
Review Article
Austin Neurosurg Open Access.2015;2(1): 1024.
Immune Cell Infiltrates in the Central Nervous System Tumors
Andrew S. Jack¹ and Jian-Qiang Lu²*
1Department of Surgery, University of Alberta Hospital,Canada
2Department of Laboratory Medicine and Pathology,University of Alberta, Canada
*Corresponding author: Jian-Qiang Lu, Department of Laboratory Medicine and Pathology, University of Alberta, 5B2.24 WCM Health Sciences Centre, 8440-112 Street, Edmonton, Alberta T6G 2B7, Canada
Received: March 31, 2014; Accepted: March 01, 2015; Published: March 05, 2015
Abstract
Major therapeutic advances resulting in overall survival increase have not been forthcoming for most Central Nervous System (CNS) tumors, such as high-grade gliomas. Over the past few decades, our understanding of CNS tumor development has greatly increased due to the introduction of new study techniques particularly in immunohistochemistry and molecular biology. These techniques have aided in the discovery and characterization of tumor-immune cell infiltration. More specifically, the infiltration of CNS tumors by immune cells, such as macrophages/microglia, lymphocytes, and eosinophils has been found to play an important role in tumor pathogenesis and progression. Increased understanding of this tumor immune microenvironment has led to the investigation and trial of immunotherapeutic agents in attempt to modulate the patient immune system and promote an anti-tumor response. In this review, we outline the current state of tumor-immune system interaction research by focusing on the latest evidence for CNS tumor-immune cell infiltration, including macrophages, lymphocytes, and eosinophils. Furthermore, immune cell interaction with the blood-brain barrier leading to infiltration and selected immunotherapeutic strategies are reviewed.
Keywords: Tumor immune microenvironment; Tumor infiltrating lymphocytes (TILs); Glioma associated macrophages/microglia (GAMs); Tumor associated tissue eosinophila (TATE); CNS tumors; Glioblastoma; Immunotherapy
Introduction
The advent and development of new molecular biological techniques has allowed the investigation and detail of different Central Nervous System (CNS) tumors. All tumors, including benign and malignant CNS tumors, have two basic components: (1) the parenchyma, made up of proliferating neoplastic cells, and (2) the supporting, host-derived, non-neoplastic stroma, made up of connective tissue, blood vessels, and host-derived inflammatory or immune cells [1]. The parenchyma of the neoplasm largely determines its biologic behavior, while the stroma is crucial to the growth of the neoplasm. Stromal cells, particularly infiltrating immune cells and neoplastic cells, carry on a two-way conversation that influences the growth of the tumor. For many malignant tumors there has yet to be a large breakthrough with respect to treatments yielding improved overall survival. For example, glioblastoma (formerly glioblastoma multiforme, GBM), the most prevalent primary malignant CNS tumor, is among the most fatal types of cancer harboring a uniformly dismal prognosis. With minor improvements in median overall survival over the past decade, more effective treatment modalities are required. Although perhaps not a new technique [2-4], the utilization of the body’s natural defense mechanisms through immunotherapy may herald such a breakthrough in tumor treatment. Increased understanding of brain tumor pathophysiology and its interaction with the patient immune system have revealed several immunotherapeutic targets of clinical relevance [5]. In this review, we focus on immune cell infiltration of CNS tumors and its clinical implications. More specifically, we will discuss infiltrating macrophages/microglia, lymphocytes, and eosinophils in the CNS tumor microenvironment.
Neuro-immunological separation, not isolation
Early studies such as those by Medawar et al. initially led to the long-held belief that CNS was an immunologically privileged site in the body [6]. This notion supported other experimental work showing that the allotransplantation of neoplasms was able to escape immunesurveillance that would normally elicit a robust immunological response [7,8]. The CNS also lacks traditional connections to the lymphatic system [9], and is separated from the intravascular space by the Blood-Brain Barrier (BBB) . However, it is now known that the immune system plays an integral role in the etiology and pathophysiology of many CNS diseases [10,11]. The CNS may be separate, but not necessarily isolated from the immune system. For example, when CNS injury occurs, leukocytic infiltration has been proposed as trafficking into the CNS via three mechanisms [12,13]: i) through choroid plexus capillaries into the CSF, ii) through blood into the subarachnoid space, and iii) from blood into the parenchyma. Here, we review how systemic immune cells may penetrate into the CNS parenchyma.
The BBB is one of the key components responsible for separating the CNS extracellular space from the cardiovascular system and its potentially deleterious agents (with the exception of capillaries of the circumventricular organs). The BBB is a selectively permeable barrier, which is composed of the cerebrovascular endothelial cells with characteristic tight intercellular junctions, pericytes, and astrocytic end-feet that make up the glia limitans. Those astrocytes ensheath blood vessels at the one end and at the other end communicate with neuronal processes in formation of a functional unit, the gliovascular unit, which plays a prominent role in maintaining hemostasis of the BBB [14]. Disturbances in this unit may seriously damage the BBB. Moreover, the BBB is not necessarily uniform in structure throughout every segment of the cerebrovasculature. For example, post-capillary venules contain a lower density of tight junctions [15,16] surrounded by a perivascular space. The astrocytic end-feet are also separated here from the endothelial cell wall by up to three basement membrane layers [15,17]. It is here, in the post-capillary venules, that leukocytes will preferentially migrate into the perivascular space [17-19]. The pericyte and vascular endothelial cell coordination helps to regulate this process. The communication between the two cell types requires cytokines such as Platelet-Derived Growth Factor-B (PDGF-B) and transforming growth factor-β (TGF- β) [17,20] which results in decreased CNS immune cell infiltration [21].
