Editorial
Austin J Nanomed Nanotechnol. 2014;2(5): 1030.
Mechanisms Behind Plasmonic Enhancement of Photocurrent in Metal Oxides
Ying-Chih Pu and Jin Z Zhang*
Department of Chemistry and Biochemistry, University of California, USA
*Corresponding author: Jin Z Zhang, Department of Chemistry and Biochemistry, University of California, Santa Cruz, CA95064, USA
Received: August 10, 2014; Accepted: August 12, 2014; Published: August 13, 2014
Abstract
Surface plasmon resonance (SPR) of metal nanostructures has been proposed as a promising strategy to enhance the photoactivity of metal oxides for photovoltaic and photoelectrochemical applications. While several studies have suggested that metal nanostructures could act as photosensitizers to improve the photocurrent of metal oxides, the mechanism behind is still not well established. Here we briefly review some of the recent research progress and discuss several possible mechanisms underlying the observed plasmonic enhancement of photocurrent in metal-metal oxide systems.
Introduction
Photoelectrical conversion is a promising and environmentally friendly technology to solve faced energy crisis because sunlight delivers a power of 106 TW/year continuously [1,2]. In photoelectrical conversion, semiconductors play an important role in capturing light to generate electron-hole pairs for subsequent electricity or chemical fuel production [3]. For instance, since Fujishima and Honda demonstrated the photocatalytic water splitting using anTiO2 photoanode in a photoelectrochemical (PEC) cell [4], an enormous amount of work has been carried out in order to achieve photoassisted water splitting and develop sustainable methods for producing chemical fuel [5-10]. Metal oxide (MO) semiconductors afford advantages of low cost, high stability, and suitable oxidation potential for water oxidation. However, the limitation of most MOs is the intrinsically wide bandgap, resulting in poor ability to capture solar energy in the visible and near-infrared (NIR) regions. It is thus highly desired to develop MOs with small bandgaps or strategies for sensitizing MOs with large bandgaps so they can respond effectively to sunlight for enhancing solar energy conversion.
Surface plasmon resonance (SPR) is an unique optical property of noble metal nanoparticles (NPs) such as Au, Ag and Cu, and it arises from collective coherent oscillation of electrons in the NPs induced by light irradiation [11]. The SPR oscillation frequency is highly sensitive to the size and shape of the metal NPs as well as the dielectric constant of the surrounding environment, which often manifests as strong absorption in the visible and NIR regions [12,13]. One interesting question is: can metal NPs with strong SPR in the visible sensitize MO semiconductors for enhancing photoelectrical conversion applications?
To answer this question, a number of studies have been carried out with focus on interface charge transfer [14,15] as well as photoelectron conversion using plasmonic metal-MO nano hetero structures [16-19]. Several studies suggested that some of the hot electrons from SPR excitation can participate in chemical reactions. For example, Au-MO or Ag-MO nanocomposites have been proposed as visible light driven photocatalysts to degrade pollutant [20-23]. Likewise, plasmonic metal NPs have been demonstrated to sensitize MOs to generate photocurrent in photovoltaic (PV) cells [24-26].
