
Research Article
J Plant Chem and Ecophysiol. 2016; 1(2): 1010.
Licorice (Glycyrrhiza glabra) Root Extract Alleviated TCDD-Induced Toxicity in Primary Rat Hepatocytes
Dela Cruz J¹, Chu XT² and Hwang SG³*
¹Department of Basic Veterinary Sciences, University of the Philippines Los Banos, Philippines
²Department of Animal Biotechnology, Hankyong National University, Korea
³Department of Animal Life and Environmental Science, Hankyong National University, Korea
*Corresponding author: Seong Gu Hwang, Department of Animal Life and Environmental Science, Hankyong National University, Korea
Received: May 18, 2016; Accepted: June 13, 2016; Published: June 14, 2016
Abstract
2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic member of a class of planar, halogenated aromatic hydrocarbons. Recently it raises great public concern about its impact on human health. It has been suggested that TCDD induce hepatocyte toxicity by a mechanism involving the generation of ROS through CYP1A1 (cytochrome P450 1A1) activation. Many studies indicated that Licorice (Glycyrrhiza glabra) Root Extract (LRE) exhibits chemopreventive and detoxicative properties. The aim of this study was to assess the effect of ethanolic LRE against TCDD induced toxicity in a primary culture of rat hepatocytes.
Primary cultured hepatocytes were treated with TCDD (10nM) with or without LRE (0-400μg/ml) for 24 and 48 hours. Treatment with TCDD alone lowered hepatocyte viability, but the toxic effect of TCDD on cell viability was ameliorated by the treatment of LRE dose dependently. Free radical scavenging activity by DPPH assay showed that LRE has a strong antioxidant activity. RT-PCR and Western Blot analysis, both showed that TCDD toxicity related genes (AhR, ARNT and CYP1A1) were subsequently down-regulated by LRE treatment in a dose dependent manner. DNA fragmentation assay also showed the protective effect of LRE against TCDD mediated DNA damage. These data suggested that LRE can alleviate the toxic effects of TCDD in cultured hepatocytes by its strong antioxidant activity as well as the down regulation of CYP1A1 expression followed by the suppression of AhR and ARNT genes. In conclusion, LRE can be used as a potential toxicity alleviating agent against TCDD induced hepatocyte toxicity.
Keywords: Licorice; TCDD; Toxicity; Antioxidant; Hepatocytes; AhR
Introduction
2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic member of a class of planar, halogenated chemicals, which are widespread and persistent environmental pollutants. It is extremely stable, both to environmental and biological breakdown, leading to its persistence in the environment and its bioaccumulation in the food chain [1]. In experimental animals and humans, TCDD causes a variety of toxic responses ranging from the induction of xenobioticmetabolizing enzymes such as cytochrome P450 1A1 (CYP1A1) to wasting syndrome, hepatotoxicity, immunotoxicity, teratogenicity, mutagenesis and carcinogenesis [2,3].
The liver is a dioxin target organ in many species. TCDD promotes liver cancer [4] and induces degeneration of hepatocytes [5]. All these disorders involve a strong oxidative stress after TCDD exposure. It has been suggested that TCDD induced hepatocyte toxicity by a mechanism involving generation of Reactive Oxygen Species (ROS) through CYP1 enzymes and oxidative DNA damage [6,7]. Many studies have revealed that virtually all major toxic effects of TCDD are mediated by the specific binding of TCDD to a cytosolic protein, AhR. The classically accepted toxicity mechanism with respect to TCDD treatment was that TCDD first bind to Aryl Hydrocarbon Receptor (AhR), and the liganded AhR translocated to the nucleus. After dimerization with Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT), the complex binds to Dioxin Responsive Elements (DREs) at the promoter region and activates transcription of certain genes including CYP1 genes [8-10].
Licorice root (Glycyrrhiza glabra) is the most popular ingredient used in over 70% of Chinese medicines and has been used by human beings for at least 4000 years. This plant contains many flavonoids and pentacyclic triterpene saponins, including liquiritin, liquiritigenin, liquiritin apioside, glycyrrhizin, isoliquiritigenin, and glycyrrhizic acid [11]. Constituents of this plant have been reported to have a wide range of bioactivities, e.g., antimicrobial, anti-inflammatory, and cardiovascular protective activities [12-14].
