Institute of Nephrology, Southeast University, China
*Corresponding author: Bicheng Liu, Institute of Nephrology, Zhong Da Hospital, Medical school, Southeast University, Nanjing 210009, China
Received: September 29, 2014; Accepted: October 20, 2014; Published: October 22, 2014
Citation: Tang R, Han Y, Wu M, Zhu D and Liu B. The Effects of Endothelial Injury in Renal Fibrosis Progression. Austin J Nephrol Hypertens. 2014;1(5): 1021.
Citation: Bucci J and Hansen KE. Should we treat Secondary Hyperparathyroidism in Patients with Pre-Dialysis Chronic Kidney Disease?. Austin J Nephrol Hypertens. 2015; 2(4): 1046. ISSN : 2381-8964
Endothelial injury is very present in Chronic Kidney Disease (CKD) at a very early stage. Previous studies have focused mainly on the fibrotic mechanism in vascular smooth muscle cells, epithelial cells, mesangial cells, and podocytes, whereas the pathogenic role of endothelial cells in the fibrosis has been neglected. This study reviews the relationship among endothelial injury with CKD, renal hypoxia, renal capillary loss, and renal fibrosis progression, with a particular emphasis of the Endothelial-Mesenchymal Transition (EndMT) in renal fibrosis.
Keywords: Endothelial cells; Endothelial-Mesenchymal transition; Renal fibrosis; Chronic kidney disease
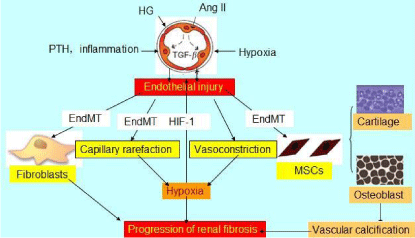
Renal fibrosis is the key reason for progression from Chronic Kidney Disease (CKD) to renal failure. Previous studies mainly focused on the fibrotic mechanism in glomerular and tubular injury, but the pathogenic role of renal vessels in fibrosis was neglected. It is known that the vessel wall can be divided into three layers: the tunica intima, tunica media and tunica adventitia. The tunica intima has one layer of EnCs. The tunica media chiefly consists of concentric layers of helically arranged smooth muscle cells. The tunica adventitia principally consists of type I collagen and elastic fibers. This study mainly discusses the role of endothelial injury in renal fibrosis. The concept of the endothelium was proposed by the physiologist His for the first time in 1865. The endothelium layer is considered to the largest body’ s organ, with a total number of approximately 1.2 x 108, a total area of approximately 400 m2 and a total weight of 1.5 kg in adults. Glomerular EnCs, as a part of the vascular endothelium, together with the glomerular basement membrane, endothelial surface layer, podocytes and sub podocyte space, constitute the five layers of the glomerular filtration barrier. Moreover, there are many round or ovoid Tran’s cellular holes, which are referred to as fenestrations, throughout the most attenuated part of the endothelial cytoplasm. The total area of the holes accounts for 30% of the area of the endothelium. Fenestrations are the defining characteristic of the glomerular EnCs both morphologically and functionally [1]. Although a proportion of other EnCs possess these transcytoplasmic holes, the cells of the glomerular endothelium have a unique constellation of structural features (including the absence of a diaphragm and the retention of a basal lamina). Furthermore, these cells perform a unique and vital physiological function in allowing filtration of the blood in the glomerulus. Without the cells, the kidney could not perform its primary function of clearing low molecular-weight waste products from the circulation [2]. Studies found that EnCs from different parts had different roles in pathologic injury and are closely related to the occurrence, development and outcome of diseases (Figure 1) [3].
