospitalized in this same unit. This indicates that, from the point of view of molecular epidemiology, this area would be the main niche for the exchange and recombination of genes coding for ESBL and carbapenemases in Gram-negative bacteria and represents the area of greatest risk and distribution of multiresistant clones.
E. coli strains are genetically diverse, so the differences between pathogenic and commensal isolates are based on their phylogenetic background. The commensal strains belonging to groups A and B1 are considered of low virulence power, while the extraintestinal pathogens are found in the phylogroups B2 and D [26]. However, in this study, ESBLs- and carbapenemases-producing E. coli strains were almost equally distributed among the four major phylogenetic groups (A, B1, B2 and D). We did not find any relationship between the clinical or epidemiological characteristics of patients and the distribution of phylogroups. However, all strains had the fimH gene with or without association with other virulence factors. The fimH gene encoding an adhesin or fimbria type 1, which is one of the key indicators of virulence in E. coli uropathogenic strains [27]. These results suggest that the movement of the ESBL genes does not interfere significantly with the distribution of virulence factors within a particular phylogroup. In fact, regardless of the number and type of beta-lactamases detected, all strains of K. pneumoniae were classified into the phylogenetic group KpI. K. pneumoniae KpI is best known as an opportunistic cause of healthcare-associated infections [12,28], so that the possibility of acquisition of both resistance and virulence traits via horizontal gene transfer, could potentially lead to the emergence of untreatable infections. The resistance–virulence link is complex [13, 26-28]. However, the knowledge of correlations between resistance pattern, virulence factors and phylogroups are of great value for clinical diagnosis, treatment and predictive prognosis of CAUTI.
In conclusion, although the sample size was small, we found that the incidence of CAUTI is alarmingly high. This finding was especially true for patients older than 56 with prolonged catheterization. Moreover, this study documents the prevalence of candiduria and non-albicans Candida species as the predominant pathogen causing CAUTI. Multidrug-resistant Gram-negative bacilli were also isolated as an important etiological agent for this infection. CTX-M-15 was the common ESBL in Enterobacteriaceae, which coexisted in some strains with other ESBLs and carbapenemases as KPC-2 and VIM- 2, while that P. aeruginosa and A. baumannii were producers of metallo-beta-lactamase type VIM-2. Several urovirulent factors were found in E. coli, but all strains harbored the fimH gene. Therefore, these multiresistant strains pose a double threat: on one hand, they reduce therapeutic options and have a substantial capacity for extra intestinal virulence and, on the other hand, they have the property of being more easily disseminated in hospital areas with high risk of infection.
The findings in this study suggest that beside appropriate strategies for the rational use of antibiotics according to local resistance patterns, it is also urgent to adopt criteria that justify a prolonged catheterization as well as rigorous hygiene protocols for the installation, care and catheter maintenance aimed at reducing the incidence of CAUTI.
Acknowledgement
This study was partially supported by the Council of Scientific, Humanistic, Technological and Arts of University of The Andes (CDCHTA-ULA), Mérida, Venezuela (grant CVI-ADG-FA-02-97) and Fundación Empresas Polar, Caracas, Venezuela (Project N° 140275).
References
- Hooton MT, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Society of America. Clin Infec Dis. 2010; 50: 625-663.
- Dudeck MA, Weiner LM, Allen-Bridson K, Malpiedi PJ, Peterson KD, Pollock DA, et al. National healthcare safety network (NHSN) report, data summary for 2012, device-associated module. Am J Infect Control. 2013; 41: 1148- 1166.
- Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014; 3: 23.
- Pigrau C. Infecciones del tracto urinario nosocomiales. Enferm Infecc Microbiol Clin. 2013; 31: 614-624.
- Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute care setting. 2009.
- Mody L, Greene MT, Meddings J, Krein SL, McNamara SE, Trautner BW, et al. A national implementation project to prevent catheter-associated urinary tract infection in nursing home residents. JAMA Intern Med. 2017.
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 27th edn. Informational Supplement. CLSI Document M100-S27. Clinical and Laboratory Standards Institute, Wayne, PA.USA. 2017.
- Clinical and Laboratory Standards Institute, Reference method for broth dilution antifungal susceptibility testing of yeast. 4° informational supplement. CLSI Document M27-S4. Wayne, PA, USA. 2012.
- Flores-Carrero A, Labrador I, Paniz-Mondolfi A, Peaper DH, Towle D, Araque M. Nosocomial outbreak of ESBL-producing Enterobacter ludwigii coharboring CTX-M-8, SHV-12 and TEM-15 in a neonatal intensive care unit in Venezuela. J Glob Antimicrobial Resist. 2016; 7: 114-118.
