
Review Article
Austin J Neurol Disord Epilepsy. 2021; 7(1): 1044.
Meropenem Monotherapy is a Valid Alternative in Community Acquired Adult Acute Bacterial Meningitis (ABM)
Johansson B1, Lindquist L1 and Fohlman J2,3*
1Department of Infectious Diseases, Karolinska Institute, Sweden
2Department of Research and Development, Region Kronoberg, Sweden
3Department of Infectious Diseases, Central Hospital, Sweden
*Corresponding author: Jan Fohlman, Department of Research and Development, Region Kronoberg, Box 1223. 35112 Vaxjo, Sweden
Received: February 22, 2021; Accepted: March 06, 2021; Published: March 13, 2021
Abstract
Background: Meropenem (Merrem®, Meropenem®) is a broad-spectrum antibacterial carbapenem with indication for Acute Bacterial Meningitis (ABM). We wanted to specifically evaluate the effect of meropenem monotherapy, compared to standard recommended therapy.
Method: A Swedish ongoing internet based quality register for ABM was started 1994 and covers the whole country with roughly 30 infectious disease clinics. After ethical approval data were extracted from the database and evaluated using conventional statistical methods (IBM SPSS Statistics 23).
Results: The register contained 1708 patients altogether. All 770 patients (45%) given meropenem or cefotaxime/ampicillin were selected for this study. The age of the studied population was from 18-91 with a mean of 55 and median 60. Overall ABM mortality was 12.1% (197/1708). In the meropenem and cefotaxime/ampicillin subgroup 7.8% (60/770) died, p=0,035 and in the non-selected group 14,6 % (137/938), p<0.001. For meningococci the mortality was only 2% and for pneumococci 9% (p=0,025) and are record low figures in this selected population. The mortality increased 1.3 times (Odd’s ratio 1.3, p<0.001) with each decade of life and 1.5 times with each RLS stratification level (Odd’s ratio 1.5, p<0.001).
Conclusion: There was no statistical significance in mortality (p=0.67) or sequelae rate between meropenem and cefotaxime/ampicillin treatment. Favourable outcome depends on speedy and correct antibiotic therapy as well as inclusion of betamethasone. Age, RLS and bacterial species, are factors, which cannot be influenced. Meropenem is a valid antibiotic choice in the Swedish population (with limited resistance problems).
Keywords: Community acquired acute bacterial meningitis in the adult; Meropenem monotherapy; Carbapenem; Prognosis
Summary
Meropenem was shown to be non-inferior to cefotaxime/ ampicillin therapy in acute adult bacterial meningitis and both regimens were superior to other treatment (p<0.01). Prognosis is dependent on speedy and correct antibiotic therapy, addition of betamethasone, age, RLS and bacterial species.
Introduction
Acute Bacterial Meningitis (ABM) is a dreaded disease with sometimes very rapidly fatal outcome [1,2]. Meningococci cause ABM as well as severe sepsis, but the total mortality is lower than for pneumococcal etiology. This may be due to the rapid disease progress and early hospital care and the fact that it affects younger individuals; peak around 14 years of age with an observant surrounding and no co morbidities. However, in adults and geriatric patients pneumococci predominate and they cause a more protracted disease with much higher mortality and sequelae as compared to meningococci. Despite extensive health care in advanced countries, overall mortality is between 16-25 % [3] and the frequency of sequelae 20-50 %, like cognitive impairment, which can be very disabling in modern societies.
Since 1994 all ABM patients have been reported to a national quality register in Sweden, now in a web based format. Conclusion from these data as well as practice guidelines are available [4]. Almost two thousand patients have been entered and are thought to represent an absolute majority of all ABM cases occurring in Sweden. Meropenem monotherapy was earlier given to a minority; 7%, but now has increased dramatically to about 50% for unknown reasons, forcing us to secure the effectiveness of empiric meropenem monotherapy. Earlier reports have suggested the value of meropenem in ABM due to its penetration into CNS, broad spectrum and absence of neurotoxicity [5-8].