The roles of LRE against TCDD-induced hepatotoxicity have not been studied so far. Therefore, the aim of this study was to investigate the protective effect of ethanolic LRE against TCDD-induced toxicity in a primary culture of rat hepatocytes.
Primary cultured hepatocytes were treated with TCDD (10nM) with or without LRE (0-400μg/ml) for 24 and 48 hours. Treatment with TCDD alone lowered hepatocyte viability, but the toxic effect of TCDD on cell viability was ameliorated by the treatment of LRE dose dependently. Free radical scavenging activity by DPPH assay showed that LRE has a strong antioxidant activity. RT-PCR and Western Blot analysis, both showed that TCDD toxicity related genes (AhR, ARNT and CYP1A1) were subsequently down-regulated by LRE treatment in a dose dependent manner. DNA fragmentation assay also showed the protective effect of LRE against TCDD mediated DNA damage. These data suggested that LRE can alleviate the toxic effects of TCDD in cultured hepatocytes by its strong antioxidant activity as well as the down regulation of CYP1A1 expression followed by the suppression of AhR and ARNT genes. In conclusion, LRE can be used as a potential toxicity alleviating agent against TCDD induced hepatocyte toxicity.
Keywords: Licorice; TCDD; Toxicity; Antioxidant; Hepatocytes; AhR
Introduction
2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic member of a class of planar, halogenated chemicals, which are widespread and persistent environmental pollutants. It is extremely stable, both to environmental and biological breakdown, leading to its persistence in the environment and its bioaccumulation in the food chain [1]. In experimental animals and humans, TCDD causes a variety of toxic responses ranging from the induction of xenobioticmetabolizing enzymes such as cytochrome P450 1A1 (CYP1A1) to wasting syndrome, hepatotoxicity, immunotoxicity, teratogenicity, mutagenesis and carcinogenesis [2,3].
The liver is a dioxin target organ in many species. TCDD promotes liver cancer [4] and induces degeneration of hepatocytes [5]. All these disorders involve a strong oxidative stress after TCDD exposure. It has been suggested that TCDD induced hepatocyte toxicity by a mechanism involving generation of Reactive Oxygen Species (ROS) through CYP1 enzymes and oxidative DNA damage [6,7]. Many studies have revealed that virtually all major toxic effects of TCDD are mediated by the specific binding of TCDD to a cytosolic protein, AhR. The classically accepted toxicity mechanism with respect to TCDD treatment was that TCDD first bind to Aryl Hydrocarbon Receptor (AhR), and the liganded AhR translocated to the nucleus. After dimerization with Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT), the complex binds to Dioxin Responsive Elements (DREs) at the promoter region and activates transcription of certain genes including CYP1 genes [8-10].
Licorice root (Glycyrrhiza glabra) is the most popular ingredient used in over 70% of Chinese medicines and has been used by human beings for at least 4000 years. This plant contains many flavonoids and pentacyclic triterpene saponins, including liquiritin, liquiritigenin, liquiritin apioside, glycyrrhizin, isoliquiritigenin, and glycyrrhizic acid [11]. Constituents of this plant have been reported to have a wide range of bioactivities, e.g., antimicrobial, anti-inflammatory, and cardiovascular protective activities [12-14].
The roles of LRE against TCDD-induced hepatotoxicity have not been studied so far. Therefore, the aim of this study was to investigate the protective effect of ethanolic LRE against TCDD-induced toxicity in a primary culture of rat hepatocytes.
Materials and Methods
Chemicals and reagents
TCDD and collagenase were purchased from Sigma-Aldrich (St. Louis, MO, USA). Minimum Essential medium, Medium 199, Hanks balanced salt solution and fetal bovine serum were purchased from GIBCO BRL (Grand Island, NY, USA). Cell counting kit-8 (CCK-8) reagent was purchased from Dojindo (Kumamoto, Japan). Trizol was purchased from Invitrogen (Carlsbad, CA, USA). Ethidium bromide was purchased from Bio basic Inc. (South Korea) and Maxime PCR Premix (i-Taq) was purchased from iNtRON Biotechnology (Seoul, Korea). Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Abcam (Cambridge, UK).
Preparation of LRE
Dried licorice (Glycyrrhiza glabra) roots were freeze-dried and pulverized. The dried powder (500 g) was then soaked in 80% ethanol for 24 h. The extracts were collected and the same process was repeated three times. The total extract was collected, filtered, and evaporated under reduced pressure. The end product was freezedried and the powdered extract was kept in a deep freezer (-70oC).
