1Department of Primary and Community Care, Radboud University Medical Centre, Netherlands
2Department of Nephrology, Radboud University Medical Centre, Netherlands
*Corresponding author: Scherpbier-de Haan ND, Department of Primary and Community Care, Radboud University Medical Centre, Nijmegen, postal route 166, PO Box 9101, 6500 HB Nijmegen, the Netherlands
Received: October 28, 2014; Accepted: November 26, 2014; Published: November 28, 2014
Citation: Scherpbier -de Haan ND, Vervoort GMM, Van Weel C, Mulder J, Wetzels JFM and de Grauw WJC. Abnormal Serum Parathyroid Hormone, Calcium or Phosphate in Patients with Chronic Kidney Disease in Primary Care. Austin J Nephrol Hypertens. 2014;1(6): 1027.
Citation: Bucci J and Hansen KE. Should we treat Secondary Hyperparathyroidism in Patients with Pre-Dialysis Chronic Kidney Disease?. Austin J Nephrol Hypertens. 2015; 2(4): 1046. ISSN : 2381-8964
Background: In Chronic Kidney Disease (CKD), abnormalities of mineral metabolism can occur early in the disease process. Changes in calcium and phosphate homeostasis and secondary hyperparathyroidism are metabolic complications of CKD that have impact on cardiovascular health and bone turnover.
Objectives: To determine the prevalence of mineral metabolism disturbance in CKD in primary care.
Methods: In a cross sectional study in nine primary care practices in the Netherlands we evaluated abnormalities of mineral metabolism in CKD patients and identified predictors of these abnormalities.
In patients with an eGFR < 60 ml/min/1.73 m2, identified during their evaluation for hypertension or diabetes mellitus, we determined the prevalence of abnormal values of parathyroid hormone (PTH), calcium and phosphate. Predictors of abnormal PTH levels were assessed.
Results: A total number of 174 patients in primary care was investigated. Mean eGFR was 50.3 ml/min/1.73 m2. An increase in PTH level above normal occurred in 40% of these patients with early stage of CKD in primary care. Although eGFR predicted abnormal PTH levels, its predictive value was low. Calcium and phosphate abnormalities were infrequent.
Conclusion: PTH testing deserves attention in patients with CKD in primary care. Prospective studies should clarify whether PTH lowering affects cardiovascular prognosis of these patients. Awaiting this evidence, we suggest to follow the K-DOQI guideline that advises PTH testing in patients with CKD stage 3 or worse and to treat patients with elevated PTH levels with vitamin D.
Keywords: Chronic renal insufficiency; Metabolic bone disease; Primary health care; Secondary hyperparathyroidism
CKD: Chronic Kidney Disease; PTH: Parathyroid Hormone; CKD-MBD CKD: Mineral and Bone disorder; K-DOQI: National Kidney Foundation Kidney Disease Outcomes Quality Initiative; GP: General Practitioner; eGFR: Estimated Glomerular Filtration Rate; KEEP: Kidney Early Evaluation Program
Chronic Kidney Disease (CKD) is a highly prevalent condition especially in patients with diabetes or hypertension [1]. In primary care, the awareness of CKD in patients with diabetes and hypertension has increased due to regular testing of serum creatinine and albuminuria in these patients and due to reporting of estimated glomerular filtration rate (eGFR) [2,3]. CKD is not only a risk factor for end stage renal disease, but may also lead to complications, among which are cardiovascular morbidity and mortality [4].
One of the complications of CKD is a change in calcium and phosphate homeostasis characterised by elevated serum intact parathyroid hormone (PTH) levels or abnormal values of calcium or phosphate. Abnormalities in these entities are considered part of the spectrum of CKD-Mineral and Bone disorder (CKD-MBD). A strong association has been established between PTH, calcium and phosphate abnormalities and increased risk of hypertension, CKD progression, adverse cardiovascular events and mortality [5,6]. This was also seen in CKD stage 3 and 4 where there was a significant increase in the prevalence of cardiovascular disease with increasing PTH levels [7]. Although controversy exists, prognosis of patients with secondary hyperparathyroidism can be improved by vitamin D treatment both in dialysis-patients and in non-dialysis-patients, which calls for early recognition of patients with CKD-MBD [5,8,9].