Bechmann et al. classified these differentially regulated steps as i) leukocyte passage across post-capillary venules into Virchow-Robin spaces, and ii) subsequent migration across the glia limitans into the neuropil [15]. There is no definitive model; however, detailing the biomolecular stages of how exactly circulating immune cells cross the BBB into the brain parenchyma. One model consists of a multi-step process, akin to that seen in other extra-cerebral sites, of cells “rolling, sticking, and then migrating” [22,23]. Lymphocytes will first slow in the capillary lumen by transiently binding to endothelial cell surface receptors through selectin glycoproteins [24]. This in turn activates more permanent binding between lymphocytes and the vessel wall via integrin glycoproteins, and ultimately results in trans- or paracellular endothelial leukocytic migration [25]. The vasculature found within a tumor’s microenvironment is substantially different from what is found in normal parenchyma. The BBB may be deficient in many areas due to abnormalities found pertaining to the endothelial cell wall, its tight junctions, and basement membrane [26]. The “blood-tumor barrier” has been thought to be more porous than the intact BBB. Abnormal immune cell trafficking may also be associated with the invasion of the capillary wall endothelial cells by tumor cells, possibly disrupting molecules that critical for cellular homing [12]. As a result, it is unclear as to whether the intra-tumoral dysfunctional capillaries would result in increased or decreased immune cell infiltration. Investigation of abnormal brain tumor vasculature has led to the identification of tumor capillaries as a potential immunotherapeutic target. The use of Vascular Endothelial Growth Factor (VEGF) antibodies has been shown to recondition the immunosuppressive tumor microenvironment and shift the expression of immune cell infiltrates toward an anti-tumor phenotype [27].
After migrating through the endothelial cell wall into the perivascular space, parenchymal infiltration becomes another issue. In order to cross the glia limitans, signaling pathways mediated by chemokines such as Monocyte Chemoattractant Protein (MCP)-1 and the expression of Matrix Metalloproteinase (MMP) are thought to be required [15]. Although the mechanisms underpinning the second step of leukocytic migration into the neuropil may not be well understood, an inflammatory reaction is thought to result in astrocytic end-feet retraction, degeneration, and allow for this breach to occur. Furthermore, as previously mentioned, the intra-tumoral BBB is dysfunctional in many areas which may extend into the glia limitans. Inflammatory/immune cell infiltration and homing toward CNS tumors may be then simply a product of chemokine and cytokine signaling, as outlined for astrocytic tumors by Bajetto et al. who have found the expression of multiple CXC chemokines in the brain tumors [28].
Macrophages and Microglia
Macrophages and microglia are derived from monocytes after myelocytic cell differentiation. One of their main functions is as a professional Antigen-Presenting Cell (APC) that triggers an adaptive immune response. They act to clean up cellular and pathogenic debris through phagocytosis, and are intricately involved in the inflammatory process. Microglia are thought to be the resident CNS macrophages acting as an APC, although it is still unclear as to whether or not they elicit a similar response [29]. Although their exact function is still somewhat ambiguous, it is clear that macrophages and microglia make up the primary component of immune cell infiltrates in CNS tumors particularly astrocytic tumors [30]. Table 1 lists the recent studies (using the relatively specific and clinical commonly used immunohistochemical markers) that demonstrate infiltrating CD68+ macrophages/microglia in astrocytic tumors [31-36].
*Perivascular
Intratumoral
Tumors
CD68+
CD3+
CD8+
CD20+
CD68+
CD3+
CD4+
CD8+
CD20+
References; Notes
Pilocytic astrocytomas (WHO grade I)
33.3% (6/18)
0% (0/18)
0% (0/18)
33.3% (6/18)
33.3%‡ (6/18)
0% (0/18)
Hewedi et al. [31]
86% (n=28)
71% (n=28)
14.3% (4/28)
86% (n=28)
75% (n=28)
0% (0/28)
Yang et al. [32]; positive expression (%) including cases with focal infiltrates
Diffuse astrocytomas (WHO grade II)
7.7% (1/13)
7.7% (1/13)
7.7% (1/13)
38.5% (5/13)
0% (0/13)
0% (0/18)
Hewedi et al. [31]; no statistically significant difference found in CD68+ cells between diffuse astrocytomas and pilocytic astrocytomas
7.9% (n=9)
Li et al. [33]; the infiltration of CD68+ and Iba+ cells (statistically significant, p <0.01) positively correlated with that of CD133+ glioma-initiating cells
Anaplastic astrocytomas (WHO grade III)
14.3% (3/21)
0% (0/21)
0% (0/18)
42.9%‡ (9/21)
0% (0/21)
0% (0/18)
Hewedi et al. [31]; no statistically significant difference found in CD68+ cells between anaplastic astrocytomas and diffuse astrocytomas
19.7% (n=12)
Li et al. [33]; CD68+ and Iba+ cells (statistically significant, p <0.01) correlated with the grades of astrocytomas; the infiltration of CD68+ and Iba+ cells (statistically significant, p <0.01) positively correlated with that of CD133+ glioma-initiating cells
Glioblastomas (WHO grade IV)
71.4% (15/21)
14.3% (3/21)
0% (0/18)
85.7% (18/21)
28.6% (6/21)
0% (0/18)
Hewedi et al. [31]; §perivascular and intratumoral CD68+ cells significantly (statistically, p <0.05) more in glioblastomas than in anaplastic astrocytomas, diffuse astrocytomas, and pilocytic astrocytomas
41% (25/61, +cells 10~%) vs. 59% (36/61, +cells <10%).
10% (6/62, +cells 10-30%) vs. 90% (56/62, +cells <10%)
11% (7/63, +cells 1-3%) vs. 89% (56/63, +cells <1%)
Kmiecik et al. [34]; CD3+ T-cell infiltration (statistically significant, p <0.05) associated with prolonged patient survival; a trend for CD8+ cells and no association of CD4+ cells with patient survival
97% (n=63)
5% (3/63)
46% (n=63)
0% (0/63)
100% (n=63)
5% (3/63)
73%‡ (n=63)
0% (0/63)
Yang et al. [32]; positive expression (%) including cases with focal infiltrates; §perivascular and intratumoral CD68+ cells significantly (statistically, p <0.05) more in glioblastomas than in pilocytic astrocytomas.
