Research Article
Austin J Med Oncol. 2014;1(1): 4.
Concurrent Chemoradiation Followed by Adjuvant Temozolomide in the Management of Malignant Gliomas
El Khoury C1*, Kamar FG2, Nehme-Nasr D1 and Nasr E1
1Department of Radiation Oncology, Hotel Dieu de France Hospital, University of Saint Joseph, Faculty of Medicine, Lebanon
2Division of Neuro-Oncology, Clemenceau Medical Center, Lebanon
*Corresponding author: El Khoury C, Department of Radiation Oncology, Hotel Dieu de France University Hospital, Alfred Naccache Boulevard, Achrafieh, Lebanon
Received: August 18, 2014; Accepted: September 15, 2014; Published: September 17, 2014
Abstract
Purpose: The current standard of care for newly diagnosed glioblastoma remains surgical resection followed by concurrent chemoradiation. The same treatment is often applied to other malignant gliomas. In this study, we report outcomes of patients with malignant gliomas treated with concomitant chemoradiation in the adjuvant setting at our institution.
Patients and Methods: We retrospectively reviewed the records of 59 patients treated at our center between March 2003 and May 2009 for malignant gliomas. Median follow up was 19 months (3-61). Variables reviewed for analysis were histology (glioblastoma 61%, anaplastic astrocytoma 20%, mixed anaplastic oligoastrocytoma 10%, and anaplastic oligodendrogioma 9%), tissue sampling technique (open surgery 66%, stereotactic biopsy 34%), and extent of resection in the open surgery group: gross total resection (GTR) 44%, partial resection (PR) 22%. Radiotherapy was delivered to the tumor bed using 3D-conformal radiation for a total dose of 60 Gy in 30 fractions with concurrent and adjuvant temozolomide in all cases.
Results: Median overall survival was 21 months (95% CI, 17.8-24.3). The two-year survival rate was 43% for the entire cohort and 68% in the subset of patients who had debulking surgery and therefore better than those who had only stereotactic biopsy (p=0.026).
Conclusion: Outcomes of patients with malignant gliomas treated with concurrent chemoradiation followed by adjuvant temozolomide in our institution was comparable to historical data from the literature. Patients who had debulking surgery upfront and prior to concurrent and adjuvant treatment had better survival than those who had stereotactic biopsy in the same setting.
Keywords: Malignant glioma; Concurrent chemoradiation; Temozolomide
Abbreviations
RT: Radiotherapy; OS: Overall Survival; GBM: Glioblastoma; WHO: World Health Organization; PS: Performance Status; EORTC: European Organization for Research and Treatment of Cancer; NCIC: National Cancer Institute of Canada; TMZ: Temozolomide; MGMT: O6-methylguanine-DNA methyl transferase; GTR: Gross Total Resection; PR: Partial Resection; 3D-CRT: Three-Dimensional Conformal External-Beam Radiation.
Introduction
Despite recent progress in the treatment of brain tumors with multimodality approaches, survival of patients with malignant glioma remains poor. While treatment improves survival, the overall prognosis is dismal. Disease progression or recurrence is the rule and its resulting neurological morbidity is high, often leading to death in the short term.
Advances in neuroimaging, neurosurgical stereotactic navigation systems, and improved tumor control with targeted radiotherapy (RT), and more recently concurrent chemoradiation have significantly prolonged disease free survival and overall survival (OS) but have not resulted in a cure. Patients receiving best supportive care, corticosteroids and anticonvulsant drugs, have median survival duration of approximately 23 weeks or less [1]. Median survival is 10- 15 months for glioblastoma (GBM) and 30-50 months for anaplastic (World Health Organization [WHO] grade III) astrocytoma [2]. Survival is inversely correlated with age and performance status (PS) [3]. The standard of care for GBM has been surgery followed by RT with concomitant and adjuvant temozolomide (TMZ) chemotherapy since a large phase III trial performed by the European Organization for Research and Treatment of Cancer and the National Cancer Institute of Canada (EORTC/NCIC) showed better survival and longer progression-free survival for postoperative radiochemotherapy compared with RT exclusively for GBM [4]. This study has been updated and long term follow up published with several subset analyses reporting survivals at two years, at five years and longer [5]. The same regimen is also applied to other malignant glioma subtypes.