Incident-photon-to-current efficiency (IPCE) of optimized Au-TiO2 and electrolyte based PV as high as 26% under SPR excitation was reported [24]. In addition, a solid-state plasmonic solar cell fabricated using metal NPs, TiO2 and hole conductor Spiro-OMeTAD showed photocurrent density of ~2.5 mA/cm2 as compared to dye sensitized solid-state solar cell (0.5 mA/cm2) under high intensity visible LED illumination [26]. On the other hand, the same effect of photosensitization of MOs using metal NPs based on hot electron injection has also been proposed for application in PECs [27-29]. Lee et al. reported that the plasmonic photoanode made by a dense array of Au nanorods capped with TiO2 could generate photocurrent and produce H2 under visible light illumination [29]. Fuel production efficiency is up to 20-fold higher in the visible region than in the UV region. In another study, plasmonic Au NPs were found to play dual roles in enhancing the efficiency of CdS-Au-TiO2 PEC for hydrogen generation [30]. As the incident light shorter than 525 nm, Au NP served as an electron relay to facilitate the charge transfer between CdS and TiO2. For wavelength from 525 nm - 725 nm, the Au NP acted as a photosensitizer to improve solar-to-hydrogen conversion. Likewise, Au-decorated TiO2 nanowire (NW) electrodes for PEC water oxidation have been investigated recently [31]. Enhancement in photocurrent was observed in the entire UV-Vis region from 300 to 800 nm by controlling the cover density and shape of the decorating Au nanostructures including both Au NPs and nanorods (NRs). Although the most improved photoactivity of TiO2 was found in the UV region, which is attributable to effective surface states passivation by Au NPs, the enhancement in visible region of TiO2 photoelectrodes matched well the SPR absorption of Au NPs and Au NRs. The mixture of Au NPs and NRs on TiO2 was able to enhance the photocurrent in the entire UV-Vis region from 300 to 800 nm, even though the efficiency of photoelectron conversion efficiency was very low, just ~0.01-0.02% in IPCE measurements.
The suggestion of metal NP-sensitized MOs with high IPCE in PV is at first somewhat surprising since the lifetime of hot electrons in Au and Ag NPs are usually very short, only 1~2 ps [32]. Thus, for photosensitization or hot electron transfer to be effective, the charge transfer rate has to be much faster than 1-2 ps. Indeed, plasmon-induced electron transfer from Au, to TiO2 was reported to be 240 fs based on transient absorption spectroscopy with an IR probe [14]. Similarly, the half-lifetime of intra-band signal of hot electron from Au to CdS nanorod by SPR excitation was recently demonstrated to be 1.83 ± 0.22 ps [33]. While ultrafast charge transfer seems to be possible, its branching ratio or quantum yield with respect to other hot electron relaxation processes, such as photothermal conversion through electron-phonon coupling, is often not determined unambiguously. We have investigated the charge carrier dynamics in Au NP-decorated Zn OMWs by ultrafast transient pump-probe spectroscopy with the attempt to determine if there is direct evidence for hot electron injection from Au, to ZnO [34]. The observed hot electron lifetime of Au NP on ZnO NWs (3.2±0.5 ps) did not change noticeably from that of Au NPs alone. Moreover, the exciton lifetime in ZnO is essentially the same with and without Au decoration. The results indicate no charge transfer between ZnO NW and Au NPs or weak interaction between them. Based on the ultrafast study of hot electron lifetime in ZnO, we estimate that the quantum yield of hot electron injection is no more than 10%, based on the uncertainty of our measurement. This is consistent with the low photo current observed in our Au-TiO2 work that also suggests a very low yield of hot electron injection, if injection is indeed responsible for the photocurrent observed in the visible [31]. Our results seem to be in interesting contrast to the much higher IPCE reported by others, as discussed earlier, which suggest that other possible enhancement mechanisms may be involved when high IPCE is observed or the IPCE may be very sensitive to the detailed experimental conditions that can easily differ for different systems studied.
