
Research Article
Austin J Lung Cancer Res. 2016; 1(2): 1007.
In Vitro Cytotoxic Activities of Leaf Extracts of Thespesia Populnea and Hygrophilla Schulli Against Daltonâs Lymphoma Ascites and Ehrlich Ascites Carcinoma Cell Lines
Chandran RP1*, Manju S2, Shaji PK3, Nair GA3 and Sukumar B4
1Department of Biotechnology, S.D.V. College of Arts and Applied Science, India
2Department of Biotechnology and Research, KVM College of Engineering and Information Technology, India
3Environmental Resources Research Centre (ERRC), India
4National Institute of Technology, Calicut, India
*Corresponding author: Chandran RP, Department of Biotechnology, S.D.V. College of Arts and Applied Science, Sanathanapuram P.O. Kalarcode, Alappuzha, Kerala- 688003, India
Received: January 25, 2015; Accepted: March 29, 2016; Published: March 31, 2016
Abstract
Phytochemical constituents of two plants, Thespesia populnea and Hygrophila schulli were extracted (cold extraction) using different solvents based on polarity (hexane, chloroform, dichloromethane, ethyl acetate, acetone, methanol and water) and the percentages of extractives were calculated. These extracts were tested for cytotoxic activities against cancer cell lines, Ehrlich Ascites Carcinoma (EAC) and Daltonâs Lymphoma Ascites (DLA). The high percentage extractive was found in methanol cold extract with 3.38 and 3.36 % in T. populnea and H. schulli respectively. In T. populnea the highest cytotoxic activity was observed in chloroform extract in EAC (95 % inhibition at 200 μg/ml concentration) and DLA cell lines (100 % inhibition at 200 μg/ml). In the case of H. schulli the same was observed in hexane extract and in EAC (98 % inhibition at 200 μg/ml) and DLA cell lines (94 % inhibition at 200 μg/ml).
Keywords: Cytotoxic activity; Thespesia populnea; Hygrophila schulli; Inhibition; Cell line
Introduction
Cancer is a disease in which the cell undergoes uncontrolled multiplication and spread within the body and may eventually cause death of the host. With changing life style including food habits and exposure to different types of chemicals and radiation, as also due to availability of curative treatment for many infectious diseases, cancer is surpassing other illnesses as a principle cause of morbidity and mortality even in developing countries [1]. Cancer is the second largest common disease and a major global health burden [2,3]. Approximately 14 million new cases and 8.2 million cancer related deaths have been reported in 2012 and the number of new cases is expected to rise by about 70% over the next 2 decades [4]. Medicinal plants play a prominent role as a source of effective anticancer agents and it is significant that 60% of currently used anticancer drugs are derived from natural sources including plants [5,6].
Thespesia populnea (L.) Soland. ex Correa, commonly known as âPortia treeâ or âIndian tulip treeâ of family Malvaceae is a small to medium sized tree with a pan tropical distribution, normally found along the coastal stretches. The tree grows to a height of 10 to 15 m. Its leaves are simple and heart-shaped, with a distinct tip. Flowers are bisexual, solitary or in cymes, showy and yellow. Fruits are globose brown capsules. The tree yields valuable pink to dark red close-grained wood and also an oil from its seeds. Though the tree is adapted to grow in a wide range of soil types in the coastal environments, it prefers near neutral soils (pH of 6 - 7.4). T. populnea is one of the common trees in the coastal lowlands and midlands of Kerala, which is often cultivated in the home gardens and in other agro forestry systems for its multifarious uses [7]. It is traditionally claimed to possess useful medicinal properties such as antifertility, anti-inflammatory, antioxidant, purgative and hepatoprotective [8] activities and its bark, leaves and flowers are useful in cutaneous infections such as scabies, psoriasis, eczema, ring worms, guinea worm [9], anti-inflammatory for poultice as a folk medicine etc. In addition to these, T. populnea has been scientifically proved to possess medicinal properties such as antibacterial, antifertility, and antinociceptive activities [10].
