Research Article
Austin J Nanomed Nanotechnol. 2014;2(4): 1022.
Detection of [Ca2+]I Changes In Sub–Plasma Membrane Micro Domains in A Single Living Cell By an Optical Fiber–Based Nanobiosensor
Wang S1,2, Ye F3, Lang X3, Fei D4, Turner APF2,5 and Ge Y2*
1College of Life Science and Technology & Advanced Biomaterials and Tissue Engineering Center, Huazhong University of Science and Technology, China
2Centre for Biomedical Engineering, Cranfield University, UK
3Division of BioEngineering, School of Chemical and Biomedical Engineering & Center for Advanced Bionanosystems, Nanyang Technological University, Singapore
4Leicester School of Pharmacy, Hawthorn Building, De Montfort University, UK
5Biosensors and Bioelectronics Centre, IFM-Linköping University, Sweden
*Corresponding author: Ge Y, Centre for Biomedical Engineering, School of Engineering, Cranfield University, Cranfield, Bedfordshire, MK43 0AL, UK
Received: May 26, 2014; Accepted: May 28, 2014; Published: May 29, 2014
Abstract
An optical fiber-based nanobiosensor, for advanced detection of [Ca2+]i (i.e. intracellular Ca2+ concentration) changes in sub–plasma membrane microdomains in a single living smooth muscle cell and a single living cardiomyocyte, was successfully prepared by coating silver and then immobilizing Calcium Green–1 Dextran, a calcium ion sensitive dye, on the distal end of the nanoprobe. The constructed nanobiosensor was capable of detecting ultra–low and local intracellular calcium ion concentration within the nanomolar range, which is around the physiological level of free cytosolic calcium ion in a single living cell. The response time was less than milliseconds enabling the detection of transient elementary calcium ion signaling events associated with calcium ion microdomains. The effects of stimulants such as high potassium buffer solution and norepinephrine solution were also investigated. The resulting system could thus greatly facilitate the development of an advanced nano–diagnostic platform for in vivo and real–time sensing/diagnosing of [Ca2+]i at the single cell level.
Keywords: Nanobiosensor; Optical fiber; Intracellular Ca2+ Concentration; Nano–diagnosis; Single cell level detection
Introduction
Calcium ion (Ca2+) is broadly recognized as one of the most versatile and universal signaling agents in the human body. It serves as an intracellular messenger, relaying information within cells to regulate their activity [1–3]. The evaluation of Ca2+ level is imperative not only because of its importance in signaling but also due to its lethality when prolonged increase of Ca2+ concentration ([Ca2+]) is presented.
In the spatial aspect of calcium ion channels, Ca2+ is organized into distinct microdomains (i.e. localized regions with a [Ca2+] significantly different from the bulk cytoplasm) to regulate various cellular processes in localized regions within single cells. Although Ca2+ microdomains are a key element of Ca2+ signaling, there remains a great challenge for the research community to develop a monitoring methodology with very high spatial and temporal resolution for more efficient studies of Ca2+ microdomains [4,5]. It becomes more challenging for the detection since the residing and moving area of Ca2+ in the cellular organelle and membrane microdomains is only about 200–300 nm diameter. In addition, it is of great interest to detect Ca2+ level by employing the technology of single–cell analysis, which is important not only to complement conventional bulk cell assays but also to perform dynamic analysis of interactions within individual living cells that are critical for mapping and deciphering cell signalling pathways and networks.
Many types of biosensors, such as the genetically encoded biosensors (chimeric proteins) [6], green fluorescent protein–based biosensors [7], and FRET (fluorescence resonance energy transfer)– based biosensor [8], have been designed and successfully applied to detect Calcium. More recently, by taking advantage of the unique properties of nanomaterials, nanobiosensors have been developed and exploited at an amazing rate, showing a much faster and sensitive performance as well as affordable, robust and reproducible properties [9,10]. Zamaleeva and his co–workers very recently reported a smart cell–penetrating nanobiosensor for pointillistic intracellular Ca2+ transient detection [11].