International guidelines put emphasis on testing of metabolic disorders, but vary in defining the patients to be tested. The guidelines of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K-DOQI ) and the KDIGO guidelines recommend that all patients with eGFR below 60 ml/min/1.73 m2 undergo evaluation for CKD-MBD by measuring PTH, calcium and phosphate [10,11]. The NICE-guideline in the UK advices these measurements in patients with eGFR below 30 ml/min/1.73 m2 [12]. The Dutch interdisciplinary CKD-guideline advices evaluation in patients < 65 year with eGFR < 60 and in patients ≥ 65 year with eGFR < 45 ml/min/1.73m2 [13]. It is not clear which testing strategy is most effective in finding patients with elevated PTH.
Data on the prevalence of metabolic disorders in primary care patients are limited. The few empirical data that are available, point to a limited uptake of MBD-assessment in primary care [14,15]. Most data on metabolic disorders in CKD originate from patients referred to nephrologist care, indicating that PTH rises early in the course of CKD [16-18]. A better understanding of the actual prevalence and predictors of MBD in the CKD-population under care of general practitioners may enhance the uptake of testing in primary care.
The purpose of our cross sectional study was to evaluate the prevalence of disorders in PTH, calcium and phosphate in patients with CKD who are under control in primary care for their diabetes or hypertension. In addition, we analysed which factors predicted metabolic disorders in these patients.
In this cross-sectional study we described and analysed the data of patients with CKD in a primary care setting. These data are part of a cluster randomised controlled trial in which the effectiveness of a shared care model between General Practitioner (GP), nurse physician and nephrologist was compared with usual care. The study was performed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983. Ethical approval was not required according to the accredited Medical Research Ethics Committee Arnhem/Nijmegen (ABR NL16590.091.07). Trial registration: Sharing study: SHARed care for patients In Nephrology and General practice; Netherlands Trial Registration TC 1140.
The study took place in nine general practices that are part of the Academic Practice-based Research Network of the Radboud University Nijmegen Medical Centre in 2008 [19]. These practices have a total of 54.231 patients. From the electronic patient files we selected all adult patients with hypertension and/or diabetes mellitus type 2 who were under care of the GP for their diabetes or hypertension treatment. Patients were eligible if the yearly measurement of eGFR was below 60 ml/min/1.73 m2. GPs could also include patients if eGFR below 60 ml/min/1.73 m2 was newly found at the annual diabetes or hypertension control. Patients with serious medical or psychiatric conditions and patients under specialist care for CKD were excluded. Eligible patients were invited to take part in the study when they visited the practice for a regular consultation. In the patients who agreed to participate, eGFR was measured again and if the result was found to be below 60 ml/min/1.73 m2 the patients were included in the study.
We collected baseline data regarding demographic and clinical characteristics as mentioned in Table 1.
Samples for PTH-analysis were put on ice immediately after blood sampling and analysed within two hours. If this was not possible, samples were centrifuged for 10 minutes at a minimum of 3000 rotations per minute and the serum was saved in a refrigerator until analysis. All clinical chemical analyses were performed by the Laboratory of Clinical Chemistry of the Canisius Wilhelmina Hospital in Nijmegen, the Netherlands. Creatinine, calcium, phosphate and PTH were measured on a routine chemistry analyzer (Roche Modular PE Analytics). PTH was measured with the Sandwich principle using an ECLIA technique (Elecsys PTH reagens, Roche). The normal value for PTH was < 6.9 pmol/l. Serum creatinine was measured enzymatically. Calibration was traceable to isotope dilution mass spectrometry. The eGFR was calculated from the Modification of Diet in Renal disease (MDRD) equation: eGFR=175x (serum creatinine (μmol/l) x0.0113) -1.154 x (age) -0.203 x (0.742 if female) x (1.210 if black) [20]. Calcium levels were corrected for albumin levels.
Albuminuria was defined as an albumin/creatinineratio ≥ 2.5 mg/mmol in male or ≥ 3.5 mg/mmol in female patients. Blood pressure was measured three times after a five-minute rest with an oscillometric device. The mean of the two last measurements was used for the analysis.