In this study, we discuss the current treatment modalities and outcomes in the management of newly diagnosed malignant glioma at our institution, with particular emphasis on the current status and future potential of the therapies for this disease.
Materials and Methods
Methods
All consecutive patients with newly diagnosed WHO grade IV or III glioma (GBM, anaplastic astrocytoma, anaplastic oligodendroglioma, or mixed anaplastic oligoastrocytoma) treated at the radiation oncology department of Hotel Dieu de France in Beirut between March 2003 and May 2009 were retrospectively reviewed. Patients included were 18 years or older at diagnosis, had pathologically proven high grade glioma, and received concomitant radiochemotherapy and sequential TMZ. 59 patients were identified. A detailed review of their medical file was performed for the demographic and treatment related information.
Data captured included age, gender, WHO Performance Status (WHO PS) [6], topographic location of the tumor (lobe), extent of resection, salvage therapies, and survival. All data were collected retrospectively and in accordance with institutional ethical policies.
Histologic diagnosis was based on the 2007 modified WHO classification system [7]. Expression of O6-methylguanine-DNA methyltransferase (MGMT) was assessed only in a minority of cases for research purposes, and consequently it was not possible to evaluate its prognostic and predictive significance in the current study. Extent of maximal safe surgical resection was recorded as stereotactic biopsy or resection which was further defined as gross total resection (GTR) or partial resection (PR) on postoperative imaging. Biopsies were carried out only when surgical resections were contraindicated by anatomical sites or location in an eloquent area. RT consisted of a conventional fractionation at a dose of 2 Gy per fraction given once daily on weekdays, for a total dose of 60 Gy. Three-dimensional conformal external-beam radiation (3D-CRT) was delivered with a Varian 2100 C linear accelerator with nominal energy of 6 or 18 MV. Follow up was reviewed in private physician’s offices and subsequent data was not accessible for review.
Statistical analysis
The Kaplan-Meier method was used to estimate OS, defined as the interval from histologic diagnosis until death from any cause. Patients alive or lost to follow-up were censored for survival. Survival curves were compared using the log-rank test. Multivariate analysis was performed with the Cox regression model. Differences between subgroups of patients were calculated using the Chi-square test. All analysis was performed using SPSS version 17.0, commercially available software package (SPSS Inc. Chicago, IL).
Results
Patient characteristics (Table 1)
Median age was 52 years (range 20-77). The male / female ratio was two to one. Most tumors were located in the frontal lobe (32%), parietal lobe (22%), or temporal lobe (19%). The majority of the patients had good performance status at the time of diagnosis (WHO PS = 0 in 59%, 1 in 29% and 2 in 12%). The majority underwent debulking surgery, including GTR (44%) and PR (22%). The remainder (33%) had stereotactic biopsy. Histology was GBM in 61%, anaplastic astrocytoma in 20%, anaplastic oligodendroglioma in 9%, and mixed anaplastic oligoastrocytoma in 10%. 90% of the patients completed both RT and TMZ as planned. TMZ had to be discontinued transiently in five patients because of hematological toxicity. All cases had adjuvant TMZ and completed at least four cycles. The main reason for not completing adjuvant TMZ therapy was disease progression. No grade 4-5 treatment related toxicities were observed.
Feature
No. (%)
Number of patients
59 (100)
Gender
Male
40 (68)
Female
19 (32)
WHO PS
0
35 (59)
1
17 (29)
2
7 (12)
Histotype
Glioblastoma Multiforme
36 (61)
Anaplastic Astrocytoma
12 (20)
Anaplastic Oligodendroglioma
5 (9)
Mixed Anaplastic Oligoastrocytoma
6 (10)
Tumor site
Frontal lobe
19 (32)
Parietal lobe
13 (22)
Temporal lobe
11 (19)
Occipital lobe
6 (10)
Thalamus
3 (5)
Others
7 (12)
Extent of surgical resection
Debulking surgery
39 (66)
GTR
26 (44)
PR
13 (22)
Stereotactic Biopsy
20 (34)
Table 1: Patients Clinical Features.