Hygrophila schulli (Buch.-Ham) M.R. & S.M. Almeida is a thorny sub-shrub of the family Acanthaceae that grows widely throughout India, Sri Lanka, Myanmar, Indo-China, Tropical Africa and Malaya [11]. The synonyms of H. schulli include H. auriculata (K. Schum) Heine and Astercantha longifolia (L.) Nees. The erect armed subshrub with purplish stem generally has eight leaves and six spines at each node. Leaves are whorled, linear-lanceolate, with undulating margins. Flowers form in axillary sessile whorls, with leafy bracts and bracteoles, and pink corolla. Capsules are 1 cm long and seeds orbicular. H. schulli (Marsh barbel) is a commonly found associated with and wetlands, where it forms large stands by easily colonizing in waterlogged areas [11]. Traditionally, the leaves are used in diuretic, jaundice, antibacterial, dropsy, rheumatism and anasaraca. It is also used to cure the diseases of urinogenital tract, leucor, sweet, sour, bitter, tonic, oleaginous, aphrodisiac, hypnotic, diarrhea, dysentery, urinary calculi, urinary discharge, anti inflammatory, joint pain and biliousness. They are very effective to cure eye disease, ascites, abdominal troubles, anemia, anuria, gleets, cough, demulcent, stomachic, lumbago, arthritis, gastric disorder and leucorrhoea [12].
The purpose of the present study was to determine the percentage of extractive and to check the cytotoxic activity of T. populnea and H. schulli extracts against Daltonâs Lymphoma Ascites (DLA) cells and Ehrlich Ascites Carcinoma (EAC) cell lines.
Materials and Methods
Chemicals
All the solvents used for the extraction process were of analytical grade and procured from SD Fine Chemicals, Mumbai, India
Collection and identification of plant material
Healthy leaf samples of Thespesia populnea and Hygrophilla schulli were collected from Kuttanad wetlands after authenticating the taxonomic identity of the source plant by Dr. T. Shaju, Scientist, Division of Plant Systematics and Evolutionary Science, Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI), Palode, Kerala, and the voucher specimens of the samples (leafy twigs with flowers) were deposited in the Herbarium of Environmental Resources Research Centre (ERRC), Thiruvananthapuram, Kerala. The plant materials were washed and shade dried and pulverized to coarse powder using electric grinder. The powder was then stored in airtight bottles for further studies.
Preparation of extracts
Preparation of extracts Thirty grams of leaf powder was subjected to cold extraction using 250 ml of solvents in the increasing order of polarity (hexane, chloroform, dichloromethane, ethyl acetate, acetone, methanol and water). The final filtrate of each extract concentrated using a rotary vacuum evaporator (IKA, RV 10 digital, Germany) was collected, evaporated to dryness and stored at 4ºC for further studies. The percent extractive of cold extracts of all the seven solvents were calculated using the formula.
Maintenance of cell lines
Daltonâs Lymphoma Ascites (DLA) cells and Ehrlich Ascites Carcinoma (EAC) cells which are murine lymphoid cancer cell lines, were purchased from Amala Cancer Research Centre, Thrissur, Kerala, and were maintained by weekly intraperitoneal inoculation of 106 cells/per mouse.
Preparation of cell suspension
Peritoneal fluid 1 ml was withdrawn from DLA/EAC cell line bearing mice. This fluid was diluted with 9 ml of PBS (Phosphate buffer Saline) and mixed well. Then it was centrifuged at 2000 rpm for 10 min. Supernatant was removed and settled cells were again resuspended with 9 ml of PBS. This cell suspension was used for further procedure.
Trypan blue dye exclusion test
The tumor cells aspirated from the peritoneal cavity of tumor bearing mice were washed thrice with PBS or normal saline and the cell viability was determined by trypan blue exclusion method. It is based on the principle that live cells possess intact cell membranes that exclude the dye while the dead cells do not and have blue coloured cytoplasam under light microscope. Viable cell suspension (1x106 cells in 0.1ml) was added to eppendorf tubes containing various concentrations of the test compounds (10, 20, 50, 100, 200 μg/ ml) and the volume was made up to 1 ml using 10% DMEM media. These assay mixtures were incubated for 3 hour at 37ºC. Control tube contained only cell suspension. After incubation, cell suspension was mixed with 0.1 ml of 1 % trypan blue and kept for 1-2 minutes and loaded on a haemocytometer. Dead cells take up the blue color of trypan blue while live cells do not take up the dye. The-number of stained and unstained cells was counted separately [13].