Optical fiber–based nanobiosensors, evolved from optical fiberbased mircrobiosensors, [12] have recently been developed to monitor apoptosis, [13,14] lactate release [15] and telomerase over–expression [16] demonstrating some distinct advantages such as submicron probe size, dramatically reduced sample volume, absolute detection limit and response time. In this paper, we describe the development of an optical fiber–based nanobiosensor, to detect [Ca2+]i (i.e. intracellular Ca2+ concentration) changes in sub–plasma membrane microdomains in a single living smooth muscle cell and a single living cardiomyocyte for the first time. With a temporal resolution of less than 1 millisecond, the constructed nanobiosensor was demonstrated to be well suited for intracellular monitoring of [Ca2+] in individual living cells.
Materials and Methods
Reagents and chemicals
Glucose, (3–aminopropyl) triethoxysilane (APTES) and poly–LIntroduction lysine hydrochloride (15,000–30,000 Da) were purchased from Sigma (St.Louis, MO). 50 % (v⁄v) glutaraldehyde in water was purchased from Merck (Hohenbrunn, Germany). Silver nitrate (AgNO3) was obtained from Fisher Scientific (UK). Calcium Green–1 Dextran (3000 Da) was obtained from Molecular Probes (Eugene, OR). High potassium buffer solution (105 mM) contained a final concentration of 40 mM NaCl, 105 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose and 10 mM HEPES (titrated to pH 7.4). Norepinephrine (NPPH) solution (1μM) was prepared in PBS. The deionized water (18.2 MΩ•cm) was collected from a Millipore Milli–Q water purification system. All other reagents were of analytical grade.
Cell cultures
Smooth muscle cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (PAA Laboratories, Pasching, Austria) supplemented with 10 % heat inactivated fetal bovine serum (PAA laboratories) and 50 U⁄mL penicillin⁄streptomycin (Gibco, Grand Island, NY). Cell cultures were grown in a humidified incubator at 37 °C in an atmosphere with 5.0 % CO2. Cells were seeded at a density of 6 × 106 cells⁄cm2 onto poly–L–lysine (5μg⁄mL) coated coverslips for cell attachment prior to calcium detection by our optical fiber based nanobiosensor.
The hearts of 1 to 3–day–old Sprague–Dawley rats were quickly removed into chilled Hanks’ Balanced Salt Solution (HBSS buffer, Sigma), containing (per liter): 8 g NaCl, 0.048 g Na2HPO4, 1 g D–glucose, 0.4 g KCl, 0.06 g KH2PO4, 0.35 g NaHCO3, 0.14 g CaCl2, 0.1 g MgCl2·6 H2O, 0.1 g MgSO4·7H2O, with addition of penicillin 50 U⁄ml and streptomycin 50 U⁄ml. The ventricles were cut into 1 to 2 mm cubes and dissociated by the three–step treatment with (1) 0.1 % trypsin digestion for 20 minutes at 180 rpm at 37 °C; and (2) addition of fetal bovine serum in the digestion solution (20 %) for 1–2 minutes with gentle pipetting at 24 °C; (3) dissociated cells were collected in cold low glucose Dulbecco’s Modified Eagles Medium (DMEM) medium supplemented with 3% fetal bovine serum and 1 % Insulin–Transferrin–Selenium. The above treatment was usually repeated for several rounds to obtain sufficient number of cells. The collected cell suspension was then centrifuged at 1200 rpm and 4 °C for 5 minutes. The resulting cell pellet was subsequently suspended in the supplemented DMEM medium and plated in a Petri dish for 1–1.5 hours at 37 °C to allow fibroblast cells to attach onto the Petri dish. The myocyte–containing supernatant was then collected and centrifuged (4 °C and 1200 rpm for 5 minutes). The myocyte pellets were then re–suspended and plated onto coverslip at a density of 2.5×105 cells⁄ml and cultured in the supplemented DMEM mediumat 37 °C and 5 % CO2.