The normal structure and function of vascular EnCs is very important for maintaining body homeostasis. The main functions of EnCs include the following: (1) Vascular barrier functions. Vascular EnCs can maintain the smoothness of the tunica intima and prevent the adhesion of platelets and leukocytes and the invasion of harmful substances from the vascular wall. The integrity of the endothelial structure also has the function of preventing lipid deposition. (2) Anticoagulation and fibrinolysis functions. Nitric Oxide (NO) and prostacyclin synthesized and released by EnCs have vasodilatory and anti-platelet aggregation functions. EnCs have a large amount of heparin sulfate on the surface combined with the secretion of antithrombin III, which can inactivate Xa and thrombin. At the same time, tissue factor inhibitor is mainly synthesized by EnCs. EnCs can also release tissue-type plasminogen activator and urokinase, promoting the conversion of plasminogen into plasmin, which is involved in fibrinolysis. (3) The regulation of vascular tone. Through the release of NO, prostaglandin and thromboxane A2 vasodilator substances, such as endothelin and vasoconstrictor substances, EnCs can regulate vascular relaxation and contraction. (4) Involvement in the inflammatory response. In the mediation of various adhesion molecules expressed by EnCs, intravascular leukocytes migrate to inflammatory sites, activated by a sequence of cytosolic protein tyrosine phosphorylation, and play proinflammatory roles. (5) Involvement in vascular remodeling. Blood flow acts on G protein on the EnCs surface, which can cause various phosphorylation processes within EnCs, leading to the rearrangement of vascular EnCs along the direction of the blood flow and the regulation of nuclear factor κB. These events can regulate the expression of multiple genes in EnCs, leading to changes in vascular structure and function [4].
EnCs not only serve as a barrier to the transvascular exchange of material but also secrete various vasoactive substances. Endothelial dysfunction induced by endothelial injury at the onset correlates closely with the development of numerous cardiovascular diseases, including hypertension, coronary heart disease, diabetes, chronic heart failure and chronic renal failure [5-7]. Rabelink et al proposed that the activation of tubular EnCs can lead to the progressive development of CKD [8]. It has been reported that endothelial dysfunction constitutes the pathophysiological basis of CKD [9,10]. Renal fibrosis in CKD is closely related to progressive obstruction of the microvasculature and to a loss of nephron capillary function induced by endothelial dysfunction. Reductions in the synthesis of NO, adipocytokines and uremia-related factors can all induce endothelial dysfunction [11]. The imbalance between local angiogenesis in the kidney and EnCs-related factors constitutes the main mechanism of progressive endothelial injury in CKD. In addition, the improvement of endothelial dysfunction and angiogenesis and the protection of renal EnCs have certain implications for improving renal function and mitigating renal histological damage [12,13].
The theory of glomerular hyper filtration proposed by Brenner is the classic mechanism of chronic renal failure. The theory holds that with the reduction of nephrons, the GFR decreases and the efferent arterioles of the residual nephrons constrict, increasing systemic and glomerular hydrostatic pressure, inducing endothelial and mesangial cell injury, and eventually leading to glomerular fibrosis. The mechanism is primarily that the dilation of the afferent arteriole is more significant than that of the efferent arteriole. Additionally, a decrease in the sensitivity of the afferent arteriole to Angiotensin II is related to increased local NO secretion and the relatively higher sensitivity of the efferent arteriole to Angiotensin II. Glomerular endothelial injury also leads to platelets’ aggregation, activation and release of cytokines, such as platelet-derived growth factor, enhancing the intraglomerular coagulation and micro thrombosis. These events will further lead to progressive reduction in the residual nephrons, aggravate renal injury and promote the development of CKD [14-16].
The maintenance of renal microcirculation plays a critical role in preventing the progression of CKD. There are two sets of capillary networks in the kidney: namely, the glomerular capillary network and the peritubular capillary network. Preservation of the number of glomerular capillaries contributes to the stability of the GFR, whereas the number of peritubular capillaries is an important factor in ensuring the tubulointerstifial oxygen and nutrient supply [17]. The endothelial repair capacity decreases and apoptosis increases after endothelial injury in the renal microcirculation, causing a loss of renal microvasculature. The inconsistency between glomerular capillary injury and subsequent neovascularization increases the apoptosis of EnCs and leads to glomerular sclerosis. A study by Advani et al [18] suggested that high glucose can cause capillary injury and the progression of diabetic nephropathy through direct action on the glomerular endothelium and the interaction between the endothelium and podocytes. In the development of CKD, the number of peritubular capillaries also decreases, similar to glomerular endothelium loss. Studies have shown that in patients with diabetic nephropathy, peritubular capillaries are also injured, resulting in a reduction in capillary density and promoting the progression of diabetic nephropathy [19]. Interstitial blood reduction induced by peritubular capillary injury has been identified as an important cause of renal interstitial fibrosis. Kang et al [20] found that peritubular capillary injury correlated with interstitial fibrosis and that such a correlation was independent of blood pressure and proteinuria action.