Results and Discussion
The highest percentage extractive value of 3.38 % was obtained in cold methanol extract of T. populnea and the lowest was found in acetone extract with a value of 0.82%. Whereas in H. schulli the highest percentage extractive value of 3.26% was obtained in cold methanol extract of T. populnea and the lowest was found in ethyl acetate extract with a value of 0. 56 %. The percentage extractives of T. populnea and H. schulli leaf extracted in the other solvents are given in Table 1.
Plants
Hexane    (%)
Chloroform (%)
Dichloro methane (%)
Ethyl acetate (%)
Acetone (%)
Methanol (%)
Distilled water (%)
T. populnea
2.0
3.36
1.58
0.64
0.82
3.38
2.02
H. schulli
1.1
1.54
0.7
0.56
0.66
3.26
2.1
Table 1: Percent extractives of cold leaf extracts of T. populnea and H. schulli
In vitro cytotoxic activity of T. populnea
In trypan blue dye exclusion method, T. populnea chloroform extract at 200 μg/ml concentration showed 95 % inhibition against EAC cell lines and the lowest was 12% in aqueous extract at 200 μg/ ml concentration. In DLA cell lines 100 % inhibition was recorded in chloroform extract at 200 μg/ml and the lowest was12 % in aqueous extract. The percentage inhibition of other extracts against EAC and DLA cell lines are given in Figures 1 and 2.
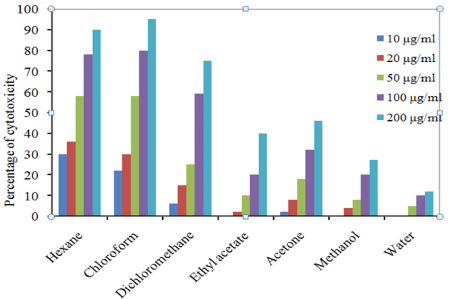
Figure 1: Percentage of cell viability of T. populnea leaf in EAC cell line.
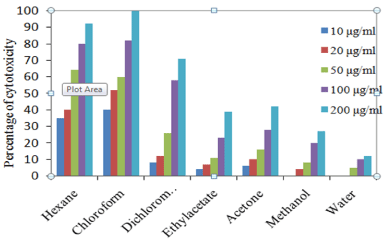
Figure 2: Percentage of cell viability of T. populnea leaves in DLA cell line.
Khairunnisa and Karthik [14] reported the cytotoxic activity of methanolic extract of the bark of Hymenodictyon excelsum (Roxb.) Wall on DLA cells by trypan blue dye exclusion method and observed 100 % cytotoxicity at 200 μg/ml and in the present study in DLA cell lines 100 % inhibition was recorded in T. populnea chloroform extract at 200 μg/ml. In addition to this the extracts of T. populnea (except aqueous extract) showed antibacterial activity against human pathogens such as Bacillus subtilis (MTCC 619), Bacillus cereus (MTCC 430), Staphylococcus epidermidis (MTCC 3615), Escherichia coli (MTCC 729), Pseudomonas aeruginosa (MTCC 4676), Klebsiella pneumoniae (MTCC 432), and Proteus mirabilis (MTCC 425). T. populnea extracts showed antifungal activity against Candida albicans (MTCC 183) [7].
The IC50 values of hexane, chloroform and dichloromethane extracts of T. populnea was found to be 38.94 μg/ml, 41.32 μg/ml, 86.88 μg/ml respectively for EAC cell line and 32.85 μg/ml, 18.55 μg/ ml, 87.42 μg/ml for DLA cell lines. The hexane extract was found to be more active against EAC cell lines and chloroform extracts was found to be more active against DLA cell lines. Whereas ethyl acetate, acetone, methanol and aqueous extract showed very low cytotoxic activity against both the cell lines (Table 2).
