Review Article
Austin Neurosurg Open Access. 2014;1(3): 1011.
Management of Diffuse Lesions in Traumatic Brain Injury in Brazil
Robson Luis Oliveira de Amorim1*, Almir Ferreira de Andrade1, Wellingson Silva Paiva1, Rodrigo Moreira Faleiro2, Ruy Monteiro3 and Manoel Jacobsen Teixeira1
1Division of Neurosurgery, Hospital das Clinicas University of Sao Paulo Medical School, Brazil
2HJXXIII Trauma Center, Hospital Felicio Rocho, Belo Horizonte-MG, Brazil
3Emergency Department, Hospital Miguel Couto; Brazilian Neurotrauma Group (Neurotrauma Brazil), Brazil
*Corresponding author: Robson LO Amorim, Division of Neurosurgery, Hospital das Clinicas University of Sao Paulo Medical School, 718 Alves Guimar&aTilde;es St, ap 112, Zip Code 05410-001, S&aTilde;o Paulo, SP, Brazil
Received: March 05, 2014; Accepted: May 25, 2014; Published: May 26, 2014
Abstract
Diffuse traumatic brain injuries are due to tangential forces that cause encephalic rotation inside the skull. This leads to distension⁄rupture of axons or even vascular structures in different encephalic regions. Diffuse injury is the most common and complex lesion in traumatic brain injury. The management includes intracranial monitoring, advanced intensive care and decompressive surgeries. In this paper the authors review the classification and management of patients with diffuse injuries and present the guidelines adopted by the Brazilian Neurosurgery Society and the Brazilian Medical Association.
Keywords : Traumatic brain injury, Diffuse axonal injury, Decompressive craniectomy, Guidelines
Introduction and Background
Traumatic brain injury (TBI) is the main cause of death and morbidity in children and young adults in Western industrialized countries [1]. Kelly [2] have estimated an incidence of five hundred thousand of new head trauma cases per year, and a prehospital mortality rate of fifty thousand cases. A large number of these patients have diffuse injuries and need a multidimensional support, usually in an intensive care unit. Traffic accidents, falls and assaults are the main causes of TBI. In Brazil, we verified an incidence of 456/100,000 and mortality of 37.99⁄100.000 [3], which represents a true epidemic, with great social burden and significant costs to the health system. In this paper, the authors present a summary of the guidelines recommended by the Brazilian Neurosurgery Society and the Brazilian Medical Association and review, with the best evidence available, each treatment performed.
The bibliographic search used the PubMed database (National Library of Medicine) and Lilacs, covering the period from 1940 to 2013. The keywords used were: “head trauma”, “brain injury”, “diffuse axonal injury”, “diffuse lesion”, “intracranial pressure monitoring” and the combinations of terms. It was considered the best evidence available for each treatment modality. The exclusion criteria were articles not written in english or portuguese.
Terminology
Diffuse TBI are classified as mild, classic concussion and diffuse axonal injury (DAI) [4]. Generally, severe diffuse TBI is associated with DAI. Patients sustaining mild concussion have a history of transient reversible neurological disturbance with no unconsciousness period and might be divided in three subtypes. The first group is comprises patients with transient mental disturbance that persists for a few seconds, without amnesia. Patients with retrograde amnesia of five to ten minutes are classified in the second group. The third group comprises patients with retrograde and posttraumatic amnesia [5].
Classic concussion is characterized by an unconsciousness period of up to six hours posttrauma, always with some degree of retrograde and posttraumatic amnesia. The duration of posttraumatic amnesia period is an important factor on prognostic evaluation. Patients with concussion may develop persistent headache in 79% of cases, posttraumatic amnesia in 59% and, 34% of patients do not return to work [4].
DAI is clinically considered when the patient remains more than six hours in coma after other known causes of neurological deterioration have been excluded. It is subdivided in mild, moderate and severe [5]. In this paper, we discuss the management of diffuse TBI in coma patients.
Pathophysiology
Diffuse TBI are due to tangential forces that cause encephalic rotation inside the skull [5]. DAI, which refers to extensive lesions in white matters tract, is a histological representation of diffuse TBI. The pathology of DAI in humans is characterized histologically by widespread damage to the axons of the brainstem, parasagittal white matter of the cerebral cortex, corpus callosum, and the gray–white matter junctions of the cerebral cortex.