Fabrication of silver–coated optical fiber–based nanoprobes
Optical fiber based nanoprobes were fabricated from F–MCC–T multimode fibers with 200 μm diameter core (Newport Corporation, Irvine, CA) by heating and pulling method using a laser based micropipette pulling device (Model P2000, Sutter Instruments, Novato, CA). The lateral wall of pulled nanofiber was coated with silver via the silver mirror reaction as we reported previously [17]. The aperture at the nanosized tip was left uncoated.
Immobilization of calcium green–1dextran on the silvercoated optical fiber–based nanoprobes
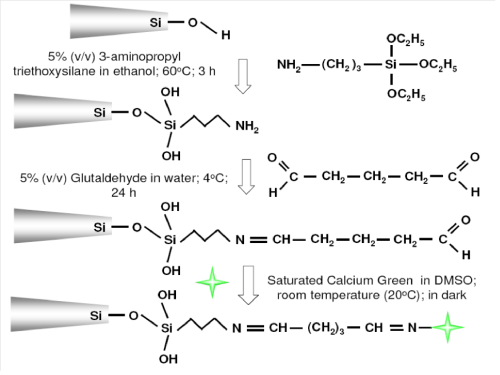
As illustrated in Figure 1, the silver–coated nanoprobes were first cleaned by immersing in pure ethanol for 45 min followed by several rinsing with deionized water and then allowed to air dry at room temperature. The air dried nanoprobes were silanized by treating with 5% (v⁄v) (3–aminopropyl) triethoxysilane (APTES) in ethanol at 60 °C for 3 h. The silanized nanoprobes were washed with ethanol and dried in a vacuum oven at 135 °C for 3 h. After drying, the nanoprobes were activated by immersing in 200 μL of 5% (v⁄v) glutaldehyde [12] at 4 °C for 12 h followed by rinsing with deionized water. Finally, the activated nanoprobes were incubated in 100 μL saturated Calcium Green–1 Dextran in DMSO solution for 24 h at 25 °C.
Figure 1: Schematic representation of immobilization of Calcium Green-1 and its molecular structure. ( represents Calcium Green -1 Dextran) on the silver coated optical fiber-based nanoprobes.
Assembly of an optical fiber–based nanobiosensor
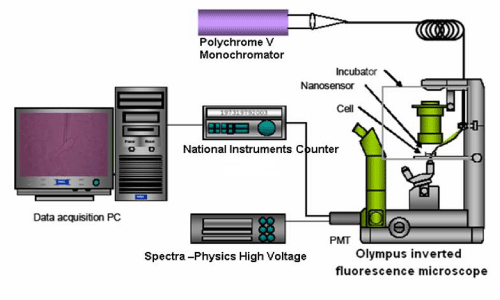
The sensor system was assembled as represented in Figure 2 with main components including Polychrome V monochromatic (7 mW), IX–71 Olympus Inverted microscope, and Photomultiplier Tube (PMT), data acquisition card (National instruments) and a personal computer. To perform the intracellular [Ca2+] detection, 488nm light from the monochromator was launched into the delivery fiber that was terminated with a Subminiature A (SMA) connector. The nanoprobe was coupled to the delivery fiber through the SMA connector and secured to the microscope via nanomanipulator. The fluorescence emitted when Calcium Green–1Dextran chelates with Ca2+ inside the smooth muscle cell cardiomyocyte was collected by the microscope objective and passed through a long–pass filter to be focused onto the PMT for detection. The output from the PMT was recorded by the data acquisition card and displayed on the computer in voltages.
Figure 2: Assembly of an optical fiber-based nanobiosensor for detection of Δ [Ca2+]i in single cells.
