
Editorial
Austin J Nanomed Nanotechnol. 2016; 4(1): 1042.
Nanotechnology- A Promising Approach for Suicide Gene Therapy
Kumar SU¹ and Gopinath P1,2*
¹Centre for Nanotechnology, Indian Institute of Technology Roorkee, India
²Department of Biotechnology, Indian Institute of Technology Roorkee, India
*Corresponding author: Gopinath P, Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee, Uttarakhand, India
Received: April 11, 2016; Accepted: April 13, 2016; Published: April 14, 2016
Editorial
Cancer is one of the world’s most dreadful diseases and the battle against cancer continues till date [1]. Suicide gene therapy for cancer is one of the best approaches for annihilation of cancer [2]. In brief, suicide gene codes for an enzyme which converts a nontoxic prodrug into toxic metabolites and subsequently mediates death of host cells itself on account of which it is named “suicide” gene therapy [3]. These suicide gene when constitutively expressed by the cells not only mediates death of host cells but also inflicts strong bystander effects on neighboring cells by predisposing them to toxic downstream metabolites. Due to such advantages, they manifest minimal systemic toxicity and are also effective against many drug resistance cancer cells. Among all existing suicide genes, Cytosine Deaminase (CD) and Herpes Simplex Virus-thymidine kinase (HSVtk) have shown promising results initially and has been investigated extensively since long. The HSVtk enzyme initially phosphorylates the prodrug Ganciclovir (GCV) to its monophosphate form, which is subsequently phosphorylated again by endogenous cellular kinase to generate nucleotide analogs (di- and triphosphate forms of GVC). Triphosphate form of GCV is then readily incorporated into DNA during the course of DNA synthesis and acts as a chain terminator to prevent further DNA synthesis, which ultimately induces cell death [4].
The therapeutic efficacy of HSVtk suicide gene therapy is often limited by cell-to-cell contact which is a prerequisite for transport of downstream metabolic byproducts of ganciclovir to neighboring cells so as to attain bystander-killing effect. As an outcome of such drawbacks, HSVtk suicide gene does not seem to be effective against different cell types [5]. In contrary to this, Cytosine Deaminase (CD) efficiently converts prodrug 5-Fluorocytosine (5- FC) into therapeutically active anticancer agent 5-Fluorouracil (5- FU), which subsequently permeates across the cell membrane to mediate bystander killing effects on adjacent neighboring cells [6]. Thus, 5-FC/CD system attains suicide gene therapy much more efficiently as compared to other counterparts. Although 5-FC/CD system attains better therapeutic outcomes, it is ineffective against 5-FC resistant cancer cells and thus its anticancer potential could not be generalized for all cancer types. In order to overcome such drawback, Gopinath et al. have designed Cytosine Deaminase-Uracil Phosphoribosyltransferase (CD-UPRT) bifunctional suicide gene construct in which Uracil Phosphoribosyltransferase (UPRT) acts upon product of CD i.e. 5-FU and converts it further into other toxic metabolites [7].
The therapeutic effect of suicide genes can be enhanced by combinatorial approaches. In combination therapy, two or more drugs with similar or different mode of action are employed to realize synergistic anticancer therapeutic potentials. Such synergistic anticancer potential of combination of radiation therapy and 5-FC/ CD plus UPRT gene therapy was demonstrated by Kambara et al. against malignant gliomas [8]. Apart from this, the combination therapy also provides scope for exploiting radio sensitizing properties of 5-FU and by stander effects during the course of treatment [9- 11]. Many research groups have reported the use of suicide gene in combination with chemotherapy and radiation to enhance the therapeutic effect and to overcome the drug resistance. Gopinath et al. were the first to report the applications of silver nanoparticles for synergizing the therapeutic effect of suicide gene [12]. They have also reported the synergistic therapeutic effect of suicide gene with anticancer drug curcumin. One of the major challenging tasks in suicide gene therapy is lack of suitable vectors for targeted delivery of suicide gene to cancer cells. The application of such DNAbased therapeutics is largely limited due to poor cellular uptake, degradation by serum nucleases and rapid renal clearance following systemic administration. In addition to these, organ specific targeted DNA therapy has been a major challenge to overcome off-target gene therapy. In order to circumvent these limitations, numerous organ specific targeted nanocarriers have been developed recently for systemic administration.