29.2% (n=11)
Li et al. [33]; CD68+ and Iba+ cells (statistically significant, p <0.01) correlated with the grades of astrocytomas; the infiltration of CD68+ and Iba+ cells (statistically significant, p <0.01) positively correlated with that of CD133+ glioma-initiating cells.
App. 9 cells/mm2 (secondary, n=11) vs. app. 22 (primary, n=54) cells/mm2
App. 4 cells/mm2 (secondary, n=11) vs. app. 8 (primary , n=54) cells/mm2
Lohr et al. [35]; elevated numbers of intratumoral effector T-cells (cytotoxic and helper) significantly correlated with a better survival in primary glioblastomas; regulatory T-cells not associated with glioblastoma patient outcome
43% (3/7)
29% (2/7)
43% (3/7)
Kuppner et al. [36]; the cytolytic activity expressed by tumor infiltrating lymphocytes against autologous tumor cells significantly greater (p<0.001) than that obtained by the corresponding peripheral blood lymphocytes cultured in a similar manner.
Abbreviations: app: approximately; vs: versus; n: number of cases studied
Markers: CD68, macrophages/microglia; CD3, T-lymphocytes; CD4, T-lymphocytes; CD8, cytotoxic T-lymphocytes; CD20, B-lymphocytes; studies using different immunohistochemical markers for the immune cells are not listed.
Positive expression (%) of immunoreactive cells: scored as “intermediate” and “extensive” on semi quantitative assessments of immunohistochemical staining
* Immune cell infiltrates within Virchow-Robin spaces: perivascular; within the tumor tissue: intratumoral.
‡ Statistically significant (p < 0.05) difference from the perivascular counterpart; “not statistically significant” results not shown.
§Statistically significant (p < 0.05) differences between tumor types or grades.
Table 1: Immune cell infiltrates in astrocytic tumors: frequency and clinical correlation.
As shown in this Table 1, the frequency of infiltrating CD68+ microphages/microglia varies with tumor WHO grade. Both the perivascular and intratumoral CD68+ cells have been found significantly more in glioblastomas (WHO grade IV) than in anaplastic astrocytomas (WHO grade III), diffuse astrocytomas (WHO grade II), and pilocytic astrocytomas (WHO grade I) [31-33]. No statistically significant differences in infiltrating CD68+ cells have been found between pilocytic astrocytomas and diffuse astrocytomas or between diffuse astrocytomas and anaplastic astrocytomas [31]. According to those studies, the percentage of intratumoral CD68+ macrophages/microglia is up to 86% (including the tumors with focal, infiltrates or extensive infiltrates) in WHO grade I pilocytic astrocytomas, up to 38.5% in WHO grade II diffuse astrocytomas, up to 42.9% in WHO grade III anaplastic astrocytomas, and up to 100% in WHO grade IV glioblastomas. Moreover, infiltrating CD68+ and Iba+ macrophages/microglia have been positively correlated with CD133+ glioma-infiltrating cells [33]. Figure 1 exhibits the infiltration of inflammatory/immune cells in an Atypical Teratoid/ Rhabdoid Tumor (AT/RT), in which Figure 1C displays frequent CD68+ macrophages/microglia in this AT/RT [37]. Macrophages/ microglia have been noted to comprise up to 34% of the gliomainfiltrating immune cells [38]; it is likely that the majority of these cells are infiltrating macrophages and not resident microglia [39].
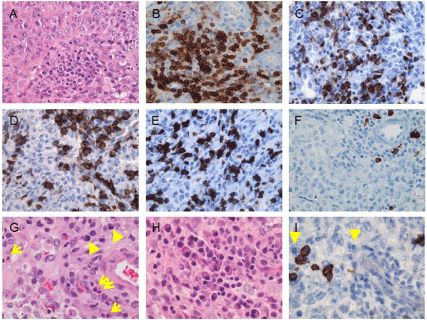
Figure 1: Infiltration of immune/inflammatory cells in an atypical teratoid/rhabdoid tumor of the fourth ventricle in a 13-month-old boy. The tumor exhibits (A)
moderate infiltration of immune/inflammatory cells which are (B) immunoreactive for CD45 and contain (C) focally frequent CD68+ macrophages/microglia, (D)
CD4+ cells and (E) CD8+ T-cells, but (F) sparse CD20+ B-cells. (G, arrows) Eosinophils and (G, arrowheads) plasmacytoid cells are often seen. The plasmacytoid
cells are (H) focally frequent, and (I, arrowheads) mostly negative for the plasma cell marker CD138. Original magnifications: x 400 (A-I). [Figure reproduced by
courtesy of the Canadian Journal of Neurological Science; Lu et al. [37].
Although the exact function of macrophages/microglia within the tumor microenvironment is still not entirely clear, it seems as though these cells are actively immunosuppressive [40,41]. A few of the problems encountered in sorting this out stems from difficulty in precisely differentiating microglia, macrophages, and Myeloid- Derived Suppressor Cells (MDSCs) [42-48]. Their immunosuppressive and tumor-supportive nature appears to be related to these cells being directed down the M2 line of differentiation. This prevents the expression of cytokines required for CD8+ T cells and Th1 CD4+ T cells, and supports production of CD4+ regulatory T-cells (Tregs) [49]. Although numerous immunosuppressive cytokines are secreted by gliomatous tumors (IL-10, IL-4, IL-6, M-CSF, macrophage inhibitory factor, among others), the predominant immunosuppressive IL-10 signaling seems to originate from macrophages/microglia [41,49- 54]. These signals skew macrophagic differentiation toward an M2 response and away from an M1 phenotype (responsible for anti-tumor responses such as tumor-antigen presentation, pro-inflammatory cytokine production, and phagocytosis) [49,55]. M2 macrophages may also promote glial tumor progression through growth factor expression. VEGF, PDGF and Fibroblast Growth Factors (FGFs) have all been shown to result in increased tumor vascularity and growth— all of which are also produced by Glioma-Associated Macrophages/ Microglia (GAMs). Furthermore, Fas-Ligand expression by GAMs could also promote T-cell apoptosis and tumor-cell survival [41,56]. The net result of GAM influence on tumor growth or suppression is likely determined by the relative amount of M1 versus M2 cells that are found within the glioma microenvironment [49].