Detection of [ca2+]i changes in sub–plasma membrane microdomains in single living smooth muscle cells and single living cardiomyocyte
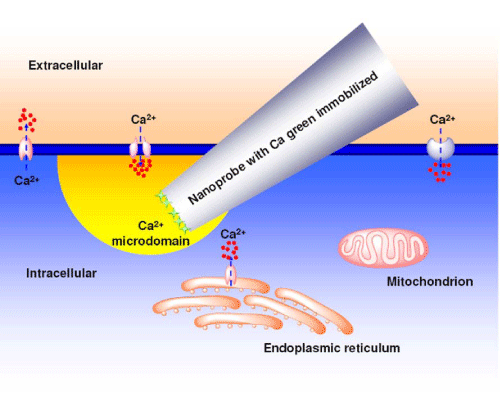
The cytomembrane of a single living cell (single living smooth muscle cell or single living cardiomyocyte) was penetrated by a functionalized nanoprobe with immobilized Calcium Green– 1Dextran via a nanomanipulator (Figure 3). The nanotip was carefully located near the inner membrane. The excited fluorescence was collected to determine the background [Ca2+]i level in cytoplasm prior to stimulation. The fluorescence intensity is quantitatively correlated to calcium ion level. Stimulants, such as high potassium buffer solution and norepinephrine solution are applied for the single living smooth muscle cell and the single living cardiomyocyte, respectively. After several seconds (60 sec and 120 sec for the single living smooth muscle cell and the single living cardiomyocyte, respectively), a stimulant solution was injected into the chamber to stimulate the cell. And the data acquisition was continued for 30 seconds and 600 seconds for high potassium buffer solution and norepinephrine solution, respectively.
Figure 3: Illustration of a functionalized nanoprobe which is inserted into a single living cell to monitor [ca2+]i change in the sub-plasma membrane microdomain.
Results and Discussion
Calcium Green–1Dextran, a sensitive and selective calcium ion indicator⁄dye, is able to emit fluorescence (a single fluorescence maximum at 530 nm with a quantum yield of 0.75) under an irradiation of 488 nm when it combines with free calcium ions. Its binding affinity (Kd) to calcium ion is as high as 190 nM. Compared with other calcium ion indicators, such as fura–2, indo–1 and fluo– 3 etc., changes in cytosolic calcium levels by using Calcium Green– 1Dextran can be accurately measured without having quenching effect and leaking from the cell [18].
As discussed by Wightman et al. [19] the amine derivative of Dextran was used in the conjugation of Calcium Green–1 Dextran to enable chemical attachment to Calcium Green–1. After the conjugation, there are still sufficient amines groups remain on the Dextran portion of Calcium Green–1Dextran, thus enabling a classic imine formation with the free aldehyde groups of the glutaraldehydes. Hence, after the step–by–step chemical functionalization of a silver–coated optical fiber–based nanoprobe with (3–aminopropyl) triethoxysilane and glutaraldehyde, Calcium Green–1Dextran was chemically immobilized on the distal end of the nanoprobe (Figure 1). Hence, Calcium Green–1Dextran was used and chemically immobilized on the distal end of a nanoprobe, showing excellent sensitivity in physiological range of intracellular calcium ion.