Patients and Methods
All patients in the Swedish national quality register were evaluated by the use of a database from 1994 to date 2013. Statistics were calculated using IBM SPSS Statistics version 23 (frequency distribution, Chi square, Fischers exact test, logistic regression, correlation and variance analysis etc.). The study was approved by the ethics committee of Karolinska Hospital.
The Swedish recommendations for ABM therapy is cefotaxime 3gx3-4 plus ampicillin 3gx3-4 i.e. or meropenem 2gx3 i.e. Addition of betamethasone 0.12mg/kg x 4 i.e. during 4 days has been suggested since many years.
This was a retrospective study where all patients in the register were retrieved and the focus was on the patients who received recommended therapy, altogether constituting 45%. Patients who received other therapy were compared and it is speculated why they did not follow the recommendations.
Results
In total 1708 patients were evaluated, indicating an ABM incidence of 0.9 per 100,000 people. Only the patients (n=770, 45%) who received either meropenem monotherapy or cefotaxime/ampicillin were further evaluated (Figure 1). During the first years few patients were included, probably due to lack of clear recommendations and other therapy given, but averaged 49/year for the past 15 years. The number of patients who got meropenem thus has gone up from below 10% to around 50% during the final 5-10 years. The age varied from 18-91 years and the average/median age was 55/60 years (Figure 2).
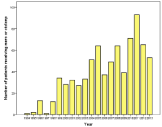
Figure 1: Distribution of the selected patients receiving either meropenem
monotherapy (mero) or cefotaxime plus ampicillin (ctx/amp) during the study
period, averaging 49 for the last 15 years.
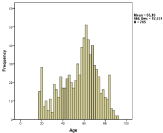
Figure 2: Age distribution of the ABM patients treated with recommended
therapy had equal sex distribution (not shown).
The total mortality was 12.1% (197/1708), and in the group not receiving recommended therapy it was 14.6% (137/938). The mortality among selected patients given recommended therapy was 7.8% (60/770), which was significantly lower, p<0.001. In the patients treated with meropenem 8.3% (22/242) died vs. 7.5% (38/468) for the cefotaxime/ampicillin arm, p=0.67; a not significant finding. It could also be seen (Figure 3) that with increasing use of meropenem during the study period, the mortality actually decreased, again clearly indicating that meropenem was non-inferior to other therapy.
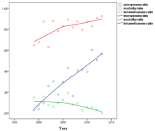
Figure 3: Meropenem and betamethasone usage increased over time.
Mortality ratio showed a declining trend.
There were exactly equal numbers of both sexes. Pneumococci were most prominent 52% (397/770) with meningococci at 13% (98/770) and H influenza at 7% (55/770), the latter a diminishing number due to vaccination, which is also reflected in the distribution in higher (non-vaccinated or failing immunity) age groups (Figure 4). Meningococci clearly affect the younger age groups to a higher extent and the reverse is true for pneumococci. However all age groups are affected by these three organisms. The mortality and sequelae risk varies extensively with bacterial etiology (Table 1).
Recovered
Sequelae
Dead
Total
p
Pneumococci n
18
176
37
397
%
46,3
44,3
9,3
100
Meningococci n
70
26
2
98
%
71,4
26,5
2
100
<0,01
H influenza n
35
18
2
55
%
63,6
32,7
3,6
100
<0,01
Other bacteria n
168
86
21
275
%
61,1
31,3
7,6
100
Total n
422
288
60
770
%
54,8
37,4
7,8
100
Table 1: Risk of death and sequelae depends on bacterial etiology.
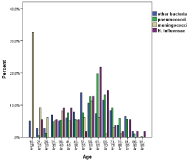
Figure 4: Distribution of microbiological aetiology with age.