Gliomas and GAMs have been shown to interact with one-another through multiple cytokine, chemokine, and growth factor signaling pathways. Gliomas are capable of attracting GAMs, promoting proliferation of GAMs, and influencing their differentiation toward an M2, tumor promoting, and phenotype. In turn, as previously mentioned, GAMs are able to support glial tumor survival, promote their growth, and infiltration into surrounding parenchyma. Microglia are first recruited for tumor infiltration through a variety of signaling pathways [57-60]. Among these include Chemokine (C-C motif) ligand 2 (CCL2 or MCP-1) [59,61-63], Stromal-Derived Factor-1 (SDF-1) [60,64], and the triggered expression of Membrane Type 1 Metalloprotease (MT1-MMP) on GAMs that then induces glioma-derived pro-MMP-2 resulting in GAM-tumor infiltration [65-67]. Glioma cells can then stimulate GAMs to secrete a variety of factors that promote glial tumor migration such as cochaparone stress inducible factor 1 (STI1), TGF-β1, CXCT1, EGF, VEGF, Colony Stimulating Factor 1 Receptor (CSF-1R), to name a few [49,57-64,66-74]. There is a complex interplay between gliomas and GAMs suggesting the potential for new immunotherapeutic modalities. Although certain pathways have been elucidated, the heterogeneous and extremely plastic microenvironment found within high-grade gliomas will make a “one-therapy-fits-all” strategy virtually impossible. More likely, ongoing study results and patient/ tumor specific molecularly directed modalities will be needed to overcome these great challenges.
Lymphocytes
Lymphocytes, which are subdivided into T-cells, B-cells, and natural killer cells, are responsible for both cell mediated immunity and humoral immunity. Dated back to 1960, Bertrand and Mannen were among the first to find lymphocytic infiltration in gliomas; their study in cadaveric specimens revealed 36.6% (63/172) astrocytomas harboring perivascular lymphocytes despite “weak” infiltrates in the majority of cases [75]. Subsequent to this seminal work, several other groups went on to confirm the findings of lymphocytic infiltration in both post-mortem and living patient specimens of astrocytomas [76,77]. The ‘definite’ degree of Tumor Infiltrating Lymphocytes (TILs) was found in around 30% (28 – 31%) of grade IIIV astrocytomas and predominantly perivascular in distribution, but not in oligodendrogliomas, medulloblastomas, and ependymomas [76]. These studies, however, failed to establish any correlation with clinical prognosis [12].
Since these studies, much work has been done to characterize the specific phenotype of lymphocytes found. Table 1 also lists a few recent studies demonstrating infiltrating lymphocytes in astrocytic tumors [31-36]. Compared to older studies [75-77], these recent studies have immunophenotyped infiltrating lymphocytes by using individual specific markers. There has been a wide variation in the findings of studies attempting to classify the lymphocytic infiltration, as shown in the Table, which is at least partially attributed to the different techniques being used [12,78-81]. Among infiltrating lymphocytes, CD4+ cells and CD8+ cytotoxic T-cells represent the predominant cell subtypes [12,36,82,83]. One study by Farmer et al. [82] has found that the proportion (41.2%) of cytotoxic T-cells was increased within TIL population as compared to the corresponding peripheral blood lymophocyte populations (30.8%, statistically significant), as were CD4+ helper T-cells and natural killer cells (mostly also positive for CD8). Unlike infiltrating macrophages/microglia, the frequency of infiltrating lymphocytes appeared not to vary with tumor WHO grade. Instead, Lohr et al. have noted that elevated numbers of intratumoral effecter T-cells (cytotoxic and helper) were significantly correlated with a better survival in primary glioblastomas [35]. Similarly, Kmiecik et al. have revealed that CD3+ T-cell infiltration was associated (statistically significant) with prolonged survival of patients with glioblastomas [34]. There appear to be no significant infiltration of CD20+ B-lymphocytes in CNS tumors particularly astrocytomas (Table 1). Figure 1D, 1E, and 1F exhibit an AT/RT containing frequent CD4+ T-cells, frequent CD8+ cytotoxic T-cells, and rare CD20+ B-cells, respectively [37].
Regulatory T-cells (Tregs) have become the focus of a number of studies. These circulatory and local TILs, expressing CD4+/CD25+/ FoxP3, have been shown to play an integral part in the tumor microenvironment with a predominantly immunosuppressive role [37,41,84,85]. This is analogous to their role in other types of cancers [86-98]. FoxP3 is a protein specific to Tregs that is critical to T-cell development, and these CD4+/CD25+ TILs seem to suppress proliferation of CD4+/CD25- lymphocytes [12,84]. Although their origin is still unclear, T-cell immunosuppression by Tregs has been suggested as being responsible for systemic immunosuppression seen in glioblastoma patients as well [41]. Simultaneous local and systemic proliferation of Tregs is unlikely as glioblastoma-related factors are not increased in patient serum [99,100], nor are excessive numbers of TILs found consistently in all glioblastoma specimens [101,102]. Similarly to Tregs, circulating MDSCs have also been found in abundance in glioblastoma patients [41,99,103]. These cells have been shown to induce apoptosis of activated T-cells, stimulate Tregs proliferation, and alter T-cell recognition [104-106]. A number of cells, including Tregs and MDSCs, likely all contribute to a systemic and tumor-specific immunosuppressive microenvironment promoting tumor progression and immune system evasion. This has led to new tumor models being proposed such as that by Parney in 2012 whereby systemic-tumor interactions result in an overall lymphocytic inhibition [41]. Ultimately, findings such as those outlined here may result in new targets for immunotherapy of astrocytomas as evidenced by the use of anti-CD25 antibodies [107-109] or TLR stimulation by DNA CpG sequences [110-115].