Statistical differences between the variables were calculated by Student’s t-tests for continuous outcomes and Chi-square tests for dichotomous outcomes using SAS version 9.2. For the application of the Student’s t-test Pooled standard error or Satterthwaite approximation were chosen based on the F-test for variances. Stepwise logistic regression was used to determine risk factors for abnormal PTH-levels. For this model variables were selected with a univariate p-value <0.15. We considered a p-value of less than 0.05 statistically significant.
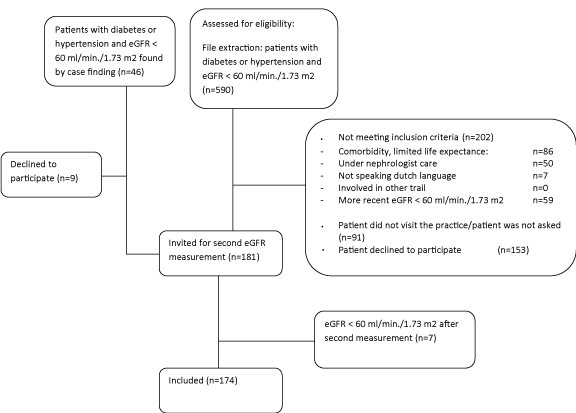
4393 patients with diabetes and/or hypertension were under GP care. Figure 1 gives an overview of the inclusion process. When comparing the included group to the non-included group of patients with an eGFR below 60 ml/min/1.73 m2 no differences were found besides vitamin D treatment and heart failure (Table 1). Relatively more male patients were included. Table 1 shows the distribution of patients over CKD-stages.
In 11 patients a PTH-analysis could not be performed due to practical barriers. Mean PTH-level was 7.2 (SD 3.7) pmol/L. An elevated PTH level (PTH ≥ 6.9 pmol/L) was found in 40.5% of the patients (Table 2). Patients with an elevated PTH level had a significantly lower eGFR than those with a normal PTH level (difference 4.5 ml/min/1.73 m2).
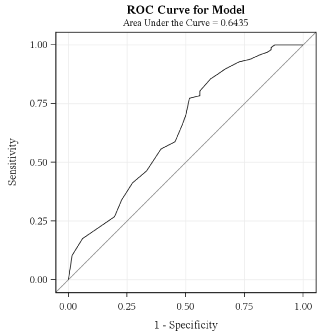
Variables that entered the equation for stepwise logistic regression modelling were eGFR, triglycerides, heart failure and diuretics as factors predicting abnormal PTH, with p-values in univariate analysis of 0.0004, 0.0008, 0.06 and 0.07 respectively. For diuretics the odds ratio was 2.7 (95% confidence interval 0.9 to 7.9); for heart failure the odds ratio was 4.7 (95% confidence interval 0.9 to 24.3). Age, sex, myocardial infarction, body mass index, blood pressure, fasting glucose, HbA1c, haemoglobin, LDL-cholesterol, HDL-cholesterol, potassium and urine albumine-creatinine ratio did not enter the equation because p-values in univariate analysis were >0.15. By stepwise logistic regression eGFR and triglycerides were identified as predicting abnormal PTH-levels (chi-square statistic 0.0012 and 0.0017). The Receiver Operating Characteristic curve of eGFR predicting abnormal PTH was very flat and not of any help in defining eGFR cut-off values (Appendix 1).
We additionally performed stepwise linear regression to assess what factors contributed to the absolute level of PTH. eGFR, heart failure and triglycerides contributed (partial R-square 13.5%, 6.0% and 2.0% respectively). Use of diuretics showed multicollinearity with heart failure.
All serum calcium levels were above 2.1 mmol/l. Three subjects showed a calcium level ≥ 2.54 mmol/l. None of the subjects had an elevated phosphate level.
In this observational study in primary care we found that serum PTH levels rose early in the disease process. Increased PTH values occurred in 40 % of the patients with eGFR below 60 ml/min/1.73 m2. In contrast, abnormal values of calcium and phosphorus were rare.