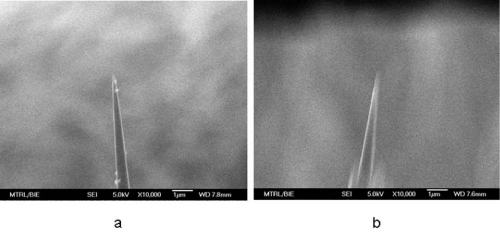
The morphology of nanoprobes was evaluated by using Field Emission Scanning Electron Microscopy (FESEM) after silver coating (Figure 4). As we previously reported, [17] the silver coating efficiently prevents light leakage thus allowing more light to be transmitted to the tip for fluorescence excitation. It was found that the tip diameter of a nanoprobe increased from 100 nm to 250 nm while retaining a smooth taper. The result was in a good agreement of our previous study. Since the diameter of the nanoprobes tip is significantly smaller than the wavelength of light that used to excite Calcium Green–1 Dextran, the photons cannot escape from the tip of the nanoprobe via conventional optics. Instead, after the photons travel as far down the nanoprobe as possible, the evanescent fields continue to travel through the remainder of the tip, providing excitation for Calcium Green–1 Dextran in the vicinity of the biosensing layer. The evanescent field provides a highly localized excitation area (distances > 0.5 λ). Thereby, the excitation of interfering fluorescent species within other locations of individual living cells could be hindered. In other words, the sensing of free calcium ion (bound to Calcium Green–1 Dextran) could be localized in individual living cells.
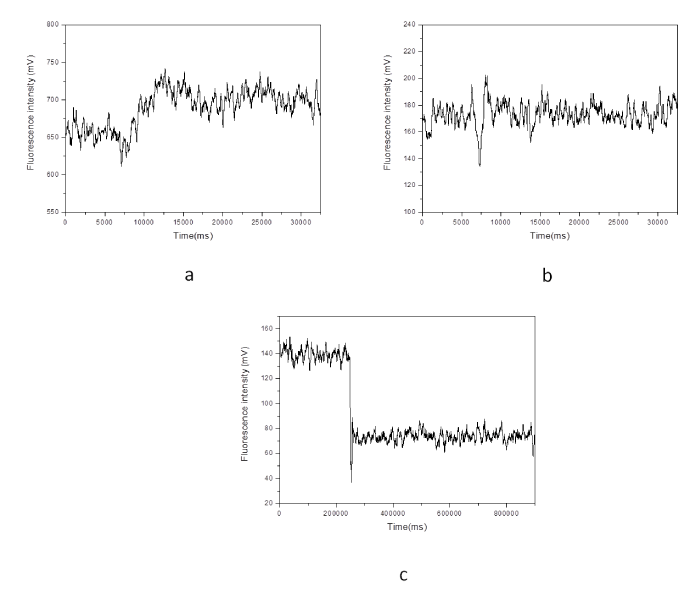
Figure 4: Field emission scanning electron micrographs of the nanoprobes. a) Bare nanotip with a diameter of 100 nm; b) diameter of the nanotip is about 250 nm after coating via a silver mirror reaction.In our study, stimulants such as high potassium buffer solution and norepinephrine solution were applied and their effects on the single living cell detection of [Ca2+]i changes in sub–plasma membrane microdomains by an optical fiber–based nanobiosensor were investigated. The control experiment, where a cell culture media containing no stimulate was applied, showed merely a fluorescence baseline. Elevation of concentrations of such stimulants has been proven to depolarize single cells thus stimulating voltage–dependent calcium channels. As can been seen in Figure 5, three types of cell response of a single living smooth muscle cell are represented: 1) upon the stimulation of high potassium buffer solution, the [Ca2+] i level increased and then remained at a high level (Figure 5A); 2) upon the stimulation of high potassium buffer solution, the [Ca2+]i level decreased and then quickly returned to its starting level (Figure 5B); 3) a third cell response was further observed where the [Ca2+] i level was dramatically decreased and then remained at a low level upon the stimulation of high potassium buffer solution (Figure 5C). These cell responses could be attributed to different sensing locations of nanoprobe within individual living cells. Their correspondingbiological and physiological mechanisms⁄functions are further under investigation.