Animals
Male rats of Sprague-Dawley strain, 4 weeks old, purchased from Samtako Bio Korea Co., Ltd., (Osan, South Korea) were initially acclimated to laboratory conditions for one week prior to experimental use. They were allowed to have access to water and standard laboratory chow ad libitum and were maintained under a 12 hours light/12 hours dark cycle, 27-30°C environment and relative humidity of 55%. The study had received an approval from the Hankyong National University Animal Welfare Committee.
Hepatocytes isolation and cultivation
Rats were sacrificed by CO2 overdose, and the livers were removed immediately. Hepatocytes were isolated using the collagenase perfusion method [15]. The liver was perfused with calcium-free Hanks balanced salt solution through the hepatic portal vein to remove blood. As soon as the liver became grayish brown, a second buffer solution supplemented with collagenase was perfused to break up the liver. It was then minced into 3-4 mm pieces using a sterile scalpel. Following mechanical dissociation, the pieces were filtered through a cell strainer and centrifuged at 1500rpm for 5min. The cells were suspended with the medium and filtered again; washed three times, centrifuged and tested by trypan blue for viability. The isolated hepatocytes were cultured in a mixture of 75% minimum essential medium and 25% medium 199 (supplemented with FBS, penicillin, insulin and BSA). The medium was changed 3-4 h later.
Cell viability analysis
Cell counting kit-8 was used to determine cell viability, according to the manufacturer’s instructions. Cells were seeded in a 96-well plate (1 × 104 cells/well) and incubated at 37oC in 5% CO2 for 24 h. The cells were treated with 50 nM TCDD and LRE (0, 50, 100, 200, and 400 μg/mL) for 24 and 48 h. After the allotted treatment time, the medium was removed and replaced with fresh media containing 10μL of CCK-8 solution and incubated at 37oC for 3 h. Absorbance at 450 nm was measured with an ELISA plate reader. The viability of treated cells is expressed as percentage of that of control cells.
RNA isolation and reverse transcription-polymerase chain reaction
Total RNA was isolated from TCDD- and LRE-treated cells using Trizol reagent according to the manufacturer’s protocol. RNA samples were reverse-transcribed with M-MuLV reverse transcriptase (Fermentas, Vilnius, Lithuania) and specific primers were used to amplify AhR, ARNT and CYP1A1. The optimum number of cycles for each gene was determined experimentally. The housekeeping gene β-actin was used to verify that equal amounts of RNA were added to the PCR reaction.
Western blot
The cells treated with TCDD and 0-400 μg/mL LRE for 24 h were lysed using a protein extraction solution (INtRON Biotechnology). Total protein concentration was determined by the Bio-Rad protein assay. Next, 25 μg of protein was diluted and heated at 95oC for 10 min prior to analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% and 12%). The proteins were then transferred to nitrocellulose membranes and blocked overnight with 5% skim milk in TBST (20 mM Tris-HCl, pH 7.6, 140 mM NaCl, 0.1% Tween 20). The membranes were then rinsed four times with TBST and incubated for 2 h with 2% skim milk containing the primary antibody (diluted at 1:200): AhR, ARNT, and CYP1A1. After washing four times with TBST buffer, the membranes were incubated for 2 h with the horseradish peroxidase-conjugated secondary antibody (diluted at 1:2000). The membranes were washed again and developed using enhanced chemiluminescence (ECL Western Blot Analysis System Kit, Amersham Biosciences).
DPPH free radical-scavenging assay
Free radical scavenging capacity of the extract was determined by previously reported procedure using the stable 2, 2-diphenyl- 1-picrylhydrazylradical (DPPH). Briefly, 150ul DPPH ethanolic solution (0.1mM) were mixed with 50ul LRE ethanolic samples (0, 50, 100, 200, 400, 800ug/mL). The mixtures were incubated in the dark for 30 min at room temperature, and then the absorbances of the resulting solutions were measured at 517 nm. The DPPH free radical scavenging activity was calculated:
Free radical scavenging activity % = (1-A/A0) ×100%
A is the absorbance with LRE, while A0 is the absorbance without samples. 5Mm quercetin was used as the positive control.