This study carefully defined CKD-diagnosis and laboratory results. CKD-diagnosis was based on two evaluations of eGFR three or more months apart, whereas other studies in the general population only use one eGFR measurement on which to base diagnosis. One single laboratory performed the measurements and measurements were well-standardised. Furthermore we paid close attention to the pre-analytical handling of PTH samples. The laboratory assessment was performed within a few hours after sampling or blood was saved on ice [21]. The study size was relatively small, but large enough to explore quality and quantity of metabolic disturbances in a group of CKD-patients that is daily seen in primary care.
We should consider some limitations of this study. First, our research was based on patients with diabetes or hypertension. We chose to focus on this group since the majority of patients under care for CKD in general practice will have diabetes or hypertension [11,22]. Second, the protocol of the Sharing study- that asked patients to come four times to the practice- may have caused a selection of patients. Figure 1 however reveals that the included patients did not differ from the non-included patients with respect to co-morbidity. If there has been a selection, it would have been the relatively vital patients that were included. For interpretation of the results this would mean that the prevalence of metabolic abnormalities is likely to be higher than the results we found. Third, the K-DOQI guideline advises yearly measurement of serum bicarbonate to detect metabolic acidosis in patients with CKD. Bicarbonate measurement however was not possible yet in our primary care setting. Bicarbonate disorders are mainly observed in patients with eGFR < 30 ml/min/1.73 m2 [23]. As 97% of our study population had an eGFR≥30 ml/min/1.73 m2, we presume that metabolic acidosis will not play a major role in our study population.
We did not find other studies that evaluated the prevalence of disorders of mineral metabolism in known CKD patients in primary care only. The most comparable data derive from the prevalence of abnormalities in serum calcium, phosphate and PTH in a cross-sectional analysis of 1814 out-patient-clinic patients (71% primary care practices) with CKD stages 3–5 in North America [24]. Elevated PTH was present in 56% of patients with eGFR <60 ml/min/1.73m2, which is higher than the 40.5 % we found. This can be explained by the facts that mean eGFR in the US study was 40 ml/min/1.73 m2, clearly below the mean of 50 ml/min/1.73 m2 in our study. A difference in race could also have been of influence; we will comment on that further in the discussion. In line with our data, calcium and phosphate values did not become abnormal until eGFR fell below 40 ml/min per 1.73m2. In a community-based screening program -the Kidney Early Evaluation Program (KEEP)- and in epidemiological data from the National Health and Nutrition Examination Survey, Vasalotti et al found that as eGFR fell from 60 to 30 ml/min/1.73 m2, calcium level decreased, phosphate level increased, and PTH level increased [25]. In summary, the evidence available points to a rise of PTH in early stages of CKD and our study confirms that this is the case in CKD patients with diabetes of hypertension in general practice.
Secondary hyperparathyroidism can be caused by a lack of renal activation of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitaminD3 but could as well be caused by a lack in inactive vitamin D. As laboratory results on vitamin D levels were not available, we cannot comment on that aspect.
Race may be of influence on secondary hyperparathyroidism. In blacks secondary hyperparathyroidism was more prevalent than in whites [26]. We were not informed on the race of patients in our study, but know that the population in the research practices is mainly (>95%) Caucasian.
In our study, eGFR and triglycerides predicted abnormal PTH levels and heart failure contributed to the absolute PTH-height. The relation of elevated PTH with eGFR and heart failure is in line with findings in other studies. Cardiovascular disease, age, BMI ≥30, albuminuria and absence of diabetes were predicting factors in the community based study of Vassalotti et al [25]. De Boer et al. found in 218 outpatient nephrology patients that PTH was associated with eGFR and with a history of congestive heart failure and myocardial infarction [27]. The relation of triglycerides to elevated PTH was not found in other studies. In our study the overall contribution was very low and probably not of any clinical significance. Based on our results, we cannot define a PTH-testing strategy. Applications of the PTH-testing advices of the guidelines available (Appendix 2) revealed that the K-DOQI and KDIGO guidelines best enable finding patients with secondary hyperparathyroidism.
Metabolic disturbances, other than PTH, were found in a cross sectional study in veterans (>65 years) with mean eGFR of 47 ml/ min/1.73m2. 2.5% of participants had hyperkalemia and 4.4% had hyperphosphatemia [28]. Hyperphosphatemia was not found in our study. This could be due to the small number of patients tested and the fact that mean eGFR was relatively high.