Although some T-cells promote tumor progression, these infiltrating lymphocytes are overall destined for anti-tumor activity. More specifically, infiltrating CD8+ cytotoxic T-cells retain several mechanisms by which they can carry out an anti-tumor function [116]. These include perforin, granzymes, granulysin, CD95L, TNF, to name a few. Their function can also be modulated through APC expression of aEβ7 integrin [116,117] as well as expression of IFNγ and granzyme B by other T-cells. Indeed cytotoxic T-cells have also been explored as an immunotherapeutic method for treating glial tumor patients. These cells, usually generated ex-vivo through antigenic stimulation of Peripheral Blood Mononuclear Cells (PBMCs) with inactivated tumor cells [118-121] have been used in several early clinical trials with variable results. Few of these studies have shown a survival benefit [119-129]. An anti-tumor response by TILs, however, seems to be over-shadowed by the tumor escaping an immune system response through mechanisms leading to local immunosuppression such as those outlined above. Another subtype of T-cells demonstrating anti-tumor activity is the Lymphokine Activated Killer Cell (LAK) [130-137]. Similar to cytotoxic T-cells, they are harvested from PBMCs and activated ex-vivo by IL-2 [130,138-141]. LAKs have been shown to lyse tumor cells that are resistant to natural killer cells, while sparing normal brain tissue [130,132-135,137,142-145]. Their clinical use, however, has been limited due to their inability to migrate into the tumor site (requiring brachytherapeutic approaches), and labor-intensive extraction. The clinical trials that have been completed using LAKs have shown variable results, some demonstrating improved survival while others have not [130,144-153].
Eosinophils
Eosinophils are granulocytic cells derived from myeloid cells. Their wide array of functions demonstrates their importance in both the innate and adaptive immune systems and mounting an appropriate response to a given antigenic source. This includes antigen presentation, wound repair, organ development, cytotoxic clearance of pathogens, and tissue remodeling and homeostasis [154-157]. Furthermore, eosinophils influence both the innate and adaptive immune response upon activation through secretion of proinflammatory cytokines, pro-fibrotic and angiogenic factors that may ultimately alter tumor development [154,156,158]. Tumor-Associated Tissue Eosinophilia (TATE) has been increasingly reported, but the exact role of infiltrating eosinophils in tumors has not yet been defined [154,159-173]. Many studies have suggested that TATE is associated with favorable prognosis for a variety of carcinomas [130,149,161-169], whereas a few other studies have noted that TATE may have a tumor-promoting role [170]. An association with tumoral invasion of the oral squamous cell carcinomas has also been established [171-173]. Infiltrating eosinophils have been found in a number of malignant and benign tumors including oral squamous cell carcinomas, breast carcinomas, gastric cancers, uterine cervix carcinomas, penile cancers, hematologic malignancies, and colonic adenomas [159-173]. It has been noted that immune cell infiltrates in tumors often vary with tumor type and size [174,175]. TATE has been suggested to preferentially occur in tumors of epithelial origin in the colon and breast [165,176,177]. There have been only a few studies describing TATE in CNS tumors [154]. Two studies performed separately by Hayes et al. [130,149] have revealed intracavitary eosinophils in malignant astrocytomas of patients who had received the infusion of IL-2 combined with ex vivo activated autologous killer cells into the surgical resection cavity. Interestingly, eosinophils had been absent in the primary operative specimens of those patients, suggesting immunotherapy-induced eosinophilia. Lu et al. have showed the infiltration of eosinophils in all four AT/RTs (Figure 1G) that are malignant embryonal tumor with divergent differentiation, but not in a small group of glioblastomas [37]. The same group has also demonstrated eosinophil infiltration in 43% (19 out of 44) of pilocytic astrocytomas, but its absence in (10 out of 10) ependymomas [178]. Based on these observations, the infiltration of eosinophils is likely limited to some CNS tumors with certain cell origins such as astrocytic differentiation/component. The pathogenesis of cell origindependent TATE requires further investigation, although it may be at least partially attributed to different types of tumor-specific antigens present in those CNS tumors [12, 179-181].
Eosinophils are immune system effector cells involved in both pathogen clearance and tissue repair [182,183]. Although the relevance of TATE is unclear, eosinophilic production and release of Eosinophil Peroxidase and Reactive Oxygen Species (EPO and ROS, respectively) may serve to amplify oxidative damage and promote tumorigenesis [184]. Furthermore, astrocytic tumors have been found to produce GM-CSF [185-187] which may promote the role of eosinophils in malignant glial tumor evolution. However, oxidative stress has also been linked to cellular apoptosis and thus potentially results in eosinophils having an anti-tumorigenic effect. Eosinophil Derived Neurotoxin (EDN) and Eosinophil Cationic Protein (ECP) have been shown to interact with Toll-Like Receptor (TLR)-2 as part of the innate immune system [188]. In animal studies of gliomas, TLRligands have been shown to cause an increased infiltration of immune effector cells and result in enhanced tumor regression [189,190]. The tumor progression or regression is the net result of either growth or apoptosis. This is a complex interplay between a myriad of cytokines, chemokines, and immune cell-tumor cell interactions. Two key grow factors found to be mutated and involved in glial tumor evolution are Epidermal Growth Factor (EGFR) and PDGF. Eosinophils also express PDGF receptors. This may serve to activate eosinophils causing degranulation and promote apoptosis. However, in vitro experiments conducted stimulating eosinophils has resulted in ligand production amplifying EGFR and may serve to promote glial tumor growth. Curran and Bertics [154] have suggested a positive feedback loop within the tumor microenvironment in which tumor cells express GM-CSF resulting in increased eosinophil activity. This then causes increased growth-factor ligand production and enhanced glial tumor progression, and causing more GM-CSF expression.