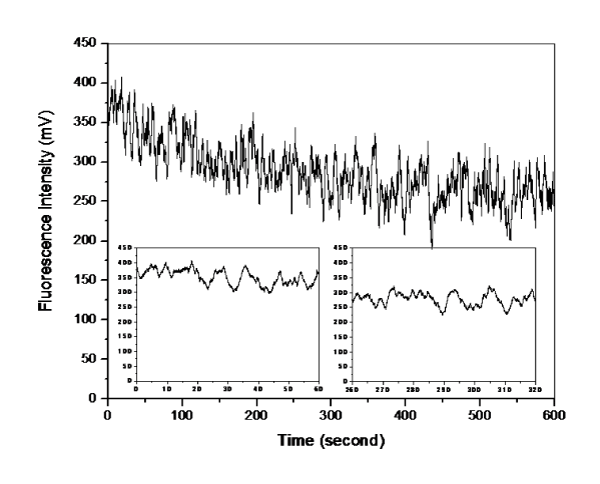
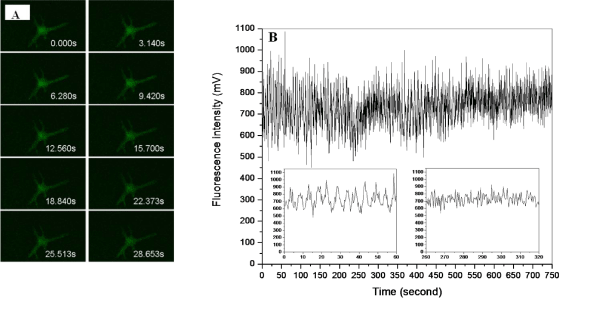
Figure 5: Effect of a stimulant (high potassium buffer solution) on the single living smooth muscle cell detection of [Ca2+]i changes in sub-plasma membrane microdomains by an optical fiber-based nanobiosensor. After 60 seconds, a high potassium buffer solution was injected into the chamber to stimulate the cell. The fluorescence intensity is quantitatively correlated to calcium ion level.Figure 5: Effect of a stimulant (high potassium buffer solution) on the single living smooth muscle cell detection of [Ca2+]i changes in sub-plasma membrane microdomains by an optical fiber-based nanobiosensor. After 60 seconds, a high potassium buffer solution was injected into the chamber to stimulate the cell. The fluorescence intensity is quantitatively correlated to calcium ion level.Figure 6 illustrates that upon the stimulation of norepinephrine solution, the [Ca2+]i level in sub–plasma membrane in a single living cardiomyocyte decreased slowly. It is worth noting that the width of signal peaks becomes narrower and more peaks (per unit) are generated after the stimulation. A parallel measurement was further conducted by a LSM510 confocal microscope to evaluate the fluorescence responses of a single living cardiomyocyte upon the stimulation of norepinephrine solution. As shown in Figure 7, with the stimulation of norepinephrine solution, narrower signal peaks and increased number of peak per unit are also observed. Since confocal microscope is an established system to reflect [Ca2+]i changes in sub–plasma membrane microdomains in a single living cell, the above agreement suggests that the resulting optical fiberbased nanobiosensor worked properly for the detection of [Ca2+]i changes in sub–plasma membrane microdomains in a single living cell. However, unlike confocal microscope, the response time of constructed optical fiber–based nanobiosensor was improved enormously from 3 data per second to 100 data per seconds. As a result, the detection of transient elementary calcium signaling events associated with calcium microdomains, which is challenging, could be achieved.