With the advent of nanotechnology, numerous nanomaterials have found promising application in health care industry [13,14]. Such nanomaterials have revolutionized cancer diagnosis and therapy and tissue engineering etc. In the recent past, several researchers developed variety of nanomaterials with high gene transfection efficiency and low toxicity (Figure 1). These nanoparticles can be targeted to cancer by passive targeting and active targeting. Passive targeting can be achieved by using polymeric nanoparticles which are known to accumulate at tumor site due to Enhanced Permeation and Retention (EPR) effect, which results in passive accumulation in solid tumor tissues. Other major advantages of polymeric nanoparticles are biodegradability and biocompatibility, and prolonged circulation time in the bloodstream. Active targeting can be achieved by incorporating tumor specific antibodies or peptides.
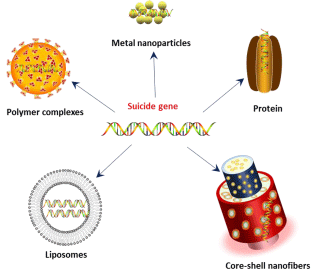
Figure 1: Different nano-carriers used for suicide gene therapy.
Only few studies have been carried out till date for delivering suicide genes using nanoparticles. Aoi et al. transduced HSVtk gene in to cancer cells using nanobubbles and ultra sound [15]. Hattori and Maitani developed a folate-linked nanoparticle for targeted delivery of HSVtk gene in to human prostate cancer and nasopharyngeal cancer cells for in vitro and in vivo suicide gene therapy [16]. Yu et al. developed poly(ethylene-glycol)-poly(γ- benzyl-L-glutamate) (PEG-PBLG) based nanocarrier for delivering HSVtk gene to Oral Squamous Cell Carcinoma (OSCC) cells and studied the therapeutic effect both in vitro and in vivo [17]. Recently, Yuan et al. used magnetic nanoparticles for the targeted delivery of suicide genes to cancer cells [18]. They have combined suicide gene therapy and magnetic hyperthermia methodology to treat cancer. Multifunctional nanoparticles hold great promise for suicide gene therapy as it can deliver suicide gene along with imaging probe for simultaneous diagnosis and therapy of cancer. Nanoparticles with such dual functions are named as theranostic nanoparticles. Sanpui et al. synthesized chitosan stabilized ZnS: Mn2+ QDs as a nanocarrier for delivery of CD-UPRT suicide gene which could be a promising approach for real-time monitoring of gene delivery [19]. Jaiswal et al. synthesized folic acid conjugated chitosan based theranostic nanocarriers for simultaneous delivery of ZnS QDs and CD-UPRT suicide gene for imaging and therapy, respectively [20]. Sahoo et al. synthesised multicolor fluorescent emitting gold nanoclusters for CD-UPRT suicide gene delivery [21]. As these metal nanoparticles are often associated with cytotoxicity, their long term application is very limited. Recently, Chen et al. transfected HSVtk gene in to prostate cancer cells using generation 5 poly (amidoamine) dendrimers as a polymeric nanocarrier [22]. The use of biofriendly polymeric nano carriers for targeted delivery of suicide gene and imaging agents could also be a promising approach for suicide gene therapy. Development of such nanomaterials has enormous potential applications and implications in cancer theranostics (diagnosis and therapy).
Serum albumin has become a promising carrier for diverse therapeutic molecules due to its biocompatibility and low immunogenicity [23,24]. In this quest, cationized albumin has been used as non-viral vector for gene delivery. The presence of cations over albumin enables stable polyplex formation with plasmid DNA by spontaneous self-assembly process and which subsequently attains efficient transfection when supplemented with chloroquine mediated endosomal escape [25]. Similarly, Faneca et al. reported use of albumin associated cationic liposomes for delivery of HSVtk/GCV suicide gene and consequently reported their synergistic antitumoral effect with vinblastine [26]. As an alternative approach, Orson et al. have synthesized PEI-albumin conjugates for improved gene delivery [27]. In the recent past, apart from PEI, dendrimer with similar functional groups is also been sought as carriers for gene delivery [28]. The Generation 5 Poly (Amidoamine) Dendrimers (G5-PAMAM-D) was observed to double the efficiency of suicide gene therapy (HSVtk /GCV fused with Cx43) against human prostate cancer cells both in vitro and in vivo [29].