Outcome and factors influencing survival
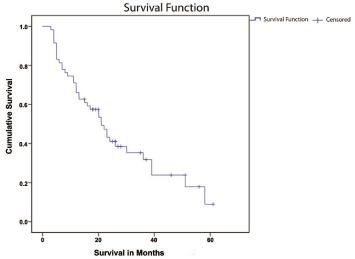
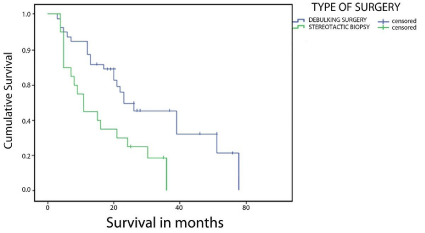
The median OS was 21 months (95% CI, 17.8-24.3). At the time of submission of the cohort, there were no surviving patients. The longest time to death was 64 months. Actuarial survival was 71% at one year and 43% at two years (Figure 1). Median follow-up was 19 months. Survival was also analyzed according to potential prognostic factors including age, gender, extent of resection, histology, and PS. The shortest survival (Figure 2) was among the subgroup of patients who underwent biopsy only, with median survival of 11 months (95% CI, 6-16), whereas median survival for patients undergoing debulking surgery including partial or total resection was 23 months (95% CI, 11-35). Sample size did not allow GTR versus PR comparison in the latter group.
Figure 1: Kaplan-Meier Estimates of Overall Survival in the current series.
Figure 2: Survival Functions stratified by extent of surgery (log-rank test, P = 0.026).
Patients with the best PS (WHO PS = 0) had the longest survival of 22 months (95% CI, 15.6-28.4) (Table 2). In cases of disease progression, further treatment was at the physician’s discretion. Patients underwent a second surgery or received chemotherapy. Salvage chemotherapy consisted of Bevacizumab plus Irinotecan. The response to salvage chemotherapy was not recorded as part of our study.
MS (95% CI)
P-value*
Type of Surgery
Debulking surgery (GTR + PR)
23 (10.9-35.2)
0.026
Stereotactic biopsy
11 (6.4-15.5)
WHO PS
0
22 (15.6-28.4)
0.03
1
20 (12.3-27.6)
2
15 (10.7-19.4)
Table 2: Median survival months following diagnosis by type of surgery and WHO performance status.
Discussion
In this study, we assessed treatment and survival in the group of patients with newly diagnosed malignant glioma and treated at our institution. We found that all patients treated with partial/total resection and stereotactic biopsy received RT with concurrent TMZ since 2003. This suggests that the current standard of care was applied in our community even before the completion and publication of the pivotal clinical trial in 2005 that showed the survival advantage of concomitant TMZ and RT compared with RT alone after surgical resection. This strategy was motivated by the phase II trial on RT and TMZ conducted by Stupp et al. [8] which served as basis for the experimental arm of the EORTC/NCIC trial. Median survival in the entire sample which included patients who underwent biopsy only, was 21 months. This is in comparison to the EORTC/NCIC randomized phase III trial which showed improved median survival with the addition of concomitant and adjuvant TMZ to the standard postoperative RT compared to postoperative RT alone; the median survival was 14.6 months with RT + TMZ and 12.1 months with RT alone establishing TMZ and RT as a standard of care [4]. This extended survival may reflect either patient selection or including those with WHO grade III histology [3,9-11]. Furthermore, GBM patients who progressed received salvage Bevacizumab plus Irinotecan chemotherapy at the time of progression which may have contributed to improved survivals [12].
There was a definite survival advantage when considering the subset of patients who had surgical resection followed by adjuvant therapy (median survival 23 months) compared to patients undergoing biopsy followed by adjuvant therapy (median survival 11 months) (log-rank test, P = 0.026). This implies that aggressive and more complete resections provided significantly longer survival time when compared to less radical surgical treatment. Our findings were comparable to previously published studies in terms of extent of resection and ultimate outcome [3,13,14].