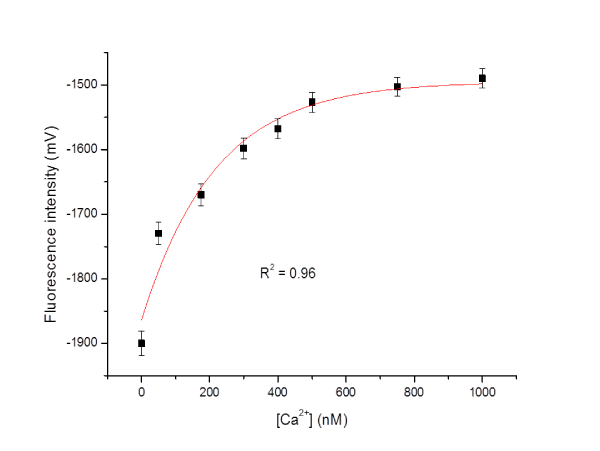
Figure 6: Effect of a stimulant (norepinephrine solution) on the single living cardiomyocyte detection of [Ca2+]i changes in sub-plasma membrane microdomains by an optical fiber-based nanobiosensor. After 120 seconds, a high potassium buffer solution was injected into the chamber to stimulate the cell. The fluorescence intensity is quantitatively correlated to calcium ion level.Figure 6: Effect of a stimulant (norepinephrine solution) on the single living cardiomyocyte detection of [Ca2+]i changes in sub-plasma membrane microdomains by an optical fiber-based nanobiosensor. After 120 seconds, a high potassium buffer solution was injected into the chamber to stimulate the cell. The fluorescence intensity is quantitatively correlated to calcium ion level.Figure 7: Effect of a stimulant (norepinephrine solution) on the single living cardiomyocyte detection of [Ca2+]i changes in sub-plasma membrane microdomains by a LSM510 Confocal Microscope. The fluorescence intensity is quantitatively correlated to calcium ion level. A) Recorded fluorescence images of single living cardiomyocyte at different intervals upon the stimulation of norepinephrine solution; B) Recorded fluorescence responses of single living cardiomyocyte upon the stimulation of norepinephrine solution.Figure 7: Effect of a stimulant (norepinephrine solution) on the single living cardiomyocyte detection of [Ca2+]i changes in sub-plasma membrane microdomains by a LSM510 Confocal Microscope. The fluorescence intensity is quantitatively correlated to calcium ion level. A) Recorded fluorescence images of single living cardiomyocyte at different intervals upon the stimulation of norepinephrine solution; B) Recorded fluorescence responses of single living cardiomyocyte upon the stimulation of norepinephrine solution.Furthermore, the detection of a range of calcium concentrations inside a single living smooth muscle cell was conducted referring to the method reported by H. Clark et al [20]. The constructed nanobiosensor was found capable of detecting ultra–low intracellular calcium concentrations within the nanomolar range (Figure 8), which is around the physiological level of free cytosolic calcium in a single living cell.
Figure 8: Detection of calcium concentrations ([Ca2+]) inside a single living smooth muscle cell.Figure 8: Detection of calcium concentrations ([Ca2+]) inside a single living smooth muscle cell.The stability of fabricated and functionalized nanoprobes with Calcium Green–1 Dextran was further evaluated. The nanoprobes were stored at 4 °C and their sensitivity was determined every 7 days until 35 days. It was found that the nanoprobes are stable for up to 28 days while retaining good sensitivity towards calcium ion.
Conclusion
In conclusion, in this paper we report the first example of detection of [Ca2+]i changes in sub–plasma membrane microdomains in a single living cell by using an optical fiber–based nanobiosensor. The nanoprobe was successfully fabricated by coating silver and then immobilizing Calcium Green–1Dextran, a calcium sensitive dye, on its distal end. The constructed nanobiosensor was capable of detecting ultra–low and local intracellular calcium concentrations in the nanomolar range which is around the physiological level of free cytosolic calcium in a single living cell. The response time was less than milliseconds enabling the detection of transient elementary calcium signaling events associated with calcium ion microdomains. The effects of stimulants such as high potassium buffer solution and norepinephrine solution were also investigated. The nanoprobes are stable for up to 28 days while retaining good sensitivity towards calcium ion.
Nanotechnology has already found its way into the applications in healthcare by helping to understand many diseases on molecular and cellular level, leading to new insights in diagnostics and therapy. Thus the resulting system could greatly facilitate the development of an advanced nanodiagnostic platform for in vivo and real–time sensing⁄ diagnosing [Ca2+]i at the single cell level. It could further offer a novel modality that holds promise to map out the architecture of calcium signal dynamics and also unravel calcium signaling mechanisms in diverse cellular processes.