Apart from these carriers, polymeric scaffolds like electrospun nanofibers and gels are also explored for controlled and sustained gene therapy [30-34]. Although nanofibers versatility for gene therapy has been studied since long, their role in suicide gene delivery was not explored until recently [35]. In pursuit of this, Sukumar et al. have fabricated core-shell bPEI-PEO nanofibers for efficient transfection of suicide gene (Cytosine Deaminase-Uracil Phosphoribosyltransferase (CD::UPRT)) and also manifested subsequent time resolved delivery of prodrug(5-Fluorocytosine (5-FC)) [36]. Such composite scaffold for simultaneous delivery for suicide gene and prodrug could efficiently manifest by-stander effects of suicide gene and attained improved anticancer therapeutic potential. As an outcome of rapid development of such diverse nano-carriers for gene delivery, anticancer efficacy of suicide gene therapy has improved drastically.
Acknowledgement
Our sincere thanks to Science and Engineering Research Board (No. SR/FT/LS-57/2012), Faculty initiation grant (MHRD, IIT Roorkee) and Department of Biotechnology (No.BT/PR6804/ GBD/27/486/2012), Government of India, for the financial support.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61: 69-90.
- Duarte S, Carle G, Faneca H, De Lima MC, Pierrefite-Carle V. Suicide gene therapy in cancer: where do we stand now?. Cancer Lett. 2012; 324: 160- 170.
- Krohne TU, Shankara S, Geissler M, Roberts BL, Wands JR, Blum HE, et al. Mechanisms of cell death induced by suicide genes encoding purine nucleoside phosphorylase and thymidine kinase in human hepatocellular carcinoma cells In vitro. Hepatology. 2000; 34: 511-518.
- Furman PA, McGuirt PV, Keller PM, Fyfe JA, Elion GB. Inhibition by acyclovir of cell growth and DNA synthesis of cells biochemically transformed with herpes virus genetic information. Virology. 1980; 102: 420-430.
- Holder JW, Elmore E, Barrett JC. Gap junction function and cancer. Cancer Res. 1993; 53: 3475-3485.
- Rowley S, Lindauer M, Gebert JF, Haberkorn U, Oberdorfer F, Moebius U, et al . Cytosine deaminase gene as a potential tool for the genetic therapy of colorectal cancer. J Surg Oncol. 1996; 61: 42-48.
- Kanai F, Kawakami T, Hamada H, Sadata A, Yoshida Y, Tanaka, et al. Adenovirus-mediated transduction of Escherichia coli uracil phosphoribosyltransferase gene sensitizes cancer cells to low concentrations of 5-fluorouracil. Cancer Res. 1998; 58: 1946-1951.
- Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005; 65: 2832-2839.
- Kievit E, Nyati MK, Ng E, Stegman LD, Parsels J, Ross BD, et al. Yeast cytosine deaminase improves radiosensitization and bystander effect by 5-fluoro-cytosine of human colorectal cancer xenografts. Cancer Res. 2000; 60: 6649-6655.
- Lawrence TS, Rehemtulla A, Ng E, Wilson M, Trosko JE, Stetson PL. Preferential cytotoxicity of cells transduced with cytosine deaminase compared to bystander cells after treatment with 5-fluorocytosine. Cancer Res 1998; 58: 2588-2593.
- Hamstra AD, Rice JD, Fahmy S, Ross DB, Rehemtulla A. Enzyme/prodrug therapy for head and neck cancer using a catalytically superior cytosine deaminase Human Gene Therapy. 2004; 10: 1993-2003.
- Gopinath P, Gogoi SK, Chattopadhyay A, Ghosh SS. Implications of silver nanoparticle induced cell apoptosis for In vitro gene therapy. Nanotechnology. 2008; 19: 075104.
- Sukumar UK, Bhushan B, Dubey P, Matai I, Sachdev A, Packirisamy G. Emerging applications of nanoparticles for lung cancer diagnosis and therapy. Int Nano Lett. 2013; 3: 45.
- Baetke SC, Lammers T, Kiessling F. Applications of nanoparticles for diagnosis and therapy of cancer. Br J Radiol. 2015; 88: 20150207.
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 2008; 26: 101-106.
- Hattori Y, Maitani Y. Folate-linked nanoparticle-mediated suicide gene therapy in human prostate cancer and nasopharyngeal cancer with herpes simplex virus thymidine kinase. Cancer Gene Ther. 2005; 12: 796-809.
- Zhu GQ. Properties of a poly (ethylene glycol)-block-poly (?-benzyl L-glutamate)-graft-poly (ethylene glycol) copolymer membrane Chem. Pap. 2010; 64: 34-39.
- Yuan C, An Y, Zhang J, Li H, Zhang H, Wang L, et al. Magnetic nanoparticles for targeted therapeutic gene delivery and magnetic-inducing heating on hepatoma. Nanotechnology. 2014; 25: 345101.