Solvents
IC50 values (μg/ml) of extracts against cell lines
EAC
DLA
Hexane extract
38.94
32.85
Chloroform extract
41.32
18.55
Dichloromethane extract
86.88
87.42
Ethyl acetate extract
Nil
Nil
Acetone extract
Nil
Nil
Methanol extract
Nil
Nil
Water extract
Nil
Nil
Table 2: IC50 values of T. populnea leaf extracts.
In vitro cytotoxic activity of H. schulli
H. schulli hexane extract showed 98 % inhibition against EAC cell line at 200 μg/ml concentrations and the lowest was 8% in aqueous extract. In DLA cell lines 94% inhibition was recorded in hexane extract at 200 μg/ml and the lowest was 7% in aqueous extract. The percentage inhibition of other extracts against EAC and DLA cell lines are given in Figure 3 and 4. In addition to this our previous studies showed that the extracts of H. schulli exhibited antibacterial activity against human pathogens such as Bacillus subtilis (MTCC 619), Bacillus cereus (MTCC 430), Staphylococcus epidermidis (MTCC 3615), Escherichia coli (MTCC 729), Pseudomonas aeruginosa (MTCC 4676) and Klebsiella pneumoniae (MTCC 432). H. schulli did not exhibit antifungal activity against the test fungal pathogens such as Candida albicans (MTCC 183), Trichophyton rubrum (MTCC 296), Cryptococcus gastricus (MTCC 1715), Aspergillus tubingensis (MTCC 2425), Aspergillus flavus (MTCC 873) and Aspergillus fumigatus (MTCC343) [11].
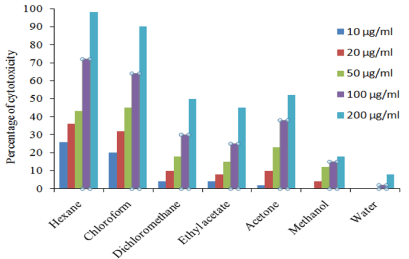
Figure 3: Percentage of cell viability of H. schulli leaves in EAC cell line.
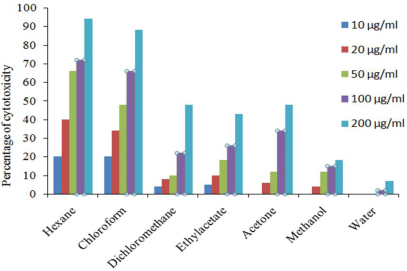
Figure 4: Percentage cell viability of H. schulli leaves in DLA cell line.
The IC50 values of hexane, chloroform, dichloromethane and acetone extracts of H. schulli was found to be 62.78 μg/ml, 63.84 μg/ml, 198.67 μg/ml and 185.16 μg/ml for EAC cell line. The IC50 value of hexane and chloroform extract was found to be 31.38 μg/ml and 55.36 μg/ml for DLA cell line. The ethyl acetate, methanol and aqueous extract showed very low cytotoxic activity against both cell lines (Table 3).
Solvents
IC50 values (μg/ml) of extracts against cell lines
EAC
DLA
Hexane extract
62.78
31.38
Chloroform extract
63.84
55.36
Dichloromethane extract
198.67
Nil
Ethyl acetate extract
Nil
Nil
Acetone extract
185.16
Nil
Methanol extract
Nil
Nil
Water extract
Nil
Nil
Table 3: IC50 values of H. schulli leaf extracts.
The results of trypan blue dye exclusion test indicated that all the cold extracts of T. populnea and H. schulli could inhibit the growth of EAC and DLA cell lines in a dose dependent manner and the cytotoxic activity of the leaf extracts could be attributed to the presence of phytochemical chemicals present in the plants. The plants can provide potential bioactive compounds for the development of new leads to combat cancer diseases [15]. Many pharmaceutical companies are showing great interest in plant derived drugs mainly due to the current widespread belief that âGreen Medicineâ is effective, safer and more reliable than synthetic drugs [16].