DNA fragmentation analysis
Cells were treated with TCDD and LRE for 24h. DNA extraction was used DNeasy Blood and Tissue kit (DNeasy kit; Qiagen, MD) by following manufacturer’s instructions. Then the extracted DNA was quantified by taking OD reading at 260 nm. Equal amounts of DNA from different concentration-treated cells were electrophoretically separated on a 1.8% agarose gel for 2 hours at 50V. Then the gel was visualized by UV after staining with 0.5ug/ml ethidium bromide for 30 min.
Statistical analysis
All experiments were performed in triplicate and the results were expressed as mean ± standard error. Differences between means were evaluated using one-way ANOVA followed by Duncan’s multiple range test; p < 0.05 was considered statistically significant.
Results
Effect of LRE and TCDD on cell viability
Before the investigation of the alleviating effect of LRE in TCDDinduced toxicity, the cytotoxicity of LRE alone was firstly evaluated on hepatocytes. LRE stimulated the proliferation of hepatocytes and no cytotoxicity was observed up to 400μg/mL (Figure 1). The effect of 10nM TCDD on the viability of hepatocytes was also measured. As the shown in Figure 2, TCDD induced hepatoxicity. The cell viability of TCDD treated cells was lower than the control group. When cells were treated with LRE, the survival curve showed that LRE had protective effect against TCDD-induced toxicity in isolated rat hepatocytes dose-and time-dependently (Figure 2).
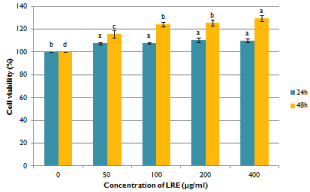
Figure 1: Effects of ethanolic licorice root extract on the cell viability of
isolated rat hepatocytes. Data are means + SE (n=3). Means with different
superscript are significantly different at p< 0.05.
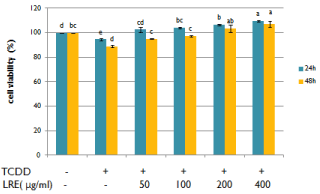
Figure 2: Effects of ethanolic licorice root extract on the cell viability of
isolated rat hepatocytes co-treated with 10nM TCDD. 0.1% DMSO was
used as a vehicle control. Data are means + SE (n=3). Means with different
superscript are significantly different at p< 0.05.
Gene expression changes induced by LRE
Reverse transcription-polymerase chain reaction analysis was performed to investigate the effect of LRE on TCDD toxicity-related genes in isolated hepatocytes (Figure 3). Compared to untreated cells, the expression level of AhR significantly increased in TCDD treated cells. In contrast, after LRE treatment for 24 h, the expression level of AhR decreased dose-dependently, as well as ARNT and CYP1A1.

Figure 3: Effect of LRE on the mRNA expressions of TCDD toxicity related
genes in TCDD treated hepatocytes. 0.1% DMSO was used as a vehicle of
control.
Effects of LRE on the expression of TCDD toxicity-related proteins
In addition, we demonstrated the cytotoxic protecting effect of LRE by Western blot analysis. Figure 4 shows the protein expressions of AhR, ARNT and CYP1A1 which are the primary molecules in the signaling pathway of hepatotoxicity induced by TCDD. All the target proteins were up-regulated by TCDD treatment, but down-regulated after LRE treatment for 24 hours.

Figure 4: Effect of LRE on the protein expressions of TCDD toxicity related
proteins in TCDD treated hepatocytes. 0.1% DMSO was used as a vehicle
control.
Effect of LRE on DPPH radical-scavenging
The free radical scavenging activity of LRE was assessed by the DPPH assay (Figure 5). The data suggests that LRE induced a concentration dependent free radical scavenging activity with IC50 value of 366.87μg/mL. At a higher concentration, LRE exhibited similar anti-oxidative activity with 5mM of quercetin. This indicates that it is a good source of natural antioxidants.
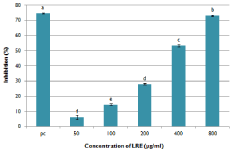
Figure 5: DPPH radical scavenging activity of LRE. 5mM quercetin was
used as a positive control. Data are means ± SD (n=3). Means with different
superscript are significantly different at p< 0.05.