A beneficial effect of steroid could not be statistically certified with this limited number of patients and dispersed distribution, although there was a trend (Figure 5).
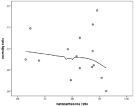
Figure 5: Trend for beneficial effect of betamethasone.
Increasing Reactory Level Scale (RLS) was associated with an increased mortality risk (Odd’s ratio 1.5) for every step in the scale (Figure 6); a clear correlation (p<0.001), but there was no difference between meropenem and cefotaxime/ampicillini treatment (Figure 7).
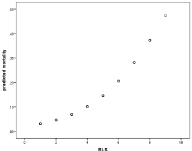
Figure 6: Increasing mortality with Reactory Level Scale, Odd’s ratio 1, 5
per step.
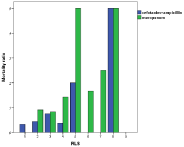
Figure 7: Mortality was augmented with increasing RLS (Reactory Level
Scale) with no difference between meropenem and cefotaxime/ampicillin.
In the first years about 10% of patients received antibiotics within 1h, which rose to >40% during study period (Figure 8) and probably contributed with steroid to improve prognosis (Figure 3). Not recommended antibiotics were associated with increased mortality and more patients were treated at a later stage (p<0.01). This has previously been described to increase the odds for unfavourable outcome by 30% for every hour of delayed therapy [9]. Strangely this effect was not clear in the selected population, but was indicated by the decreased mortality and increased speed of treatment (Figure 3 and 8) and it was also clear that treatment was started earlier during the last years (Figure 8). Thus the combined effect of antibiotics given earlier and steroid added increasingly with the registered time period probably contributed to the lowered mortality seen (Figure 3). Delayed therapy was shown to influence outcome in the non-selected group (p<0.01) but not in the study group (p=0.6).
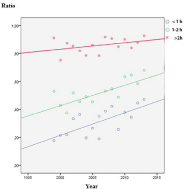
Figure 8: Time to adequate therapy.
Increased mortality was also seen with age and was associated with an Odd’s ratio of 1.3 for every decade of life (Figure 9). This also would reflect the bacterial etiology distribution, which is skewed to higher ages for pneumococci and associated with more severe disease as reflected in mortality as well as sequealae rate (Figure 4, Table 1).
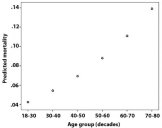
Figure 9: Increasing mortality with age group (decades), Odd’s ratio 1.3 per
decade.
Discussion
No statistical difference (p=0.67) between meropenem mono therapy vs cefotaxime/ampicillin combination treatment could be found (with or without cortisone).
No comparative prospective randomized study for treatment of ABM with meropenem monotherapy has to our knowledge been performed in an adult population [10]. The IDSA (Infectious Disease Society of America) guidelines [11] are mainly based on evidence graded as BIII, i.e., moderate evidence for support based on opinions of respected authorities and not any RCT. Since such a study would be costly and very difficult to perform, the need for a retrospective analysis is emphasized.
Many factors influence the mortality and sequelae rates in ABM
• Delayed treatment is a well-known factor but is difficult to evaluate, since the exact starting point for the individual to contract pathogen is unknown. Symptoms are thought to appear within 24-48 h [12]. Even so, the time from the mucosal phase to the septic phase may vary among patients with different immunogenetics. Natural Toll-receptors [13] and specific immune defence genes as HLA, cytokine and interleukin response and other factors will influence the course of the infection. The time to treatment was rather compressed in the selected subgroup. The population given not recommended antibiotics had a more dispersed time span with more delayed therapy and an ensuing significantly increased mortality (p<0.001).
• The aetiologic bacterial species will affect individuals differently. Pneumococci have a higher mortality and sequelae rate (p<0.01), and this was also reflected here (Table 1), which also shows Haemophilus influenzae to have a high sequaelae rate (p<0.01), but a lower risk for meningococci.