Several studies have demonstrated an inverse relationship between atopic diseases and risk of glioma [191-193]. As eosinophils have been proven to play a key role in atopic diseases [182], it stands to reason that perhaps infiltrating eosinophils in some gliomas are partially responsible for this phenomenon [154]. Although there are only few studies of TATE in CNS tumors, emerging evidence suggests that TATE plays a functional role in the development and progression of CNS tumors (especially pediatric), and this role lends credence to future novel TATE immunotherapy [154,178].
Immune cell infiltrates in pediatric versus adult CNS tumors
Children have a distinct, developing immune system that undergoes a transition from fetal to adult-equivalent immune competence, and peaks at puberty [194]. Age-related changes have been found in the circulating immune cells including eosinophils and multiple subtypes of lymphocytes [194,195]. For example, within the total lymphocyte population, the percentages of T cells including CD4+ and CD8+ subtypes increase with age; percentages of B-cells and NK cells are higher in newborn infants than in adults [195]. With particular relevance to CNS tumors, the glioma-associated antigen precursor protein profile displays different types between pediatric and adult patients [12,180,181]. These age-dependent differences may give rise to some discrepancies in immune cell infiltrates between pediatric and adult CNS tumors.
A recent study has analyzed the age difference and found infiltrating eosinophils in 18 out of 29 (62%) pilocytic astrocytomas of pediatric patients (1-18 years old) versus 1 out of 15 (7%, significantly less compared to that of pediatric patients) pilocytic astrocytomas of adult patients (20-72 years old; only one positive in a 20-year-old patient) [178]. This age-dependent finding appears to be consistent with another study demonstrating infiltrating eosinophils in AT/RTs of all four patients less than 2 years of age [37] (Figure 1G). Griesinger et al. [196] have recently characterized the frequency and phenotype of infiltrating immune cells in the most common pediatric CNS tumors. Their study has revealed the most myeloid cell-rich tumor to be pilocytic astrocytomas (31.6%), followed by ependymomas (27.1%), glioblastomas (7.6%), and medulloblastomas (4.1%). In comparison, myeloid cells were found to make up 0.4% of nontumor brain tissues. They have also found the correlation of T-cell infiltration with myeloid cell infiltration, and the highest number of infiltrating CD8+ cytotoxic T-cells in pilocytic astrocytomas (3.28%) compared to other tumors, but CD4+ T-cell infiltration higher in ependymomas (1.40%) than other tumors. These observations may imply that immunotherapeutic interventions should be tailored to individual tumor types [197], with particular consideration of distinct immunophenotypes and differences in tumor-associated antigens between pediatric and adult CNS tumors [180].
Immunotherapy
The field of immunotherapy has spawned from increased knowledge of tumor-immune system interaction, i.e., the tumor immune microenvironment. In brief, immunotherapy consists of utilizing the patient immune system to eradicate foreign tumors. This may consist of targeting tumor specific epitopes, or modulating the tumor-immune cell infiltrates such as GAMs. Immunotherapy has been recently reviewed in detail [5,198,199]. Outlined below are only a few of the many potential immunotherapeutic targets currently under investigation.
Tumor specific epitopes such as the EGFRvIII receptor exemplifies such a target. This is a particular EGFR receptor not expressed by normal cells, and unique to glioblastomas (found in 20- 30% of glioblastoma patients) [200]. Preliminary animal and patient investigations have shown increased survival upon immunization against EGFRvIII antigens [201,202]. However, multi-tumor specific epitope targeting is essential as malignant glial tumor cells are infamous for their heterogeneity and plasticity as highlighted by tumor recurrence from cells not expressing EGFRvIII mutations.
Other immunotherapeutic strategies consist of altering the tumor microenvironment by skewing macrophages toward an M1 phenotype. This has been previously shown to reduce tumor growth [203]. The studies investigating this option have focused on blocking STAT3 (signal transducer and activator of transcription 3) which has been shown to be responsible for promoting an M2 differentiation and immune system suppression [49]. Their results have demonstrated decreased levels of IL-10 and IL-6 (immunosuppressive cytokines), and GAM activation [204,205]. In addition to altering macrophage phenotype, overcoming the immunosuppressive tumor microenvironment is essential for immunotherapy success. Research focused on Tregs targeting has led to several potential pathways by which this can be achieved. The inactivation of immunosuppressive Tregs through antibodies blocking CD25, and decreased Tregs accumulation with TLR8 and TLR9 stimulation has both shown promising results [206-211].
Strategies focused on preventing tumor infiltration and immune cell attraction has also shown promise. This is accomplished through blockade of tumor-induced expression of cytokines. For example, blocking the MT1-MMP cascade and TGF-β1 receptor on GAMs has resulted in decreased glioma invasion [68,212]. Furthermore, immunotherapeutic targeting of CCL2 may prevent immune cell infiltration of the tumor, attenuate the immunosuppressive response, and promote tumor clearance [213,214].
Conclusion
Ongoing investigation into the interaction between CNS tumors and the patient immune system has led to a surge in the development of novel immunotherapeutic agents. However, the translation of these agents into clinical practice has been underwhelming, and not resulted in a drastic improvement of prognosis. Glioblastomas have been the most extensively researched of CNS tumors with respect to immune cell infiltration and immunotherapy. They have a dismal natural history that is only slightly improved with maximal therapy, currently consisting of a combination of surgical resection, chemoand radiotherapy. Despite these efforts, average survival is only 14 months. However, the amount of knowledge gained with respect to glioblastoma molecular biology and immunobiology rivals that of most other oncological entities. Ongoing basic science research has resulted in the emergence of an entirely new field of therapeutics directed at manipulating the patient immune system to combat tumor progression. New models focusing on the immune systemtumor interaction through circulating cells such as MDSCs and Tregs and their resultant immunosuppression represent novel targets for therapy. The discovery of innate and adaptive immune cells infiltrating into such tumors, their underlying function, and the mechanisms by which they penetrate the blood-brain barrier will serve to facilitate the design of new therapeutic agents. Tumor-specific antigens such as EGFRvIII, STAT3, and signaling mechanisms such as Fox P3, TGF, or CCL2 represent only a few targets for these agents. Although immunotherapeutic results have not yet translated into substantial clinical gains, continued study is crucial and will yield important information that will bring us closer to that end.