Conclusion
In the present study highest percentage extractive was observed in methanolic extracts of T. populnea and H. schulli. Higher cytotoxic activity was observed in chloroform extracts in T. populnea in both EAC and DLA cell lines and in H. schulli the same was observed in hexane extract in both EAC and DLA cell lines. Thus both the test plants exhibited potent cytotoxic activity.
Acknowledgment
We are thankful to the Department of Biotechnology (DBT): Ministry of Science and Technology, Government of India, for the award of the project âBioresources of Kuttanad Wetland Ecosystem: Inventorization, Characterization and Conservationâ (Grant no: BT/PR-13695/BCE/08/798/2010, dated 28-06-2011), under which the present study was conducted. We also sincerely thank Dr. V.V. Pyarelal, Director and Principal, K. V. M. College of Engineering and IT, Cherthala, Kerala, India for providing necessary facilities and support.
References
- Notani PN. Global variation in cancer incidence and mortality. Curr Sci. 2001; 81: 465-467.
- Kainsa S, Kumar P, Rani P. Medicinal Plants of Asian Origin Having Anticancer Potential: Short Review. Asian J Biomed and Pharma Sci. 2012; 2: 1-7.
- Ma X, Yu H. Global Burden of Cancer. Yale J Biol Med. 2006; 79: 85-94.
- World Cancer Report. International agency for research on cancer (IARC), WHO Nonserial Publication edited by Stewart. BW, Wild CP. 2014.
- Newman DJ, Cragg GM, Snader KM. Natural products as a source of new drugs over the period 1981-2002. J Nat Prod. 2003; 66: 1022-1037.
- Chandran RP, Kumar SN, Manju S, Kader SA, Kumar BSD. In vitro α-glucosidase inhibition, antioxidant, anticancer and antimycobacterial properties of ethyl acetate extract of Aegle tamilnadensis Abdul Kader (Rutaceae) leaf. Appl Biochem Biotechnol. 2015; 175: 1247â1261.
- Chandran RP, Manju S, Vysakhi MV, Shaji PK, Nair GA. Antibacterial and antifungal activities of Thespesia populnea leaf extracts against human pathogens. Int J PharmTech Res. 2014; 6: 290-297.
- Shirwaikarkumar A, Krishnan AV, Sreenivasan KK. Chemical investigation and antihepatotoxic activity of Thespesia populnea. Int J Pharmacog. 1995; 33: 305-310.
- Chopra RN, Nayar SN, Chopra IC. Glossary of Indian Medicinal Plants. CSIR, New Delhi, India. 1956.
- Vasudevan M, Parle M. Pharmacological actions of Thespesia populnea relevant to Alzheimerâs disease. Phytomedicine. 2006; 13: 677-687.
- Chandran RP, Manju S, Vysakhi MV, Shaji PK, Nair GA. In vitro antimicrobial activities of Hygrophila schulli (Buch.-Ham) leaf and root extracts against clinically important human pathogens. Biomed Pharmacol J. 2013; 6: 421- 428.
- Patra A, Jha S, Murthy PN, Satpathy S. Antibacterial activity of Hygrophila spinosa T. Anders leaves - a comparative study. Int J Pharm Tech Res. 2009; 1: 837-839.
- Phillips HJ, Terryberry JE. Counting actively metabolizing tissue cultured cells. Cell Res. 1957; 13: 341-347.
- Khairunnisa K, Karthik D. Evaluation of in-vitro apoptosis induction, cytotoxic activity of Hymenodictyon excelsum (Roxb) Wall in Daltonâs lymphoma ascites (DLA) and Lung fibroblast - Mouse L929 cell lines. J App Pharm Sci. 2014; 4: 011-017.
- Shoeb M. Anticancer agents from medicinal plants. Bang J Pharmacol. 2006; 1: 35-41.
- Sujatha S. Complementary and alternative therapies in palliative care: a transition from modern medicine to traditional medicine in India. J Cancer pain Symptom Palliation. 2005; 1: 25-29.