• It seems well established that steroid treatment is beneficial for pneumococci [1]. With the selected population it was not evident after statistical evaluation, but there was a trend (Figure 4). The beneficial effect of steroids was published in 2004, reflected in an increasing ratio of steroid treated patients (Figure 3).
Certainly lumbar puncture and bacterial culture remains the mainstay for diagnosis. However, diagnostic procedures (esp CT) delay the start of therapy. It may be wiser to emphasise a paradigm of prioritizing a shortened time to treatment. Atiological diagnosis can be secured later, e.g. by the aid of Nucleic Acid Tests (NAT) like PCR, which will ensure diagnosis even after antibiotics are instituted. NAT procedures are increasingly becoming fast, and might in the future be used to guide specific therapy, instead of guessing empiric therapy. Blood culture yields positive results in about 80% of the patients and the spinal fluid may take many hours to become negative (depending on bacteria). However for the near future and in resource-poor settings [12] we need to start with a good coverage. Our data may be an argument for using meropenem as empiric therapy, especially if ESBL abound. Coverage of MRSA/ARE/VRE etc. is not the goal for this study (we have too few cases in our population), but addition of vancomycin or linezolide (evidence is accumulating) may be unavoidable in certain settings, e.g. neurosurgical or traumatic ABM.
The main goal and first question of this paper was whether empiric meropenem monotherapy would be equivalent to other treatment. Other treatment usually means cefotaxime, mostly with the addition of ampicillin, since many patients are >50 years or immunosuppressed. However, ampicillin alone or even bensylpenicillin alone were often recorded, probably since the correct diagnosis was not suspected early enough. We hope these inferior empiric therapies will diminish (except after etiologic results become available, but then it is not empiric any more). Since the correct diagnosis may not be immediately recognized, other antibiotics may be given since undefined infection is suspected. It would not be reasonable to compare meropenem to a faulty regimen applied to resistant pathogens. We believe that the narrow spectrum monotherapy sometimes observed usually was instituted in cases after a positive culture, where ABM was not initially suspected. We decided to mainly compare meropenem with cefotaxime/ampicillin –which should be a fair comparison.
The figures do not indicate inferiority for meropenem regarding mortality nor sequelae. The theoretical prediction is that meropenem would have coverage for more pathogens, which should be reflected in mortality statistics, given a large enough data base. It may be prudent to add here that MRSA are not common ABM agents in Sweden, in fact only one case was recorded (with a fatal outcome).
By international comparison Sweden has less problems with antibiotic resistance, <1% MRSA, penicillin resistant pneumococci and ESBL, although ESBL and especially ESBL-carba increased with 80% in 2015 as compared to 2014 [14]. Strong national organizations and the presence of infectious disease specialists since >60 years have created a very good adherence to antibiotic policy documents, using drugs sparsely and with narrow directed spectrum. There is no sale of antibiotics without prescription and the veterinary use is extremely limited compared to e.g. the US (about twenty times less). No antibiotics are allowed as “growth promoters” in EU. Yet overuse of all antibiotics especially cephalosporins during the last decades and to a lesser degree quinolones, has led to increased spread of imported ESBLs. This may lead to future cephalosporin failure and escalate empiric therapy to carbapenems (and tazobactam) for many indications, since they have a broader coverage and also kill bacteria fast. As yet a limited number of carbapenemase producing strains have been found in Sweden [14], but they are rising alarmingly in many countries. A trend towards substituting piperacillin/ tazobactam for cephalosporin has been noted, perhaps motivated by better coverage, alternate use or trying to avoid a selection pressure. Carbapenems possess several advantageous properties like rapid penetration of Gram-negative bacteria, high PBP (penicillin binding proteins) affinities, unrivalled antibacterial spectra and exceptional beta-lactamase stability [8].