- Guevara A, de Waard J, Araque M. Detección del gen blaVIM-2 en cepas de Pseudomonas aeruginosa productoras de metalo β-lactamasas aisladas en una unidad de cuidados intensivos en Ciudad Bolívar, Venezuela. Rev Chilena Infect. 2009; 26: 328-333.
- Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000; 66: 4555- 4558.
- Silva M, Borges A, Vieira A, Magalhães V, De Souza A. Phylogenetic groups among Klebsiella pneumoniae isolates from Brazil: Relationship with antimicrobial resistance and origin. Curr Microbiol. 2011; 62: 1596-1601.
- Millán Y, Hernández E, Millán B, Araque M. Distribución de grupos filogenéticos y factores de virulencia en cepas de Escherichia coli uropatógena productora de beta-lactamasa CTX-M-15aisladas de pacientes de la comunidad en Mérida, Venezuela. Rev Argent Microbiol. 2014; 46: 175-181.
- Lozano D, Cisneros J, Becerril B, Cuberos L, Prados T, Ortíz C, et al. Comparison of a repetitive extragenic palindromic sequence-based PCR method and clinical and microbiological methods for determining strain sources in cases of nosocomial Acinetobacter baumannii bacteremia. J Clin Microbiol. 2002; 40: 4571-4575.
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsedfield gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995; 33: 2233-2239.
- Jahani-Sherafat S, Razaghi M, Rosenthal VD, Tajeddin E, Seyedjavadi S, Rashidan M, et al. Device-associated infection rates and bacterial resistance in six academic teaching hospitals of Iran: findings from the international nocosomial infection control consortium (INICC). J Infect Public Health. 2015; 8: 553-561.
- Salgado Yepez E, Bovera MM, Rosenthal VD, González Flores HA, Pazmiño L, Valencia F, et al. Device-associated infection rates, mortality, length of stay and bacterial resistance in intensive care units in Ecuador: international nosocomial infection control consortium’s findings. World J Biol Chem. 2017; 8: 95-101.
- Dudeck MA, Edwards JR, Allen-Bridson K, Gross C, Malpiedi PJ, Peterson KD, et al. National healthcare safety network report, data summary for 2013, device-associated module. Am J Infect Control. 2015; 43: 206-221.
- Empaire GD, Guzman ME, Rosenthal VD, Pérez F, Ruiz Y, Díaz C, et al. Multicenter prospective study on device-associated infection rates and bacterial resistance in intensive care units of Venezuela: international nosocomial infection control consortium (INICC) ?ndings. Int Health. 2017; 9: 44-49.
- Deorukhkar SC, Saini S. Medical device-associated Candida infections in a rural tertiary care teaching hospital of india. Interdiscip Perspect Infect Dis. 2016; 2016: 1854673.
- Xie DS, Lai RP, Nie SF. Surveys of catheter-associated urinary tract infection in a university hospital intensive care unit in China. Braz J Infect Dis. 2011; 15: 296-297.
- Flores-Carrero A, Paniz-Mondolfi A, Hernández E, Araque M. Candida pelliculosa blood infection in a neonate patient from Mérida, Venezuela. Intern J Adv Cases Reports. 2015; 2: 717-720.
- Mishra M, Agrawal S, Raut S, Kurhade AM, Powar RM. Profile of yeasts isolated from urinary tracts of catheterized patients. J Clin Diagn Res. 2014; 8: 44-46.
- Oteo J, Pérez-Vázquez M, Campos J. Extended-spectrum beta-lactamase producing Escherichia coli: changing epidemiology and clinical impact. Curr Opin Infect Dis. 2010; 23: 320-326.
- van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017; 8: 460-469.
- Er DK, Dundar D, Uzuner H, Osmani A. Relationship between phylogenetic groups, antibiotic resistance and patient characteristics in terms of adhesin genes in cystitis and pyelonephritis isolates of Escherichia coli. Microb Pathog. 2015; 89: 188-194.
- Subashchandrabose S, Mobley HLT. Virulence and fitness determinants of uropathogenic Escherichia coli. Microbiol Spectr. 2015; 3.
- Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016; 80: 629-661.
Citation: Quijada-Martínez P, Flores-Carrero A, Labrador I, Millán Y and Araque M. Microbiological Profile and Molecular Characterization of Multidrug-Resistant Gram-Negative Bacilli Producing Catheter-Associated Urinary Tract Infections in the Internal Medicine Services of a Venezuelan University Hospital. Austin J Infect Dis. 2017; 4(1): 1030.