As PTH is related with adverse outcomes in CKD, and the prevalence of abnormal PTH in early stages of CKD is high, the debate on PTH-testing in primary care deserves more attention. The effectiveness of vitamin D in lowering PTH levels has been proved and the effects on prognosis were positive as well in dialysis as in non-dialysis patients [5,9,29]. The K-DOQI-guideline advises correction of MBD-abnormalities by native vitamin D amongst other therapies. In patients in whom serum PTH is rising and remains above the upper limit of normal for the assay despite correction of modifiable factors, treatment with calcitriol or vitamin D analogs is suggested by the KDIGO guideline [11]. However, convincing evidence that adverse cardiovascular outcome could be prevented or delayed by early detection and treatment of elevated PTH is not available yet [8,30]. In primary care, PTH may prove to be a useful additive risk factor in identifying CKD-patients who have a higher cardiovascular risk.
Given the frequency of elevated serum-PTH in a primary care population that comes forward in this study there is a need for further research on the effects of PTH lowering in primary care on cardiovascular morbidity and mortality. Awaiting this evidence, general practitioners may consider checking PTH-levels in patients with CKD stage 3 or worse and prescribing vitamin D in patients with elevated PTH levels.
Differences between included and non-included patients with diabetes and hypertension and eGFR<60 ml/min/1.73 m2 in nine primary care practices.
|
Patients with MDRD<60 ml/min/1.73 m2 Not included in the study n = 448 (72%) |
Included in this study n = 174 (28%) |
Difference (95% CI) |
P value * |
|
Age in years (SD) |
73.5 (10.2) |
72.9 (8.1) |
0.7 (-0.9 to 2.2) |
0.39 |
|
Sex, male (%) |
|
125 (28%) |
77 (44%) |
|
<0.0001 |
Blood pressure mmHg (SD) |
Syst |
146.0(18.9) |
143.5 (21.9) |
2.5 (-1.3 to 6.2) |
0.19 |
Diast |
79.1 (10.1) |
78.0 (13.5) |
1.1 (-1.1 to 3.4) |
0.31 |
|
Hypertension (no diabetes) |
288 (64%) |
109 (63%) |
|
0.70 |
|
Diabetes, (normo- and hypertension) |
160 (36%) |
65 (37%) |
|
0.70 |
|
eGFR MDRD ml/min/1.73m2 (SD) |
49.2 (9.5) |
50.3 (7.0) |
-1.1 (-2.5 to 0.3) |
0.11 |
|
Medication |
|
|
|
|
|
Vitamin D |
23 ( 5%) |
2 (1%) |
|
0.02 |
|
Thiazides |
46 (10%) |
23 (13%) |
|
0.29 |
|
Bifosfanates |
12 ( 3%) |
1 (0.6%) |
|
0.12 ** |
|
Iron supplements |
13 ( 3%) |
3 (2%) |
|
0.58** |
|
Agents acting on the renin-angiotensin system |
284 (64%) |
123 (71%) |
|
0.09 |
|
Cardiovascular comorbidity |
|
|
|
|
|
Myocardial infarction |
48 (11%) |
19 (11%) |
|
0.94 |
|
Heart failure |
53 (12%) |
9 ( 5%) |
|
0.01 |
|
TIA |
32 ( 7%) |
13 (7%) |
|
0.89 |
|
CVA |
62 (14%) |
16 (9%) |
|
0.12 |
|
Peripheral arterial disease |
37 ( 8%) |
20 (11%) |
|
0.21 |
|
* T-test and Chi-square test for continuous and dichotomous outcomes.
**Fisher’s exact test.
Characteristics of patients with elevated PTH levels.