Effect of LRE on TCDD induced DNA damage
DNA damage was examined to further understand the mechanism of LRE against TCDD induced toxicity in isolated hepatocytes. DNA fragmentation assay was used to measure the effect of TCDD and LRE on DNA damage. It was observed that TCDD caused significant increases in the DNA fragments of primary cultures of rat hepatocytes compared to untreated cells. However, LRE was able to decrease the level of DNA damage in TCDD-treated hepatocytes.
Discussion
This study was conducted to investigate the protective effect of ethanolic LRE against TCDD induced toxicity in primary cultures of rat hepatocytes. LRE mitigate TCDD toxicity in cultured hepatocytes by strong antioxidant activity and the suppression of CYP1A1 expression followed by the down regulation of AhR and ARNT genes.
Many studies reported that TCDD induced CYP1A1 alter the formation of ROS through the activation of AhR [16,17], then cause a cascade of different disorders. Liver is one of the major target organs of TCDD. In the present study, we measured cell viability, DNA damage and the expressions of AhR, ARNT and CYP1A1 in TCDD treated rat hepatocytes. The results suggested that TCDD caused a dose and time related decrease on cell survival (Figure 2) through the up-regulation of AhR, ARNT and CYP1A1 (Figure 3 and Figure 4), then resulted in DNA damage in primary cultured rat hepatocytes (Figure 6).
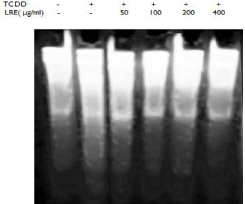
Figure 6: Effect of LRE on DNA fragmentation of TCDD treated rat
hepatocytes. 0.1% DMSO was used as a vehicle control.
Licorice root has been used for at least 4000 years and has already been identified by the National Cancer Institute [18] as having chemopreventive attributes. In our study, cell viability assay showed that LRE exerts a potent protective effect on TCDD treated rat hepatocytes. The alleviation of TCDD-induced toxicity was a result of the suppression of CYP1A1 expression and antioxidant activity of LRE. RT-PCR and Western Blot showed a down-regulation of AhR signaling pathway that mediates the expression of CYP1A1; DPPH analysis of LRE and DNA damage analysis showed that LRE had a strong antioxidant activity for scavenging free radical to protect oxidative DNA damage.
Although the mechanism underlying TCDD-induced hepatotoxicity is not completely understood, AhR seems to be a key transcriptional regulatory protein in TCDD-elicited gene expression [19]. After specific binding of TCDD to AhR, the AhRligands translocate to the nucleus and heterodimerize with ARNT. Subsequently, the complex binds to the xenobiotic-response element, leading to the expression of dioxin-responsive genes, including CYP1A1 [20]. Cytochrome P450 members are efficient intracellular sources of ROS. Upregulation of CYP1A1 produces very large amount of ROS [21]. It can cause DNA damage, loss of enzyme activity, inhibition of protein synthesis and so on, ultimately lead to cell death [22]. In the present study, we found that LRE suppressed the expression of AhR, ARNT, as well as CYP1A1 (Figure 3 and Figure 4), which are components of the primary signaling pathway that mediates the hepatotoxicity induced by TCDD.
DPPH is considered as a good kinetic model for free radicals [23]. The decrease in absorbance at 517nm shows the ability of LRE to scavenge the DPPH radicals. The results of the DPPH scavenging activity assay for LRE exhibits strong antioxidant activity. It suggested that TCDD toxicity can be decreased by free radical scavenging activity of LRE in neutralizing ROS. Therefore, oxidative stress which was induced by ROS may also be alleviated by LRE. The effect of DNA damage was measured by DNA fragmentation assay. In our investigation, DNA damage was recovered by LRE treatment. All these findings constitute evidence that the anti-oxidative properties of LRE contribute to the prevention of hepatocyte degenerations and hepatic DNA damages induced by TCDD in rats. Because effective antioxidants can be definitely protect cellular DNA from oxidative stress by supplementation with antioxidants [24].
Conclusion
In conclusion, the present study shows that TCDD induces generation of DNA damage which leads to cell death by the increased expression of CYP1A1. However, LRE showed a promising protective effect against TCDD-induced hepatotoxicity in cultured hepatocytes by strong antioxidant activity as well as the suppression of CYP1A1 expression followed by down regulation of AhR and ARNT genes. The results indicate that LRE can be used as a potential toxicity alleviating agent against TCDD-induced hepatocyte toxicity.