Increasing evidence shows that hypoxia and hypoxia-inducible factor-1 play a major role in the development of CKD [21,22]. The mechanism of local hypoxia is complex. Oxidative stress, inflammation, renal hemodynamics, urinary protein and angiotensin II can induce endothelial injury, resulting in the impairment of capillaries in the nephron and influencing the local blood supply to the kidney. These phenomena aggravate local hypoxia, resulting in microvascular dysfunction, tubular atrophy, interstitial inflammation infiltration and the increased accumulation of Extracellular Matrix (ECM), which caused renal fibrosis and further hypoxic damage, with glomerular filtration membrane destruction and a secondary decrease in the GFR [23,24].
At present, the excessive accumulation of ECM has been widely recognized as an important pathological feature of renal fibrosis [25]. In recent years, many studies demonstrated the critical role of activated fibroblasts in the synthesis and secretion of ECM [26]. Previously, it was generally believed that activated fibroblasts in the kidney mainly originated from local, intrinsic renal fibroblasts and that the activation of bone marrow fibroblast and epithelial cells required the phenotype of fibroblasts, acquired through the Epithelial-Mesenchymal Transition (EMT) [27].
In 2008, Zeisberg et al [28] of Harvard University first demonstrated that approximately 30 to 50% of resident fibroblasts in the kidney co-expressed the endothelial marker CD31 and markers of fibroblasts and myofibroblasts. The researchers implemented endothelial-lineage tracing using transgenic mice with unilateral ureteral obstructive nephropathy or streptozotocin-induced diabetic nephropathy and a model of Alport renal disease. The study strongly indicated the presence of the EndMT in the progression of renal fibrosis. Li et al [29] then demonstrated that 10.4 ± 4.2% and 23.5 ± 7.4% of renal interstitial myofibroblasts in 1- and 6-month streptozotocin-induced diabetic kidneys were of endothelial origin, compared with only 0.2 ± 0.1% of myofibroblasts in vehicle-treated Tie2-Cre;LoxP-EGFP mice. These findings suggested that the EnC is an important origin of myofibroblasts. Moreover, the EndMT contributed to the development of renal interstitial fibrosis independently of microalbuminuria injury. Li et al [30] stimulated Tie2-Cre; Loxp-EGFP-transgenic mice and a mouse pancreatic microvascular EnCs line using Advanced Glycation End-products (AGEs). The authors found that AGEs can also induce the EndMT and that Smad3 mediated this process. Moreover, using TGF-β inhibitors, such as SIS3, an inhibitor of Smad3, could retard the progression of diabetic nephropathy. Our previous study showed that high glucose can induce the EndMT via Angiotensin II action [7,31]. These findings indicated that the EndMT is prevalent and constitutes a new mechanism in the progression of renal fibrosis. Further studies demonstrated that the EndMT plays a major role in the early development and progression of diabetic renal fibrosis and cardiac fibrosis [32-34].
With the deepening of research, studies find that vascular EnCs are also the source of bone-like cells in locally ectopic calcification. Medici et al [35] showed that vascular EnCs can transform into multipotent stem-like cells by an activin-like kinase-2 receptor-dependent mechanism. These stem-like cells could be triggered to differentiate into osteoblasts or chondrocytes, which contributed to vascular calcification. In addition, Bostrom et al [36] found medial artery calcification was associated with increased osteogenesis and calcium accumulation in diabetic mice and rats, which was mediated by Bone Morphogenetic Proteins (BMP) activity. And in vitro study found that high glucose-treated aortic EnCs increased osteogenesis, as mediated by BMP-2/BMP-4. In our study, we showed that high glucose mediated endothelial-to-chondrocyte transition and parathyroid hormone induced endothelial-to-osteoblast transition in ECs [37-39]. Furthermore, snail and Wnt/β-catenin signal pathway might join in the EndMT [37-39]. These studies indicated that EndMT was involved in the vascular calcification that contributing to the renal fibrosis.
Although the effect of the EndMT is comprehensive and is an important source of renal interstitial fibroblasts in renal fibrosis, the underlying molecular mechanism of this pathological phenomenon is still not clear. Recently, the endothelium has been proposed as a special type of epithelial tissue, so the EndMT can be defined as a special type of EMT [34].
As one of the sources of activated tubulointerstifial fibroblasts, the effect of the EMT on the progression of kidney disease has been widely and deeply studied. At present, the EMT is recognized as a dynamic process triggered by pathological mediators such as Transforming Growth Factors β (TGF-β), chemokines (e.g., MCP- 1) and matrix metalloproteinase during the inflammatory reaction induced by kidney injury. This process is mainly regulated by three signal transduction pathways: namely, the TGF-β/Smads pathway, the integrin/ILK pathway and the Wnt/β-catenin pathway [40-42]. Whether the EndMT is regulated by the same signaling pathways and the potential molecular regulatory networks of the EndMT need further research.