In order to combat ABM many approaches are needed
• Vaccination has been a great success for Haemophilus influnzae in youngsters, but seems also to have diminished the number in adults. Pneumococcal vaccination may improve the situation, but is not expected to be as dramatic. Conjugated pneumococcal vaccines which might have a better effect against pneumococcal invasive disease, might be more effective [15]. In the US meningococcal vaccine has also lowered ABM incidence [15].
• Early recognition and early therapy is mandatory [16] but unfortunately leaves room for improvement, especially regarding clinical vigilance and high suspicion for ABM. Positively such a development was noted (Figure 8).
• The choice of empiric therapy is important and has been discussed at length and different recommendations are suggested in different countries. To cover for Listeria, amoxicillin has to be added if cefalosporins are chosen as empiric therapy. This could be limited to the youngest (<2y), the oldest (>50y) and the immunosuppressed. The adherence to this combination therapy has been less than optimal (45%) so far, probably mainly reflecting missed/delayed diagnosis. Furthermore, on pure theoretical grounds meropenem has a slightly broader spectrum and covers for Listeria [10,17,18], although there is limited clinical experience, since Listeria is a comparatively rare aetiology in ABM. Meropenem as first line empiric monotherapy for acute bacterial meningitis has been used increasingly at many sites in Sweden and with good result as is presented here (Figure 3). In a simulation pediatric study meropenem seemed superior [19]. A theoretic calculation also showed less therapeutic failure with meropenem [4]. Since use of meropenem has increased considerably the need for a clinical evaluation has increased significantly.
• The advent of ESBL bacteria on the scene as well as other multidrug resistance has in some countries forced heavy empiric first line therapy and may in the future accelerate in many more countries.
• Many studies have demonstrated increased mortality and sequelae with delayed therapy in sepsis [20] and this is also true for ABM [9], although proper controlled studies obviously cannot be performed. Data from animal studies would corroborate the statement and historical results, before antibiotics were available, indicate mortalities above 70%. Even in modern studies mortality up to 34% [3,21] are cited and is certainly rising with age and multimorbidity. RLS might to some extent mirror all-cause delay and showed a significant worsening (OR=1.5) with every step (Figure 6).
Carbapenems should be carefully restricted. The number of adult ABM in Sweden is <100/year, meaning an incidence <1/100,000 (9.6 million inhabitants 2013). There was not a rising incidence trend (Figure 1) in the subselection we made, which represents 45% of all ABM cases. In consequence empiric therapy for 2-3 days, meaning 2-300 DDD or <0.01% of all antibiotic doses, would not make an impact on the selection pressure, even with some overtreatment, but could be lifesaving and probably decrease ensuing morbidity/sequelae (Table 1). However, other important steps like speed of therapy, (by speeding or postponing diagnostic procedures) as well as adjunctive therapy must also be at hand to improve outcome.
The register covers historical cases, when steroids were not so clearly indicated. The increasingly lower mortality may reflect prompter institution of therapy and beneficial effect of steroids rather than lower antibiotic resistance. In fact only 1 case of MRSA may have represented non-coverage of cefotaxime/ampicillin or meropenem. Gram-negative bacteria are not very common as meningitis agents and ESBL have only become more prominent in the very last years, thus may not be reflected in the register as failure of cefotaxime/ ampicillin. RLS, age, time to adequate treatment, pathogen resistance patterns and individual comorbodity (or immune function) are the main factors that influence outcome. Sequelae were not more common in the meropenem group. Meropenem thus could be recommended as a first line empiric monotherapy in Sweden, given the low number of MRSA and an increasing number of ESBL. It might be simpler to handle for often less experienced emergency doctors than a combination therapy and better compliance may be a benefit. Cefotaxim has a metabolite (des-acetyl-cefotaxime) which penetrates well into CNS. However there is no reason to believe that meropenem would not penetrate, especially not in the initial phase when the inflamed meninges are regarded to be leaky. It is well known for imipenem/cilastatin that it has a risk of neurotoxicity and causes seizures in up to 30% of cases, which indicates that it is penetrating and affecting neuronal cells. It is for this reason not a recommended carbapenem for ABM. No such side effect has been implicated with meropenem. Studies regarding carbapenem penetration into CSF have been performed [5,22] and indicate satisfactory CNS distribution. Too little data is available for other, newer carbapenems at this stage.