Definitive posttraumatic encephalic injuries result from pathophysiological mechanisms that are triggered at the time of trauma and continue for days or weeks. Brain swelling can evolve after a diffuse injury, and can get worse if occur hypoxia and hypotension. Therefore, these brain lesions are classified as primary (caused by the initial impact to the skull) and secondary lesions (caused both by the inflammation generated following the initial impact and by external factors, as hypoxia) [6,7].
The primary lesions occur at the time of trauma. High kinetic energy causes cerebral movement that is the primary factor in diffuselesions. Important to state is that a direct impact over the skull is not a predisposing condition for the development of diffuse lesion. Brain and skull have different responses to the same forces applied during a head trauma due to their different densities. These differences in movement may lead to cerebral vein rupture and also, impact of the brain against rigid skull structures. Additionally, peripheral encephalic regions have higher amplitude of movement than central regions because of the stability given by the brainstem. Consequently, the stretches of axons and vessels may lead to temporary dysfunction or even complete rupture [8].
Secondary lesions are due to disturbances occurring after trauma and involve intra and extra cranial factors that lead to cell death. Clinical complications after trauma as hypoglycemia, hypoxia, high or low CO2 levels, hypertermia and hydroelectrolytic disturbances are the most common causes of secondary lesions. Later, other metabolic and infectious systemic disturbances are added, together with the presence of neurotoxic substances, hydrocephaly and intracranial hemodynamic alterations [8,9]. Finally there are also cellular death mechanisms, neuronal, endothelial and glial mechanisms, due to biochemical and ionic disturbances related to both primary and secondary lesions. Understanding all these factors is essential to the management of head trauma. These different mechanisms of cellular injury involving specific biochemical pathways and locations of injury may, in part, explain the lack of success in drug trials to ameliorate the prognosis of TBI patients.
Imaging Diagnosis
The brain CT scan of DAI patients may be “normal” or exhibit small hemorrhagic lesions, mainly on frontal or parietal cortex, also known as gliding contusions; other common sites of those lesions are: periventricular region, corpus callous, thalamus, basal ganglia and posterolateral portion of midbrain are [6]. When CT scan is normal, spectroscopy and diffusion weighted magnetic ressonance imaging (MRI) are indicated because of their high accuracy to detect well defined anatomic lesions as a brighten image on splenium of corpus callosum [10]. Traumatic subarachnoid hemorrhage (TSAH), acute subdural hematoma and brain contusions can be present in patients with DAI, because of its similar trauma mechanisms.
Interesting to notice is that some level of axonal injury may exist in mild TBI patients. These patients have traumatic axonal injury (TAI) and the finding of low fractional anisotropy correlates to poor clinical outcome in such patients [11,12].
Radiologic Imaging Classification
Diffuse brain lesions are common in severe head trauma patients. They may be present in moderate trauma patients and are rare amongmild head trauma patients. The Marshall classification is the most common classification of the diffuse injuries used in Brazil, where the intracranial lesions are classified based on CT scan findings [13].
- Type I diffuse lesions: no pathological findings on CT scan.
- Type II diffuses lesions: basal cisterns are present; midline shift < 5 mm and⁄or hyper dense focal lesions with a volume smaller than 25 cm3 (including bone fragments or foreign bodies).
- Type III diffuse lesions: Diffuse brain swelling with bilateral cisternal compression, midline shift < 5 mm,with no hyper dense focal lesions > 25 cm3
- Type IV diffuse lesions: Hemispheric brain swelling with midline shift > 5 mm, with no focal lesions greater than 25 cm3.
- Type V and type VI lesions of Marshall CT scan classification are those with focal lesions: lesions treated surgically are type V; non surgical lesions bigger than 25 cm3 are classified as type VI.
This classification is used to guide the management of TBI in our country. It also allows the emergency physician to identify high–risk patients for intracranial hypertension, enabling early neurosurgical intervention. The non enhanced CT scan enables identification of diffuse brain swelling in patients with head trauma by the compression (or absence) of the intracranial cerebrospinal fluid space, in the absence of other intracranial disturbances. Brain swelling may occur both associated with focal lesion and with DAI. Acute brain swelling may worsen DAI; although it does not occur in all cases of DAI, swelling may trigger deleterious effects to the primary lesion causing intracranial pressure (ICP). Acute swelling is most commonly associated with severe TBI, whereas late swelling may be associated with mild scenarios. Even though DAI and swelling are associated, they are different groups of brain injuries with different pathophysiologic mechanisms and outcomes [13].