References
- Birnbaum LS. The mechanism of dioxin toxicity: relationship to risk assessment. Environ Health Perspect. 1994; 102: 157-167.
- Hung YC, Huang GS, Sava VM, Blagodarsky VA, Hong MY. Protective effects of tea melanin against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity: antioxidant activity and aryl hydrocarbon receptor suppressive effect. Biol Pharm Bull. 2006; 29: 2284-2291.
- Mandal PK. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol B. 2005; 175: 221-230.
- Huff J, Lucier G, Tritscher A. Carcinogenicity of TCDD: experimental, mechanistic, and epidemiologic evidence. Annu Rev Pharmacol Toxicol. 1994; 34: 343-372.
- Czepiel J, Biesiada G, Gajda M, Szczepanski W, Szypula K, Dabrowski Z, Mach T. The effect of TCDD dioxin on the rat liver in biochemical and histological assessment. Folia Biol (Krakow). 2010; 58: 85-90.
- Aly HA, Domènech O. Cytotoxicity and mitochondrial dysfunction of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in isolated rat hepatocytes. Toxicol Lett. 2009; 191: 79-87.
- Park JY, Shigenaga MK, Ames BN. Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo (3,2-b) carbazole is associated with oxidative DNA damage. Proc Natl Acad Sci U S A. 1996; 93: 2322-2327.
- Nohara K, Fujimaki H, Tsukumo S, Ushio H, Miyabara Y, Kijima M, et al. The effects of perinatal exposure to low doses of 2,3,7,8-tetrachlorodibenzo-pdioxin on immune organs in rats. Toxicology. 2000; 154: 123-133.
- Fernandez-Salquero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon Receptor - Deficient Mice Are Resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996; 140: 173-179.
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008; 18: 207-250.
- Kamei J, Nakamura R, Ichiki H, Kubo M. Anti-tussive principles of Glycyrrhiza radix, a main component of the Kampo preparations Bakumondo-to (Maimen- dong-tang). Eur J. Pharmacol. 2003; 469: 159-163.
- Gupta VK, Fatima A, Faridi U, Negi AS, Shanker K, Kumar JK, et al. Antimicrobial potential of Glycyrrhiza glabra roots. J Ethnopharmacol. 2008; 116: 377-380.
- Kao TC, Wu CH, Yen GC. Bioactivity and potential health benefits of licorice. J Agric Food Chem. 2014; 62: 542-553.
- Chakravarthi KK, Avadhani R. Beneficial effect of aqueous root extract of Glycyrrhiza glabra on learning and memory using different behavioral models: An experimental study. J Nat Sci Biol Med. 2013; 4: 420-425.
- Türkez H, Yousef MI, Geyikoglu F. Propolis protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity in rat hepatocytes. Food Chem Toxicol. 2012; 50: 2142-2148.
- Aly HA, Khafagy RM. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced cytotoxicity accompanied by oxidative stress in rat sertoli cells: Possible role of mitochondrial fraction of sertoil cells. Toxicology and Applied pharmacology. 2011; 252: 273-280.
- Knerr S, Schaefer J, Both S, Mally A, Dekant W, Schrenk D. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induced cytochrome P450s alter the formation of reactive oxygen species in liver cells. Mol. Nutr. Food Res. 2006; 50: 378-394.
- Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999; 70: 491-499.
- Tuomisto JT, Viluksela M, Pohjanvirta R, Tuomisto J. The AH receptor and a novel gene determine acute toxic responses to TCDD: segregation of the resistant alleles to different rat lines. Toxicol Appl Pharmacol. 1999; 155: 71- 81.
- Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003; 1619: 263-268.
- Pereira SP, Pereira GC, Pereira CV, Carvalho FS, Cordeiro MH, Mota PC, et al. Dioxin-induced acute cardiac mitochondrial oxidative damage and increased activity of ATP-sensitive potassium channels in Wistar rats. Environ Pollut. 2013; 180: 281-290.
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. Journal of Botany. 2012; 217037: 1-26.
- Rackova L, Oblozinsky M, Kostalova D, Kettmann V, Bezakova L. Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. J Inflamm. 2007; 4: 1-7.
- Lee JH, Wada T, Febbraio M, He J, Matsubara T, Lee MJ, et al. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology. 2010; 139: 653-663.