N=163 |
|
|
|||
|
PTH <6.9 pmol/l |
PTH ≥ 6.9 pmol/l |
Difference |
P value |
|
Number |
97 (59.5%) |
66 (40.5%) |
|
|
|
Age (years)(SD) |
72.6 (8.1) |
73.6 (8.1) |
-0.9 (-3.5 to 1.6) |
0.47 |
|
Sex ,male(%) |
42 (43%) |
32 (48%) |
|
0.51 |
|
eGFR (ml/min/1.73 m2) |
51.3 (5.9) |
46.7 (9.0) |
4.5 (2.0 to 7.0) |
0.0005 |
|
BMI |
28.7 (4.9) |
29.1 (4.4) |
-0.4 (-1.9 to 1.0) |
0.56 |
|
Diabetes |
38 (39.2%) |
24 (36.4%) |
|
0.72 |
|
30-minute blood pressure(mmHg) |
Systolic |
124.0(16.6) |
126.4 (24.3) |
-2.3 (-9.5 to 4.8) |
0.52 |
diastolic |
73.7 (10.1) |
75.3 (14.0) |
-1.6 (-5.8 to 2.6) |
0.45 |
|
Albuminuria: (n, %) n=157 |
17 (18%) |
18 (29%) |
|
0.12 |
|
Hb (mmol/l) |
8.8 (0.9) |
9.0 (0.8) |
-0.2 (-0.5 to 0.1) |
0.22 |
|
Calcium (mmol/l) |
2.35 (0.1) |
2.34 (0.1) |
0.00 (-0.03 to 0.04) |
0.88 |
|
Phosphate (mmol/l) |
1.11 (0.18) |
1.08 (0.17) |
0.03 (-0.02 to 0.09) |
0.24 |
|
HbA1c (%) in patients with diabetes |
6.9 (0.84) |
7.5 (1.16) |
-0.56 (-1.1 to -0.05) |
0.03 |
|
LDL (mmol/l) |
2.88 (0.99) |
2.6 (1.1) |
0.2 (-0.12 to 0.53) |
0.22 |
|
HDL (mmol/l) |
1.35 (0.37) |
1.35 (0.52) |
-0.006 (-0.15 to 0.14) |
0.93 |
|
Triglyceride (mmol/l) |
1.57 (0.62) |
2.01 (0.90) |
-0.44 (-0.70 to -0.19) |
0.007 |
|
Medication: |
|
|
|
|
|
Calcium |
0 |
0 |
|
|
|
Thiazides |
10 (10.3%) |
11 (16.7%) |
|
0.23 |
|
High ceiling diuretics |
6 (6.2%) |
10 (15.2%) |
|
0.06 |
|
Drugs affecting bone structure and mineralization |
0 |
1 (1.5%) |
|
0.22 |
|
Cardiovascular co-morbidity |
|
|
|
|
|
Myocardial infarction |
11 (11%) |
6 (9%) |
|
0.64 |
|
Heart failure |
2 (2%) |
6 (9%) |
|
0.04 |
|
TIA |
7 (7%) |
6 (9%) |
|
0.66 |
|
CVA |
8 (8%) |
7 (11%) |
|
0.61 |
|
Peripheral arterial disease |
9 (9%) |
7 (11%) |
|
0.78 |
|
* T-test and Chi-square test for continuous and dichotomous outcomes
Receiver Operating. Characteristic curve of eGFR MDRD predicting PTH-levels.
Number and % of patients with increased PTH that would be found following the testing strategies of the K-DOQI guideline, the NICE guideline and the Dutch interdisciplinary CKD-guideline for primary care and nephrology.
|
K-DOQI: all patients with eGFR < 60 ml/min/1.73 m2 |
NICE: all patients with eGFR < 30 ml/min/1.73 m2 |
Dutch: patients<65 year AND eGFR < 60 ml/min/1.73 m2; patients ≥65 year and eGFR <45 ml/min/1.73 m2 |
Number of patients with elevated PTH/number of patients tested |
66/163 (40.5%) |
4/4 (100%) |
33/62 (53.2%) |
% of elevated PTH diagnosed |
66/66 (100%) |
4/66 (6.1%) |
33/66 (50%) |
Mean PTH (pmol/l) in tested patients (SD) |
7.2 (3.7) |
12.5 (3.1) |
8.6 (4.7) |
Range |
0.9-22.9 |
11.0-17.1 |
0.9-22.9 |
Mean PTH (pmol/l) of non-tested patients (SD) |
- |
7.0 (3.6) |
6.3 (2.5) |
PTH-range of non-tested patients |
|
0.9-22.9 |
2.8-15.0 |
inclusion process.