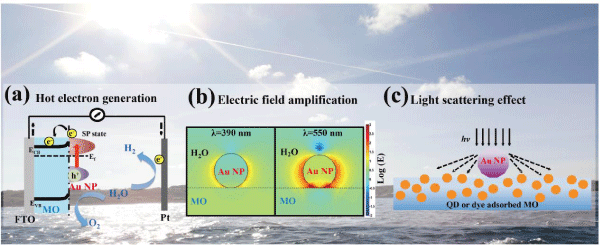
In the hot electron generation mechanism, metal nanostructures serve as photosensitizers, similar to dyes or quantum dots (QDs), that generates high energy or hot electrons with enough energy to overcome the Schottky barrier upon SPR excitation and be injected into the conduction band of MO semiconductors (Figure 1a). Hot electron injection from metal to MOs will likely occur when the metal and MOs are strongly coupled. SPR excited Au nanostructures have been found to generate hot electrons in Au-ZnO photoanode under visible light irradiation based on in-situ X-ray absorption measurements [35]. As mentioned above, based on the very short lifetime of hot electrons, the probability of electron injection should be low if possible at all. Several studies have focused on improving the hot electron injection efficiency of plasmonic metal-MO systems by optimizing the detailed structures of MOs. For instance, three-dimensional (3D) hierarchical MO nanostructures have been designed and demonstrated to exhibit not only high interfaces between metal, MO and electrolyte, but also strong light harvesting in improving the performance of PEC performance [36,37]. The plasmonic metal combined with geometric 3D-MO nanostructures showed high photoactivity in the visible region, with photocurrent density ~0.125 mA/cm2 (0.5 V vs. Ag/AgCl) and ~0.4 mA/cm2 (1 V vs. reversible hydrogen electrode or RHE, IPCE = 5.5 % at 550 nm) in dendritic Au-TiO2nanorods and Au-ZnO branched nanowires, respectively [36,37]. Similarly, the combined 3D MO structures or photonic crystals with plasmonic metal NP-MO systems were claimed to generate hot electrons for enhancing PEC performance [38-40].
Figure 1: Three possible mechanisms for enhancing photoelectric conversion in metal/MO systems: (a) hot electron generation, (b) interfacial electric field amplification, (c) light scattering effect.
In principle, besides hot electron injection, there are other possible mechanisms that may be responsible for enhancing photoelectric conversion of MOs by metal nanostructures. For example, metal nanostructures may increase photocurrent by local electric field enhancement. SPR of metal NPs could amplify the electric field near semiconductor surfaces, resulting in an increase of the light absorption rate by the semiconductor (Figure 1b). The enhancement should be observed only when the SPR of the metal spectrally overlaps with the absorption of the semiconductor. For instance, an enhancement factor of 3~4 around 600~700 nm was reported in the case of hematite coated on Au nanopillars [41,42]. Similarly, the efficiency of an N719 dye-sensitized solar cell (DSSC) was enhanced from 9.3% to 10.2% when SiO2-capped Au NPs were used as a localized electric field amplifier [43]. The increased absorption of dyes by SPR resulted in the improved photocurrent and IPCE of DSSC in the visible region. In related studies, it was suggested that the negative shift of Fermi level in the Au/TiO2 nanocomposite could increase open-circuit voltage of DSSC or improve charge separation in PEC [44]. On the other hand, the band edge absorption of most wide bandgap MOs could not overlap with the SPR of metals, such as TiO2, ZnO and SnO2, while the enhancements in PEC cells were still observed after metal NP decoration. For example, the performance of Au-deposited TiO2 films under visible light illumination for PEC water splitting was enhanced around 66-foldat 633 nm [45]. The proposed enhancement mechanism in this study was that the electrical field of SPR could amplify the contribution of absorption due to defect and impurity state within the bandgap.
In addition, metal nanostructures can serve as light scattering centers to increase the average photon path length, resulting in improved absorption and electron-hole pair formation in semiconductors (Figure 1c) [46,47]. For example, enhanced coupling of light into semiconductor thin films by scattering from plasmonic metal nanoparticle arrays has been demonstrated [48]. Within corporation of Au, Ag or Cu dense nanoparticle arrays into Si-on-insulator photodetector, a 20-fold increase in photocurrent was observed. Likewise, light scattering due to plasmonic metal nanostructures was also observed for single-crystalline Si [49], amorphous Si [50] and GaAs [51] solar cells [52]. The scattering effect is expected to become more important for larger sized metal NPs. For instance, nanorods, nanowires, and aggregates tend to exhibit stronger scattering effect. Since light scattering can occur from far field, it can happen when the metal NPs are not near or strongly coupled with the semiconductor MOs. For example, in a study of CdSe QD-sensitized Au/TiO2 hybrid mesoporous electrode for PEC applications [53], it was found that without CdSe QDs, the photocurrent of Au/TiO2was lower than that of TiO2 in the UV region, suggesting trapping of electrons photogenerated in TiO2 by Au NPs, which is expected. However, the combination of Au NPs, CdSe QDs and TiO2 nanostructures showed substantial increase in photocurrent due to Au NPs in the visible region (350-600 nm). The increase was stronger with higher Au NP loading and at shorter wavelength. The enhancement was attributed to enhanced absorption of CdSe QDs due to increased light scattering by Au NPs. The same mechanism may also be responsible for the enhancement photocurrent observed for TiO2 with Au NPs and NRs we reported recently, where the TiO2 bandgap state absorption may be enhanced due to scattering of the Au NPs and/or NRs [31].