However, it is still an early stage to finally achieve a robust and complete sensing⁄diagnosing system. Further investigations are still being undertaken within our group in terms of probe localization, sensor selectivity and reproducibility, etc. Where appropriate, the statistical methods and mathematical models with the aid of computational software will be employed for the calibrations.
Acknowledgement
The authors thank the Research Directorate–General of European Commission for supporting this work under the framework of EU FP7 Program Marie Curie Actions (PIIF–2009–254957).
References
- Berridge MJ, Bootman MD, Lipp P. Calcium--a life and death signal. Nature. 1998; 395: 645-648.
- MacLennan DH. Ca2+ signalling and muscle disease. Eur J Biochem. 2000; 267: 5291-5297.
- Clapham DE. Calcium signaling. Cell. 1995; 80: 259-268.
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006; 86: 369-408.
- Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006; 40: 405-412.
- Pinton P, Rimessi A, Romagnoli A, Prandini A, Rizzuto R. Biosensors for the detection of calcium and pH. Methods Cell Biol. 2007; 80: 297-325.
- Váradi A, Rutter GA. Green Fluorescent Protein Calcium Biosensors. Methods in Molecular Biology. Green Fluorescent Protein: Applications and Protocols. Hicks BW, editor.©Humana Press Inc, Totowa, NJ. 2002; 183: 255-264.
- Mank M, Reiff DF, Heim N, Friedrich MW, Borst A, Griesbeck O. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys J. 2006; 90: 1790-1796.
- Rai M, Gade A, Gaikwad S, Marcato PD, Durán N. Biomedical applications of nanobiosensors: the state-of-the-art. J. Braz. Chem. Soc. 2012; 23: 14-24.
- Bellan LM, Wu D, Langer RS. Current trends in nanobiosensor technology. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011; 3: 229-246.
- Zamaleeva AI, Collot M, Bahembera E, Tisseyre C, Rostaing P, Yakovlev AV, et al. Cell-Penetrating Nanobiosensors for Pointillistic Intracellular Ca2+-Transient Detection. Nano Lett. 2014.
- Tan W, Shi ZY, Smith S, Birnbaum D, Kopelman R. Submicrometer intracellular chemical optical fiber sensors. Science. 1992; 258: 778-781.
- Song JM, Kasili PM, Griffin GD, Vo-Dinh T. Detection of cytochrome C in a single cell using an optical nanobiosensor. Anal Chem. 2004; 76: 2591-2594.
- Kasili PM, Song JM, Vo-Dinh T. Optical sensor for the detection of caspase-9 activity in a single cell. J Am Chem Soc. 2004; 126: 2799-2806.
- Zheng XT, Yang HB, Li CM. Optical Detection of Single Cell Lactate Release for Cancer Metabolic Analysis. Anal. Chem. 2010; 82: 5082-5087.
- Zheng XT, Li CM. Single living cell detection of telomerase over-expression for cancer detection by an optical fiber nanobiosensor. Biosens Bioelectron. 2010; 25: 1548-1552.
- Wang SQ, Zhao H, Chen ZH, Wang Y, Li CM. Fabrication of silver coated scanning near field optical microscope probes by silver mirror reaction. Applied Physics B: Lasers and optics. 2008; 92: 49-52.
- Lee SK, Lee JY, Lee MY, Chung SM, Chung JH. Advantages of calcium green-1 over other fluorescent dyes in measuring cytosolic calcium in platelets. Anal Biochem. 1999; 273: 186-191.
- Xin Q, Wightman RM. Simultaneous detection of catecholamine exocytosis and Ca2+ release from single bovine chromaffin cells using a dual microsensor. Anal Chem. 1998; 70: 1677-1681.
- Clark HA, Kopelman R, Tjalkens R, Philbert MA. Optical nanosensors for chemical analysis inside single living cells. Anal. Chem. 1999; 71: 4837-4843.