In addition to rapid diagnosis and treatment there has been some controversy whether steroids should be used [1,23,24]. Our data from the registry show a beneficial trend but could not be shown to give a significant boost on survival (Figure 5). We have no reason to doubt that it should be used and one explanation why a beneficial effect could not be clearly demonstrated might be a low number and dispersed distribution.
Discouraging data concerning spread of ESBL and multiresistance at large [25-27] will most likely force many countries into a more or less desperate carbapenem usage. This will in a distant future cause enhanced emergence of carbapenemase production among microbial metabolic masters and there are already alarming reports of global spread of ESBL carba [28], residing on transferable plasmids. After attacks of ESBL producing strains, hospitals were required to adopt new antibiotic regimens in order to avoid selection pressure. In the future even more restrictions on antibiotic practice are foreseen. It may also be wise already now to use carbapenems with utmost care and save them for inevitable situations. We feel however that meropenem monotherapy could be well suited for a rare and dangerous situation like ABM. For sepsis other alternatives are at hand and one-sided selection pressures should be avoided, although in severe sepsis with shock, carbapenems should be one option. Including an aminoglycoside may be wise in septic shock to stop endotoxin production and increase the antibiotic spectrum. Cautioning against wide spread carbapenem use is with good cause, since there is a real concern for an ESBL carba pandemic [14].
This investigation clearly indicates that meropenem monotherapy strategy is non-inferior in our present environment, but future investigations are needed to monitor sequelae, quality of life and risk of emerging resistance. In countries with a high frequency of highly resistant gram positive bacteria (MRSA, PRP, ARE, VRE) it may be necessary to add vancomycin, rifampicin or linezolide, which is included in the established empiric regimen in many places and should be added in neurosurgical or traumatic meningitis.
Acknowledgement
Excellent working facilities the Department of Research and Development, Region Kronoberg, Central Hospital, Växjö and the Department of Infectious Diseases, Karolinska Hospital, Huddinge were a prerequisite for the evaluation. Economic support from Astra at an early stage is valued. Anna Lindgren and Per Nyberg are heartily thanked for help with statistic evaluations. All physicians at all clinics for infectious diseases who added acute bacterial meningitis patient data into the register are heartily thanked for a most valuable contribution. The funding and maintenance of the ABM register by the Swedish Society for Infectious Diseases was fundamental to this study. For expert help with figures Laszlo Illes is acknowledged.
Conflict of Interest
A stipend for dedicated work with the quality register was awarded to JF by Astra-Zeneca Drug Company. Meropenem is now available as a generic drug.
References
- van de Beek D, de Gans J, Spanjard L, Weisfelt M, Reitsma J, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004; 351: 1849-1859.
- Mace SE. Acute bacterial meningitis. Emerg Med Clin North Am. 2008; 26: 281-317.
- Tunkel A. Initial therapy and prognosis of bacterial meningitis in adults. 2015: 1-28.
- SILF. Vardprogram, bakteriella CNS infektioner. 2010.
- Nau R, Lassek C, Kinzig-Schippers K, Thiel A, Prange H, Sorgel F. Disposition and elimination of meropenem in cerebrospinal fluid of hydrocephalic patients with external ventriculostomy. Antimicrobial Agents and Chemotherapy. 1998; 42: 2012-2016.
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the bloodcerebrospinal fluid/blood-brain barrier and treatment of central nervous system infections. Clinical microbiology reviews. 2010; 23: 858-883.
- Norrby R. Neurotoxicity of carbapenem antibiotics: consequences for their use in bacterial meningitis. Journal of Antimicrobial Chemotherapy. 2000; 45: 5-7.