At present, the study of the regulatory mechanism of the EndMT is mainly focused on the TGF-β signaling pathway. In 2009, Kitao et al [43] confirmed that the EndMT in portal vein EnCs was closely related to the activation of the TGF-β1/Smad signaling pathway in idiopathic portal-hypertension patients. In 2007, Zeisberg et al [32] demonstrated the involvement of the activated TGF-β signaling pathway in the EndMT during cardiac fibrosis and the partial reversion of this process by BMP-7. Interestingly, BMP-7 can also partially reverse the process of the EMT. Kokudo et al [44] found that TGF-β2 can induce mouse embryonic stem cells to express the markers of EnCs, such as α-SMA, transgelin and calponin, but the expression of claudin-5, a marker of EnCs, decreased. Snail 1 is involved not only in the EMT [45] but also in the process of the EndMT [37], giving us more reason to propose that the EMT and EndMT might share certain common pathways [46].
The concept that the protection of endothelial function and the repair of injured EnCs can retard the progress of CKD is new [47,48]. Yamaguchi et al [49] found that the expression of the vascular endothelial adhesion molecule cadherin significantly increased in the fibrosis site of the UUO model compared with the wild type, indicating that cadherin mediated the progression of renal interstitial fibrosis. Animal experiments also showed that an angiotensin II receptor blocker could reverse arterial endothelial dysfunction, inhibit the EndMT and alleviate fibrosis [31].
In addition, as a cytokine that maintains EnCs growth, VEGF can stimulate the EnCs to produce NO. In the remnant kidney model, Kang et al [50] intervened with VEGF and found that VEGF could retard the progression of interstitial fibrosis. This phenomenon may be due to VEGF’s effects of promoting EnC proliferation and retarding interstitial microvascular reduction. However, studies [51] showed that plasma VEGF levels increased in patients with diabetic nephropathy, and animal models showed that blocking VEGF could retard the progression of diabetic nephropathy. Another study [52] found that plasma VEGF levels also increased in patients with CKD and that VEGF even played a detrimental role in the common complications of CKD, including atherosclerosis and sepsis. Therefore, the clinical applications of VEGF to maintain endothelial integrity need further investigation.
Endothelial Progenitor Cells (EPCs), which are stem cells and precursors of vascular EnCs, exist not only in the bone marrow but also in the peripheral circulation. The induction of differentiation in vitro rendered the expression of specific antigens of EnCs in EPCs [53]. Kong et al [54] cultured EPCs in vitro and injected the cells into the site of a carotid artery bulb injury in a rabbit. Four weeks later, EPCs were found around the injury site. The speed of re-endothelialization accelerated and the diastolic function of EnCs improved compared with the control, and the transplanted EPCs had no impact on the contraction of the smooth muscle cells in the injured artery. At present, the EPC-mediated repair of endothelial injury is mainly based on the transplantation of EPCs after in vitro culture. The preliminary preparation cycle for this type of cell treatment is long, and the in vivo microenvironment may result in alteration of the cell phenotype, thereby altering cellular immunogenicity and increasing the possibility of immune rejection. Therefore, the method of in vitro EPCs proliferation and the means of transplantation need improvement. EPCs transplantation, as a new means of treating vascular EnCs injury, has a promising future [55].
The loss of glomerular capillary endothelial function induced by hyperglycemia, inflammation, growth factors and oxidative stress ultimately leads to renal fibrosis, which is the striking feature of CKD. Currently, studies on CKD mainly focus on the mechanisms of glomerular and tubular interstitial fibrosis. With deep research into CKD, the EnCs of the kidney will receive more attention. An in-depth study on EnCs will have great significance for the prevention and treatment of CKD. Furthermore, repair of the renal microvascular damage caused by EnC injury will be a target in the future treatment of CKD.
This work was supported by grants from National Natural Science Foundation of China (Key Program, No. 81130010, 81370919) and Natural Science Foundation of Jiangsu Province (No. BK2011603).
Schematic view of the pathophysiological role of endothelial injury in CKD progression.
Ang II: Angiotensin II; TGF-β: Transforming Growth Factor-β; HG: High Glucose; PTH: Parathyroid Hormone; End MT: Endothelial Mesenchymal Transition; HIF-1: Hypoxia-Inducible Factor-1; MSCs: Mesenchymal Stem Cells.