References
- Stricker TP, Kumar V. Neoplasia. Robbins Basic Pathology 8th edn. Kumar V, Abbas AK, Fausto MD, Mitchell RN, editors. In: Philadelphia, PA: Saunders Elsevier. 2007; 174.
- Bast RC Jr, Zbar B, Borsos T, Rapp HJ. BCG and cancer (first of two parts). N Engl J Med. 1974; 290: 1413-1420.
- Bast RC Jr, Zbar B, Borsos T, Rapp HJ. BCG and cancer. N Engl J Med. 1974; 290: 1458-1469.
- Eilber FR, Morton DL, Holmes EC, Sparks FC, Ramming KP. Adjuvant immunotherapy with BCG in treatment of regional-lymph-node metastases from malignant melanoma. N Engl J Med. 1976; 294: 237-240.
- Yamanaka R. Gloima: Immunotherapeutic Approaches. New York: Springer Science+Business Media; 2012.
- Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948; 29: 58-69.
- Murphy JB, Sturm E. Conditions Determining The Transplantability Of Tissues In The Brain. J Exp Med. 1923; 38: 183-197.
- Shirai Y. On the transplantation of the rat sarcoma in adult heterogenous animals. Jpn Med World. 1921; 1: 14-15.
- Bertrand I MH. Etude des reactions vasculaires dans les astrocytomes. Rev Neurol (Paris). 1960; 102: 3-19.
- Siffrin V, Vogt J, Radbruch H, Nitsch R, Zipp F. Multiple sclerosis - candidate mechanisms underlying CNS atrophy. Trends Neurosci. 2010; 33: 202-210.
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010; 140: 918-934.
- Dunn GP, Dunn IF, Curry WT. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007; 7: 12.
- Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003; 3: 569-581.
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006; 7: 41-53.
- Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007; 28: 5-11.
- Nagy Z, Peters H, Hüttner I. Fracture faces of cell junctions in cerebral endothelium during normal and hyperosmotic conditions. Lab Invest. 1984; 50: 313-322.
- Hamilton A, Sibson NR. Role of the systemic immune system in brain metastasis. Mol Cell Neurosci. 2013; 53: 42-51.
- Raine CS, Cannella B, Duijvestijn AM, Cross AH. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990; 63: 476-489.
- Cross AH, Cannella B, Brosnan CF, Raine CS. Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab Invest. 1990; 63: 162-170.
- Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009; 29: 630-638.
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010; 468: 562-566.
- Engelhardt B1. Regulation of immune cell entry into the central nervous system. Results Probl Cell Differ. 2006; 43: 259-280.
- Engelhardt B1. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm. 2006; 113: 477-485.
- Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005; 26: 485-495.
- Engelhardt B, Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004; 34: 2955-2963.
- Long DM. Capillary ultrastructure and the blood-brain barrier in human malignant brain tumors. J Neurosurg. 1970; 32: 127-144.
- Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012; 109: 17561-17566.
- Bajetto A, Barbieri F, Dorcaratto A, Barbero S, Daga A, Porcile C, et al. Expression of CXC chemokine receptors 1-5 and their ligands in human glioma tissues: role of CXCR4 and SDF1 in glioma cell proliferation and migration. Neurochem Int. 2006; 49: 423-432.
- Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system's immune response. Neurol Res. 2005; 27: 685-691.
- Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006; 8: 261-279.
- Hewedi IH, Radwan NA, Shash LS, Elserry TH. Perspectives on the immunologic microenvironment of astrocytomas. Cancer Manag Res. 2013; 5: 293-299.
- Yang I, Han SJ, Sughrue ME, Tihan T, Parsa AT. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. J Neurosurg. 2011; 115: 505-511.
- Yi L, Xiao H, Xu M, Ye X, Hu J, Li F, et al. Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol. 2011; 232: 75-82.
- Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, Enger PØ, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013; 264: 71-83.
- Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res. 2011; 17: 4296-4308.
- Kuppner MC, Hamou MF, de Tribolet N. Immunohistological and functional analyses of lymphoid infiltrates in human glioblastomas. Cancer Res. 1988; 48: 6926-6932.
- Lu JQ, Wilson BA, Yong VW, Pugh J, Mehta V. Immune cell infiltrates in atypical teratoid/rhabdoid tumors. Can J Neurol Sci. 2012; 39: 605-612.
- Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000; 46: 957-961.
- Parney IF, Waldron JS, Parsa AT. Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg. 2009; 110: 572-582.
- Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010; 17: 6-10.
- Parney IF. Basic concepts in glioma immunology. Adv Exp Med Biol. 2012; 746: 42-52.
- Deininger MH, Seid K, Engel S, Meyermann R, Schluesener HJ. Allograft inflammatory factor-1 defines a distinct subset of infiltrating macrophages/microglial cells in rat and human gliomas. Acta Neuropathol. 2000; 100: 673-680.
- Deininger MH, Meyermann R, Trautmann K, Duffner F, Grote EH, Wickboldt J, et al. Heme oxygenase (HO)-1 expressing macrophages/microglial cells accumulate during oligodendroglioma progression. Brain Res. 2000; 882: 1-8.
- Badie B, Schartner J. Role of microglia in glioma biology. Microsc Res Tech. 2001; 54: 106-113.
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995; 154: 4309-4321.
- Sedgwick JD, Schwender S, Imrich H, Dörries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991; 88: 7438-7442.