In summary, the possibility of photosensitization of MOs using plasmonic metal NPs is tantalizing. While there is increasing evidence for hot electron injection, there is no general consensus to date. Further research is necessary to more firmly establish the possibility. In addition, better determination of the quantum yield or branching ratio for photosensitization with respect to other competing processes such as photothermal conversion needs to be made, which requires more quantitative studies. More work is also needed to better understand what factors determine the mechanism of operation. The strengthen of interaction between the metal NP and MOs is likely a critical factor, which may in turn depends on the detailed structures of both constituents down to the molecular or atomic level. Control and optimization of charge carrier dynamics at the interface of metal NPs and MOs will be very important for possible hot electron injection as well as other possible enhancement mechanisms such as local electric field amplification and light scattering. Both experimental and theoretical works will be needed before a complete understanding can be achieved and the full potential for application can be realized.
References
- Lewis NS. Toward cost-effective solar energy use. Science. 2007; 315: 798-801.
- Gray HB. Powering the planet with solar fuel. Nat Chem. 2009; 1: 7.
- Sivula K. Metal Oxide Photoelectrodes for Solar Fuel Production, Surface Traps, and Catalysis. The J Phy Chem Letters. 2013. 4: 1624-1633.
- Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972; 238: 37-38.
- Lin Y. Semiconductor nanostructure-based photoelectrochemical water splitting: A brief review. Chemical Physics Letters. 2011; 507: 209-215.
- Grätzel M. Photoelectrochemical cells. Nature. 2001; 414: 338-344.
- Chen HM, Chen CK, Liu RS, Zhang L, Zhang J, Wilkinson DP, et al. Nano-architecture and material designs for water splitting photoelectrodes. Chem Soc Rev. 2012; 41: 5654-5671.
- Liu C, Tang J, Chen HM, Liu B, Yang P. A fully integrated nanosystem of semiconductor nanowires for direct solar water splitting. Nano Lett. 2013; 13: 2989-2992.
- Gan J, Lu X, Tong Y. Towards highly efficient photoanodes: boosting sunlight-driven semiconductor nanomaterials for water oxidation. Nanoscale. 2014; 6: 7142-7164.
- Pu Y-C. Surface Passivation of TiO2 Nanowires Using a Facile Precursor-Treatment Approach for Photoelectrochemical Water Oxidation. The Journal of Physical Chemistry C. 2014; 118: 15086-15094.
- Kelly KL. The Optical Properties of Metal Nanoparticles-The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B. 2003; 107: 668-677.
- Chou CH, Chen CD, Wang CR. Highly efficient, wavelength-tunable, gold nanoparticle based optothermal nanoconvertors. J Phys Chem B. 2005; 109: 11135-11138.
- Zhang JZ. Biomedical Applications of Shape-Controlled Plasmonic Nanostructures: A Case Study of Hollow Gold Nanospheres for Photothermal Ablation Therapy of Cancer. J. Phys. Chem. Lett. 2010; 1: 686-695.
- Furube A, Du L, Hara K, Katoh R, Tachiya M. Ultrafast plasmon-induced electron transfer from gold nanodots into TiO2 nanoparticles. J Am Chem Soc. 2007; 129: 14852-14853.