Although no level one evidence justifies the use of postoperative TMZ in patients with newly diagnosed WHO grade III gliomas, TMZ-based chemotherapy is used widely in clinical practice. Meta-analyses have demonstrated a consistent improvement in survival of approximately 5% to 10% at one and two years in patients with high grade gliomas who received adjuvant chemotherapy in addition to RT [15-17]. For patients with anaplastic gliomas, international Intergroup phase III trials are ongoing: The EORTC/Radiation Therapy Oncology Group (RTOG) CATNON (Concomitant and Adjuvant TMZ in Nondeleted Anaplastic Astrocytoma) and the RTOG/North Central Cancer Treatment Group CODEL (1p/19q codeleted) [17].
In CATNON, patients will be randomly assigned to concomitant TMZ/RT versus RT alone, with a second random assignment for the administration of adjuvant TMZ or observation. In CODEL, patients will be randomly assigned to concomitant TMZ/RT followed by adjuvant TMZ, RT alone or TMZ alone. Probably the results of these trials will help determine if TMZ administration also improves outcomes for grade III glioma, and if concomitant TMZ administration is superior to adjuvant TMZ alone or administered only after the end of RT. Another large retrospective trial of Lassman et al suggested that PCV chemotherapy (Procarbazine, Lomustine and Vincristine) maybe more effective than TMZ in treating anaplastic gliomas [19].
One of the major factors that determine outcome or survival in malignant glioma patients is PS and it is well documented in the literature [3,20]. In our study, WHO PS stands out as having strong impact on survival. While it is as high as 22 months in WHO PS=0 patients, median survival declines significantly with lower PS scores (log-rank test, P = 0.03). In this retrospective analysis, low PS could have also led to less aggressive resection because of treatment bias.
Limitations of our study were its retrospective nature, the absence of information on salvage treatment, the small sample size and pre-selection of the patients, and lack of molecular data such as MGMT promoter methylation. The sample was not large enough to adequately represent all age groups and possible variations of pathology (WHO grade III and IV), location and sensitively distinguish between the contributions of minor variations of individual factors on patient survival.
Conclusion
In this analysis of survival in high grade glioma patients, we find that survival remains limited and aggressive surgical resection along with adjuvant treatment offers the best chance of survival. However, quality of life after tumor resection remains the most important issue; the question of whether a significant increase in survival translates into better quality of life remains unanswered. Future prospective studies must be directed at this issue. The upcoming clinical trials will stratify patients on the basis of the histologic tumor grade and on molecular markers, allowing for better understanding of the molecular pathways of glioma genesis. Given the molecular heterogeneity of malignant gliomas and the advent of innovative radiation therapy modalities, selective dose intensification should be considered according to tumor density. Thus, enhancing our understanding of this disease and allowing for rapid further improvement in outcome.
Conflict of Interest
The authors declare that they have no potential conflict of interest relevant to this article.
References
- Walker MD. Brain tumor study group: a survey of current activities. Natl Cancer Inst Monogr. 1977; 46: 209-212.
- Nieder C, Andratschke N, Wiedenmann N, Busch R, Grosu AL, et al. Radiotherapy for high-grade gliomas. Does altered fractionation improve the outcome? Strahlenther Onkol. 2004; 180: 401-407.
- Laws ER, Parney IF, Huang W, Anderson F, Morris AM, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003; 99: 467-473.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352: 987-996.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10: 459-466.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5: 649-655.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. 2007; 114: 97-109.
- Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002; 20: 1375-1382.
- Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006; 20: E1.
- Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006; 106: 1358-1363.
- Laws ER, Shaffrey ME, Morris A, Anderson FA Jr. Surgical management of intracranial gliomas – does radical resection improve outcome? Acta Neurochir Suppl. 2003; 85: 47-53.
- Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007; 25: 4722-4729.
- McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009; 110: 156-162.
- Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008; 62: 564-576.
- DeAngelis LM. Anaplastic glioma: how to prognosticate outcome and choose a treatment strategy. J Clin Oncol. 2009; 27: 5861-5862.
- Dresemann G. Temozolomide in malignant glioma. Onco Targets Ther. 2010; 3: 139-146.
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002; 359: 1011-1018.
- Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007; 25: 4127-4136.
- Lassman AB, Iwamoto FM, Cloughesy TF, Aldape KD, Rivera AL, et al. International retrospective study over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011; 13: 649-659.
- Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003; 30: 10-14.