Marshall’s type IV diffuse lesion may be isolated, but it is frequently associated with laminar acute subdural hematoma. Although it is rare, it can also be associated with focal intracranial lesions as epidural hematoma or cerebral contusion [14,15]. In conjunction to Marshall's IV lesions, the type III lesions have the worst outcome in 6 months [15].
When we assess a cranial CT scan of a severely injured patient we must look to some factors related to prognosis. The absence of basal cisterns may predict patients’ evolvement, and there is a correlation between the presence of cisterns in the first CT scan and determination of prognosis [16]. In severe head trauma, mortality rate in cases of absent basal cisterns is 77%, in compressed cisterns it is 39%, and in normal cisterns, it is 22%. The state of the basal cistern on head trauma is a critical factor to determine the risk of increase in ICP, thus the absence or compression of cisterns is associated with elevated ICP. Approximately 75% of the patients with absence of cisterns and 55% with compression of cisterns had an early and maintained increase in ICP higher than 30mm Hg. Mortality is 57% [16].
Management
After hospital admission with resuscitation by ATLS and Brazilian Neurotrauma Group protocol, patients underwent neurosurgical examination, cervical spine and plain chest radiography, and cerebral computed tomography (cervical spine CT are performed in special cases). Patients with TBI and normal hemodynamic status are generally admitted to ICU. Medical management includes intubation, normoventilation, oxygenation, head elevation (30–45°), fluid resuscitation, and sedation. Patients with severe TBI (3–8 points in Glasgow Coma Scale) with diffuse brain swelling must be under went to intracranial pressure monitoring [17].
The clinical management to control ICP in the ICU begins with preventive measures such as lifting the back of the bed to 30 degrees, keeping the patient in neutral alignment of the head and maintenance of patent airways to allow optimized ventilation. Patients are maintained in normoventilation. However, mild hyperventilation (30–35 mm Hg) is performed under controlled situation, in patients showing hyperemia (through DTC). Hypocapnia is effective in the short term to decrease CBF and CBV. If the PaCO2 decreases below 18–20 mm Hg, may lead to ischemia [18]. We have occasionally used brain oximetry and microdialysis, in special cases, but are not protocol in Brazil, because these methods present high cost in our country.
Some brazilian neurosurgical centers have performed ICP monitoring with continuous cerebrospinal fluid drainage by external ventricular drainage (EVD) in patients with head trauma. In these patients, this method allowed favorable outcome in 49.2% of cases and a mortality of 13% [17]. Continuous cerebrospinal fluid drainage when compared to intermittent, decreased levels of neuron–specific enolase suggesting that this drainage method may contribute to reduce more effectively neuronal death in children with brain injury [18]. In addition, recent studies have shown that continuous cerebrospinal fluid drainage, are related to better ICP control [19,20]. However, it may be associated with adverse events, as infection [18]. Few centers in Brazil induce mild hypothermia (34–35o C) in ICU for traumatic brain injuries. Most centers maintain hypothermia until ICP control. Hypothermia, theoretically, should be effective in reducing death and unfavorable outcomes for traumatic head injured patients, however in last Cochrane systematic review, a significant benefit was only found in low quality trials [21]. A post hoc analysis of two randomized control trials showed that hypothermia (35o C reached within 1.5h after craniotomy) seems to improve outcomes in patents with evacuated mass [22].
In patients with clinical signs of uncal herniation, dysfunction of the cerebral stem or intracranial expansive lesions requiring immediate surgery, craniotomy is indicated, evacuation of the hematoma followed by ICP monitoring [5,23].
Sedation withdraw is performed guided by the intracranial pressure and/or radiologic control with CT scan. The recommendation is to repeat CT scan in 6–12h in all patients with TBI and intracranial bleeding [5]. Other CT scan is performed according to ICP. The use of DTC is widespread in Brazil, therefore, in some centers, the sedation withdraw is guided by this exam [24].
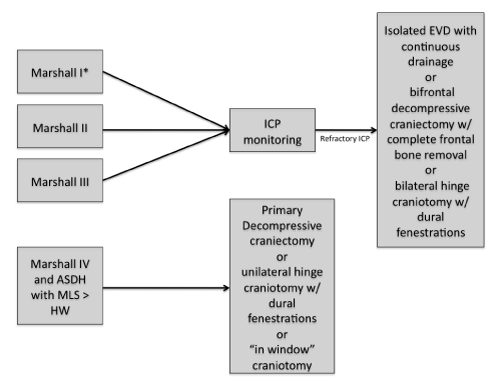
Specific management according to Marshall's classification (Figure 1)
Figure 1: Summary of the surgical management of diffuse TBI in Brazil. ASDH: Acute Subdural Hematoma; MLS: Midline Shift; HW: Hematoma Width. *ICP monitoring is considered if the patients have two of the following conditions: hypoxia, hypotension an pathologic postures.