- Du L. Ultrafast plasmon induced electron injection mechanism in gold-TiO2 nanoparticle system. Journal of Photochemistry and Photobiology C: Photochemistry Reviews. 2013; 15: 21-30.
- Li J, Cushing SK, Zheng P, Meng F, Chu D, Wu N, et al. Plasmon-induced photonic and energy-transfer enhancement of solar water splitting by a hematite nanorod array. Nat Commun. 2013; 4: 2651.
- Mubeen S, Lee J, Singh N, Krämer S, Stucky GD, Moskovits M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat Nanotechnol. 2013; 8: 247-251.
- Mubeen S, Lee J, Singh N, Krämer S, Stucky GD, Moskovits M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat Nanotechnol. 2013; 8: 247-251.
- Clavero C. Plasmon-induced hot-electron generation at nanoparticle-metal-oxide interfaces for photovoltaic and photocatalytic devices. Nature Photonics. 2014; 8: 95-103.
- Tian Y, Tatsuma T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J Am Chem Soc. 2005; 127: 7632-7637.
- Priebe JB, Karnahl M, Junge H, Beller M, Hollmann D, Brückner A, et al. Water reduction with visible light: synergy between optical transitions and electron transfer in Au-TiO(2) catalysts visualized by in situ EPR spectroscopy. Angew Chem Int Ed Engl. 2013; 52: 11420-11424.
- Chen K-H. Ag-Nanoparticle-Decorated SiO2Nanospheres Exhibiting Remarkable Plasmon-Mediated Photocatalytic Properties. The Journal of Physical Chemistry C. 2012; 116: 19039-19045.
- Kale MJ, Avanesian T, Christopher P. Direct Photocatalysis by Plasmonic Nanostructures. ACS Catalysis. 2014; 4: 116-128.
- Tian Y, Tatsuma T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J Am Chem Soc. 2005; 127: 7632-7637.
- Su Y-H. Surface plasmon resonance of layer-by-layer gold nanoparticles induced photoelectric current in environmentally-friendly plasmon-sensitized solar cell. Light: Science & Applications. 2012; 1: e14.
- Reineck P, Lee GP, Brick D, Karg M, Mulvaney P, Bach U, et al. A solid-state plasmonic solar cell via metal nanoparticle self-assembly. Adv Mater. 2012; 24: 4750-4755, 4729.
- Ingram DB, Linic S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J Am Chem Soc. 2011; 133: 5202-5205.
- Thimsen E, Le Formal F, Grätzel M, Warren SC. Influence of plasmonic Au nanoparticles on the photoactivity of Fe₂O₃ electrodes for water splitting. Nano Lett. 2011; 11: 35-43.
- Lee J, Mubeen S, Ji X, Stucky GD, Moskovits M. Plasmonic photoanodes for solar water splitting with visible light. Nano Lett. 2012; 12: 5014-5019.
- Li J, Cushing SK, Zheng P, Senty T, Meng F, Bristow AD, et al. Solar hydrogen generation by a CdS-Au-TiO2 sandwich nanorod array enhanced with Au nanoparticle as electron relay and plasmonic photosensitizer. J Am Chem Soc. 2014; 136: 8438-8449.
- Pu YC, Wang G, Chang KD, Ling Y, Lin YK, Fitzmorris BC, et al. Au nanostructure-decorated TiO2 nanowires exhibiting photoactivity across entire UV-visible region for photoelectrochemical water splitting. Nano Lett. 2013; 13: 3817-3823.
- Zhang JZ. Ultrafast Studies of Electron Dynamics in Semiconductor and Metal Colloidal Nanoparticles: Effects of Size and Surface. Acc. Chem. Res. 1997; 30: 423-429.
- Wu K, Rodríguez-Córdoba WE, Yang Y, Lian T. Plasmon-induced hot electron transfer from the Au tip to CdS rod in CdS-Au nanoheterostructures. Nano Lett. 2013; 13: 5255-5263.