- Drusano G. Meropenem: laboratory and clinical data. Clinical Microbiology and Infection. 1997; 3: 4S51-4S59.
- Koster-Rasmussen R, Korshin A, Meyer C. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008; 57: 449-454.
- Baldwin CM, Lyseng-Williamson KA, Keam SJ. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs. 2008; 68: 803-838.
- Tunkel A, Hartman B, Kaplan S, Kaufma B, Roos K, Scheld W, et al. Practice guidelines for the management of bacterial meningitis. IDSA Guidelines. Clinical Infectious Diseases. 2004; 39: 1267-1284.
- Scarborough M, Thwaites GE. The diagnosis and management of acute bacterial meningitis in resource-poor settings. Lancet Neurol. 2008; 7: 637- 648.
- Koedel U, Scheld W, Pfister H. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2002; 2: 721-736.
- Dahl A. Kraftig men väntad ökning av ESBLcarba. Läkartidningen. 2015; 112: DS7W.
- Castelblanco R, Lee M, Haburn R. Epidemiology of bacterial meningitis in the USA from 1997 to 2010: a population-based observational study. Lancet Infect Dis. 2014; 3099: 70805-70809.
- Gjini A, Stuart J, Cartwright K, Cohen J, Jacobs M, Nichols T, et al. Quality of in-hospital care for adults with acute bacterial meningitis: a national retrospective survey. 2006.
- Manfredi R, Sabbatani S, Marinacci G, Salizzoni E, Chiodo F. Listeria monocytogenes meningitis and multiple brain abscesses in an immunocompetent host. Favorable response to combination linezolidmeropenem treatment. J Chemother. 2006; 18: 331-333.
- Stepanovic S, Lazarevic G, Jesic M, Kos R. Meropenem therapy failure in Listeria monocytogenes infection. Eur J Clin Microbiol Infect Dis. 2004; 23: 484-486.
- Ellis J, Kuti JL, Nicolau DP. Pharmacodynamic evaluation of meropenem and cefotaxime for pediatric meningitis: a report from the OPTAMA program. Paediatr Drugs. 2006; 8: 131-138.
- Kumar A, Roberts D, Wood K, Light B, Parrillo J, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human shock. Crit Care Med. 2006; 34: 1589-1596.
- van de Beek D, de Gans J, Tunkel A, Wijdicks E. Community acquired bactyerial meningitis in adults. N Engl J Med. 2006; 354: 44-53.
- R N, F S, H E. Penetration of drugs through the blood-cerebrospinal fluid/ blood-brain barrier and treatment of central nervous system infections. Clinical microbiology reviews. 2010; 23: 858-883.
- Chaudhuri A. Adjunctive dexamethasone treatment in acute bacterial meningitis. Lancet Neurol. 2004; 3: 54-62.
- van de Beek D, de Gans J, McIntyre P, Prasad K. Corticosteroids in acute bacterial meningitis. Cohcrane Database Syst Rev. 2003; 3: CD004405.
- Brink A, Moolman J, da Silva MC, Botha M. Antimicrobial susceptibility profile of selected bacteraemic pathogens from private institutions in South Africa. S Afr Med J. 2007; 97: 273-279.
- Mokaddas EM, Rotimi VO, Albert MJ. Increasing prevalence of antimicrobial resistance in Streptococcus pneumoniae in Kuwait: implications for therapy. Microb Drug Resist. 2007; 13: 227-233.
- Srifeungfung S, Chokephaibulkit K, Tribuddharat C. Serotypes and antimicrobial susceptibilities of Streptococcus pneumoniae isolated from hospitalized patients in Thailand. Southeast Asian J Trop Med Public Health. 2007; 38: 469-477.
- Chen L. Klebsiella pneumoniae carbapenemase: Extended-spectrum betalactamase continues to go global. 2009.