- Sanpui P, Pandey SB, Chattopadhyay A, Ghosh SS. Incorporation of gene therapy vector in Chitosan stabilized Mn2+-doped ZnS Quantum. Mater Lett. 2010; 64: 2534-2537.
- Jaiswal A, Chattopadhyay A, Ghosh SS. Functional chitosan nanocarriers for potential applications in gene therapy. Mater Lett. 2012; 68: 261-264.
- Sahoo AK, Banerjee S, Ghosh SS, Chattopadhyay A. Simultaneous RGB emitting Au nanoclusters in chitosan nanoparticles for anticancer gene theranostics. ACS Appl Mater Interfaces. 2014; 6: 712-724.
- Chen Y, Wang G, Kong D, Zhang Z, Yang K, Liu R, et al . In vitro and in vivo double-enhanced suicide gene therapy mediated by generation 5 polyamidoamine dendrimers for PC-3 cell line. World J Surg Oncol. 2012; 10: 3.
- Bhushan B, Dubey P, Kumar SU, Sachdev A, Matai I. Gopinath P. Bionanotherapeutics: niclosamide encapsulated albumin nanoparticles as a novel drug delivery system for cancer therapy. RSC Adv. 2015; 5: 12078- 12086.
- Bhushan B, Gopinath P. Tumor-targeted folate-decorated albumin-stabilised silver nanoparticles induce apoptosis at low concentration in human breast cancer cells. RSC Adv. 2015; 5: 86242-86253.
- Fischer D, Bieber T, Brüsselbach S, Elsasser H, Kissel T. Cationized human serum albumin as a non-viral vector system for gene delivery? Characterization of complex formation with plasmid DNA and transfection efficiency. Int J Pharm. 2001; 225: 97-111.
- Faneca H, Faustino A, Pedroso de Lima MC. Synergistic antitumoral effect of vinblastine and HSV-Tk/GCV gene therapy mediated by albumin-associated cationic liposomes. J Control Release. 2008; 126: 175-184.
- Orson FM, Kinsey BM, Hua PJ, Bhogal BS, Densmore CL, Barry MA. Genetic immunization with lung-targeting macro aggregated polyethyleneiminealbumin conjugates elicits combined systemic and mucosal immune responses. J Immunol. 2000; 164: 6313-6321.
- Maruyama-Tabata H, Harada Y, Matsumura T, Satoh E, Cui F, Iwai M, et al. Effective suicide gene therapy in vivo by EBV-based plasmid vector coupled with polyamidoamine dendrimer. Gene Ther. 2000; 7: 53-60.
- Chen Y, Wang G, Kong D, Zhang Z, Yang K, Liu R, et al . In vitro and in vivo double-enhanced suicide gene therapy mediated by generation 5 polyamidoamine dendrimers for PC-3 cell line. World J Surg Oncol. 2012; 10: 3.
- Matai I, Sachdev A, Gopinath P. Self-Assembled hybrids of fluorescent carbon dots and PAMAM dendrimers for epirubicin delivery and intracellular imaging ACS Appl mater interfaces. 2015; 7: 11423-11435.
- L Dong, H Xu, Y-B Liu, B Lu, D-M Xu, B-H Li, et al. M-PEIs nanogels: potential nonviral vector for systemic plasmid delivery to tumor cells. Cancer Gene Therapy. 2009; 16: 561-566.
- Sachdev A, Matai I, Gopinath P. Carbon dots incorporated polymeric hydrogels as multifunctional platform for imaging and induction of apoptosis in lung cancer cells. Colloids Surf B Biointerfaces. 2016; 141: 242-252.
- Cao P, Sun X, Liang Y, Gao X, Li X, Li W, et al. Gene delivery by a cationic and thermosensitive nanogel promoted established tumor growth inhibition. Nanomedicine (Lond). 2015; 10: 1585-97.
- Kumar SU, Matai I, Dubey P, Bhushan B, Sachdev A, Gopinath P. Differentially cross-linkable core-shell nanofibers for tunable delivery of anticancer drugs: Synthesis, characterization and its anticancer efficacy. RSC Adv. 2014; 4: 38263-38272.
- Lee S, Jin G, Jang JH. Electrospun nanofibers as versatile interfaces for efficient gene delivery. J Biol Eng. 2014; 8: 30.
- Sukumar UK, Packirisamy G. Bioactive core-shell nanofiber hybrid scaffold for efficient suicide gene transfection and subsequent time resolved delivery of prodrug for anticancer therapy. ACS Appl. Mater. Interfaces. 2015; 7: 18717-18731.