- Tachibana Y. Ultrafast charge carrier dynamics and photoelectrochemical properties of ZnO nanowires decorated with Au nanoparticles. 2011; 8109: 81090M-81090M-10.
- Chen HM, Chen CK, Chen CJ, Cheng LC, Wu PC, Cheng BH, et al. Plasmon inducing effects for enhanced photoelectrochemical water splitting: X-ray absorption approach to electronic structures. ACS Nano. 2012; 6: 7362-7372.
- Su F, Wang T, Lv R, Zhang J, Zhang P, Lu J. Dendritic Au/TiO₂ nanorod arrays for visible-light driven photoelectrochemical water splitting. Nanoscale. 2013; 5: 9001-9009.
- Zhang X, Liu Y, Kang Z. 3D branched ZnO nanowire arrays decorated with plasmonic au nanoparticles for high-performance photoelectrochemical water splitting. ACS Appl Mater Interfaces. 2014; 6: 4480-4489.
- Kim K, Thiyagarajan P, Ahn HJ, Kim SI, Jang JH. Optimization for visible light photocatalytic water splitting: gold-coated and surface-textured TiO2 inverse opal nano-networks. Nanoscale. 2013; 5: 6254-6260.
- Zhang Z, Zhang L, Hedhili MN, Zhang H, Wang P. Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett. 2013; 13: 14-20.
- Zhang X. Coupling surface plasmon resonance of gold nanoparticles with slow-photon-effect of TiO2 photonic crystals for synergistically enhanced photoelectrochemical water splitting. Energy & Environmental Science. 2014; 7: 1409-1419.
- Gao H, Liu C, Jeong HE, Yang P. Plasmon-enhanced photocatalytic activity of iron oxide on gold nanopillars. ACS Nano. 2012; 6: 234-240.
- Thomann I, Pinaud BA, Chen Z, Clemens BM, Jaramillo TF, Brongersma ML, et al. Plasmon enhanced solar-to-fuel energy conversion. Nano Lett. 2011; 11: 3440-3446.
- Choi H, Chen WT, Kamat PV. Know thy nano neighbor. Plasmonic versus electron charging effects of metal nanoparticles in dye-sensitized solar cells. ACS Nano. 2012; 6: 4418-4427.
- Chandrasekhara N, Kamat PV. Improving the Photoelectrochemical Performance of Nanostructured TiO2 Films by Adsorption of Gold Nanoparticles. J. Phys. Chem. B. 2000; 104: 10851-10857.
- Liu Z, Hou W, Pavaskar P, Aykol M, Cronin SB. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 2011; 11: 1111-1116.
- Linic S, Christopher P, Ingram DB. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat Mater. 2011; 10: 911-921.
- Evanoff DD Jr, Chumanov G. Synthesis and optical properties of silver nanoparticles and arrays. Chemphyschem. 2005; 6: 1221-1231.
- Stuart HR, Hall DG. Absorption enhancement in silicon-on-insulator waveguides using metal island films. Applied Physics Letters. 1996; 69: 2327.
- Schaadt DM, Feng B, Yu ET. Enhanced semiconductor optical absorption via surface plasmon excitation in metal nanoparticles. Applied Physics Letters. 2005; 86: 063106.
- Derkacs D. Improved performance of amorphous silicon solar cells via scattering from surface plasmon polaritons in nearby metallic nanoparticles. Applied Physics Letters. 2006; 89: 093103.
- Nakayama K, Tanabe K, Atwater HA. Plasmonic nanoparticle enhanced light absorption in GaAs solar cells. Applied Physics Letters. 2008; 93: 121904.
- Atwater HA, Polman A. Plasmonics for improved photovoltaic devices. Nat Mater. 2010; 9: 205-213.
- Liu L. CdSe quantum dot-sensitized Au/TiO2 hybrid mesoporous films and their enhanced photoelectrochemical performance. Nano Research. 2010; 4: 249-258.