Type I, II and III diffuse lesion
In coma patients, we have considered ICP monitoring in all coma patients with type I, II and III Marshal Lesions. In type I lesions we perform ICP monitoring when patient presents two of the following: hypotension, pathological posture and age above 45 years old [5]. Leitgeb [25] described that monitoring ICP is beneficial in patients with severe head trauma. However, the only high quality RCT available showed no benefit in terms of functional outcome and mortality in patients undergoing ICP monitoring [26].
These patients can evolve with refractory ICP (ICP > 25 mmHg). In such cases, the management differs among centers: EVD with continuous drainage, bifrontal craniectomy (with complete bone removal) and dural expansion, and hinge bilateral craniotomy with dural fenestrations. The DECRA trial is the only RCT available addressing the role of craniectomy for TBI [27]. This study showed a clear reduction in ICP in the intervention group however there were no benefits in terms of functional outcome. The main criticisms of this trial were about the inclusion criteria and the surgical technique performed (bifrontal craniectomy with a bone bridge over the supeior sagital sinus). In children, a bitemporal craniectomy without dural opening may show some benefit [28].
In Brazil, not uncommon, the use of ICP monitoring is limited due to high costs. To manage such patients, they are submitted to daily CT scans and to daily DTC. If there is neurological, imaging or hemodynamic worsening, the patients are managed with deepsedation and manitol⁄hypertonic saline.
Type IV diffuse lesion or hemispheric cerebral swelling
The recommendation in cases of Type IV lesion is to perform a unilateral decompressive craniectomy (DC) – primary DC (Figure 1) [29]. In Brazil, the use of DCs for trauma seems to increase and, the presence of an expressive number of patients with skull defects has become an issue. As DC is an emergent surgery and cranioplasty is not, the number of patients with skull defects increases. We believe that in low and middle income countries, this problem tend to increase and it is up to each institution to provide the cranial reconstructions in a systematized way, as an elective surgery. Some centers in Brazil perform “in window” craniotomy or unilateral hinge craniotomy with dural fenestrations to avoid such problems [30,31]. The same rationale is used to the treatment of associated intracranial hematomas. In patients with acute subdural hematoma greater than 25 cm3, if the admission CT scan shows a midline shift greater than the width of the hematoma, the patients are treated as sustaining a type IV lesion.
Conclusion
In Brazil, the management of diffuse TBI is guided according to Marshall's classification and, in general, is in accordance with the best evidence available, which is poor. In low and middle–income countries, the guidelines should be optimized to each health system, aiming to improve outcome without compromising the funding sources.
References
- Andriessen TM, Jacobs B, Vos PE. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J Cell Mol Med. 2010; 14: 2381-2392.
- Kelly DF, Nikas DL, Becker DP. Diagnosis and Treatment of Moderate and Severe Head Injury in Adults. In: Youmans JR, editor. Neurological Surgery. 5ª edn. Pennsylvania: WB Saunders. 2004; 1618-1718.
- Maset A, Andrade AF, Martucci SC, Frederico LM. Epidemiologic features of head injury in Brazil. Arq. bras. Neurocir. 1993; 12: 293-302.
- Gennarelli TA. Cerebral Concussion and Diffuse Brain Injuries. Cooper PR, editor. In: Head Injury. 3a edn. Baltimore: Williams & Wilkins. 1993; 137-158.
- Andrade AF, Ciquini Jr. O Figueiredo EG, Brock RS, Marino Jr. R. Diretrizes do atendimento ao paciente com traumatismo cranioencefálico. Arq Bras de Neurocir. 1999; 18: 131-176.
- Mendelow AD, Teasdale G, Jennett B, Bryden J, Hessett C, Murray G. Risks of intracranial haematoma in head injured adults. Br Med J (Clin Res Ed). 1983; 287: 1173-1176.
- Paiva WS, de Andrade AF, de Amorim RL, Muniz RK, Paganelli PM, Bernardo LS, et al. The prognosis of the traumatic subarachnoid hemorrhage: a prospective report of 121 patients. Int Surg. 2010; 95: 172-176.
- Andrade AF, Paiva WS, Amorim RL, Figueiredo EG, Rusafa Neto E, Teixeira MJ. [The pathophysiological mechanisms following traumatic brain injury]. Rev Assoc Med Bras. 2009; 55: 75-81.
- Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003; 126: 515-530.
- Cecil KM, Hills EC, Sandel ME, Smith DH, McIntosh TK, Mannon LJ, et al. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J Neurosurg. 1998; 88: 795-801.
- Lipton ML, Kim N, Park YK, Hulkower MB, Gardin TM, Shifteh K, et al. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging Behav. 2012; 6: 329-342.
- Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007; 24: 1447-1459.
- Marshall LF, Marshall SB, Klauber MR. A new clasification of head injury based on computerized tomography. J Neurosurg. 1991; 75: S14-S20.
- Zumkeller M, Behrmann R, Heissler HE, Dietz H. Computed tomographic criteria and survival rate for patients with acute subdural hematoma. Neurosurgery. 1996; 39: 708-712.
- Maas AI, Steyerberg EW, Butcher I, Dammers R, Lu J, Marmarou A, et al. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007; 24: 303-314.
- Toutant SM, Klauber MR, Marshall LF, Toole BM, Bowers SA, Seelig JM, et al. Absent or compressed basal cisterns on first CT scan: ominous predictors of outcome in severe head injury. J Neurosurg. 1984; 61: 691-694.
- Andrade AF, Paiva WS, Amorim RL, Figueiredo EG, Almeida AN, Brock RS, et al. Continuous ventricular cerebrospinal fluid drainage with intracranial pressure monitoring for management of posttraumatic diffuse brain swelling. Arq Neuropsiquiatr. 2011; 69: 79-84.
- Shore PM, Thomas NJ, Clark RS, Adelson PD, Wisniewski SR, Janesko KL, et al. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J Neurotrauma. 2004; 21: 1113-1122.
- Nwachuku EL, Puccio AM, Fetzick A, Scruggs B, Chang YF, Shutter LA, et al. Intermittent versus continuous cerebrospinal fluid drainage management in adult severe traumatic brain injury: assessment of intracranial pressure burden. Neurocrit Care. 2014; 20: 49-53.
- Lescot T, Boroli F, Reina V, Chauvet D, Boch AL, Puybasset L. Effect of continuous cerebrospinal fluid drainage on therapeutic intensity in severe traumatic brain injury. Neurochirurgie. 2012; 58: 235-240.
- Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Syst Rev. 2009; CD001048.
- Clifton GL, Coffey CS, Fourwinds S, Zygun D, Valadka A, Smith KR Jr, et al. Early induction of hypothermia for evacuated intracranial hematomas: a post hoc analysis of two clinical trials. J Neurosurg. 2012; 117: 714-720.
- Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999; 90: 187-196.
- Bor-Seng-Shu E, Hirsch R, Teixeira MJ, De Andrade AF, Marino R Jr. Cerebral hemodynamic changes gauged by transcranial Doppler ultrasonography in patients with posttraumatic brain swelling treated by surgical decompression. J Neurosurg. 2006; 104: 93-100.
- Leitgeb J, Erb K, Mauritz W, Janciak I, Wilbacher I, Rusnak M; Australian Severe TBI Study Investigators. Severe traumatic brain injury in Austria V: CT findings and surgical management. Wien Klin Wochenschr. 2007; 119: 56-63.
- Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. Global Neurotrauma Research Group. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012; 367: 2471-2481.
- Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'Urso P, et al. DECRA Trial Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011; 364: 1493-1502.
- Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001; 17: 154-162.
- de Andrade AF, Marino R, Ciquini O, Figueiredo EG, Machado AG. Guidelines for neurosurgical trauma in Brazil. World J Surg. 2001; 25: 1186-1201.
- Valença MM, Martins C, da Silva JC. "In-window" craniotomy and "bridgelike" duraplasty: an alternative to decompressive hemicraniectomy. J Neurosurg. 2010; 113: 982-989.
- Andrade AF, Amorim RL. Paiva WS, Sousa LM, Santana D, Teixeira MJ. New Technique for Surgical Decompression: Merging Two Concepts to Prevent Early and Late Complications of Unilateral Decompressive Craniectomy with Dural Expansion. Poster presentation, ICP 2012, Barcelona. 2012.