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12: 253-268.
- Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008; 83: 1136-1144.
- Wei J, Gabrusiewicz K, Heimberger A. The controversial role of microglia in malignant gliomas. Clin Dev Immunol. 2013; 2013: 285246.
- Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008; 216: 15-24.
- Bach JP, Deuster O, Balzer-Geldsetzer M, Meyer B, Dodel R, Bacher M. The role of macrophage inhibitory factor in tumorigenesis and central nervous system tumors. Cancer. 2009; 115: 2031-2040.
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011; 59: 1169-1180.
- Qiu B, Zhang D, Wang C, Tao J, Tie X, Qiao Y, et al. IL-10 and TGF-β2 are overexpressed in tumor spheres cultured from human gliomas. Mol Biol Rep. 2011; 38: 3585-3591.
- Zhang L, Handel MV, Schartner JM, Hagar A, Allen G, Curet M, et al. Regulation of IL-10 expression by upstream stimulating factor (USF-1) in glioma-associated microglia. J Neuroimmunol. 2007; 184: 188-197.
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008; 13: 453-461.
- Badie B, Schartner J, Prabakaran S, Paul J, Vorpahl J. Expression of Fas ligand by microglia: possible role in glioma immune evasion. J Neuroimmunol. 2001; 120: 19-24.
- Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012; 18: 519-527.
- Held-Feindt J, Hattermann K, Müerköster SS, Wedderkopp H, Knerlich-Lukoschus F, Ungefroren H, et al. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs). Exp Cell Res. 2010; 316: 1553-1566.
- Okada M, Saio M, Kito Y, Ohe N, Yano H, Yoshimura S, et al. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol. 2009; 34: 1621-1627.
- Wang SC, Hong JH, Hsueh C, Chiang CS. Tumor-secreted SDF-1 promotes glioma invasiveness and TAM tropism toward hypoxia in a murine astrocytoma model. Lab Invest. 2012; 92: 151-162.
- Desbaillets I, Tada M, de Tribolet N, Diserens AC, Hamou MF, Van Meir EG. Human astrocytomas and glioblastomas express monocyte chemoattractant protein-1 (MCP-1) in vivo and in vitro. Int J Cancer. 1994; 58: 240-247.
- Jantaratnotai N, Choi HB, McLarnon JG. ATP stimulates chemokine production via a store-operated calcium entry pathway in C6 glioma cells. BMC Cancer. 2009; 9: 442.
- Wang H, Zhang L, Zhang IY, Chen X, Da Fonseca A, Wu S, et al. S100B promotes glioma growth through chemoattraction of myeloid-derived macrophages. Clin Cancer Res. 2013; 19: 3764-3775.
- Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010; 120: 694-705.
- da Fonseca AC, Badie B. Microglia and macrophages in malignant gliomas: recent discoveries and implications for promising therapies. Clin Dev Immunol. 2013; 2013: 264124.
- Markovic DS, Glass R, Synowitz M, Rooijen Nv, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005; 64: 754-762.
- Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci U S A. 2009; 106: 12530-12535.
- Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M, et al. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion--an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008; 27: 918-930.
- Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol. 2012; 189: 444-453.
- Lin HC, Song TY, Hu ML. S-Adenosylhomocysteine promotes the invasion of C6 glioma cells via increased secretion of matrix metalloproteinase-2 in murine microglial BV2 cells. Toxicol Sci. 2009; 112: 322-330.
- Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007; 8: 610-622.
- Kerber M, Reiss Y, Wickersheim A, Jugold M, Kiessling F, Heil M, et al. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008; 68: 7342-7351.
- Fonseca AC, Romão L, Amaral RF, Assad Kahn S, Lobo D, Martins S, et al. Microglial stress inducible protein 1 promotes proliferation and migration in human glioblastoma cells. Neuroscience. 2012; 200: 130-141.
- Färber K, Synowitz M, Zahn G, Vossmeyer D, Stragies R, van Rooijen N, et al. An alpha5beta1 integrin inhibitor attenuates glioma growth. Mol Cell Neurosci. 2008; 39: 579-585.
- Bertrand I, Mannen H. Etudes des reactions vasculaires dans les astrocytomes. Rev Neurologia. 1960; 102: 3-19.
- Ridley A, Cavanagh JB. Lymphocytic infiltration in gliomas: evidence of possible host resistance. Brain. 1971; 94: 117-124.
- Takeuchi J, Barnard RO. Perivascular lymphocytic cuffing in astrocytomas. Acta Neuropathol. 1976; 35: 265-271.
- Paine JT, Handa H, Yamasaki T, Yamashita J, Miyatake S. Immunohistochemical analysis of infiltrating lymphocytes in central nervous system tumors. Neurosurgery. 1986; 18: 766-772.
- Saito T, Tanaka R, Yoshida S, Washiyama K, Kumanishi T. Immunohistochemical analysis of tumor-infiltrating lymphocytes and major histocompatibility antigens in human gliomas and metastatic brain tumors. Surg Neurol. 1988; 29: 435-442.
- Yasuda K, Alderson T, Phillips J, Sikora K. Detection of lymphocytes in malignant gliomas by monoclonal antibodies. J Neurol Neurosurg Psychiatry. 1983; 46: 734-737.
- Yu JS, Lee PK, Ehtesham M, Samoto K, Black KL, Wheeler CJ. Intratumoral T cell subset ratios and Fas ligand expression on brain tumor endothelium. J Neurooncol. 2003; 64: 55-61.
- Farmer JP, Antel JP, Freedman M, Cashman NR, Rode H, Villemure JG. Characterization of lymphoid cells isolated from human gliomas. J Neurosurg. 1989; 71: 528-533.
- Sawamura Y, Hosokawa M, Kuppner MC, Kobayashi H, Aida T, Abe H, et al. Antitumor activity and surface phenotypes of human glioma-infiltrating lymphocytes after in vitro expansion in the presence of interleukin 2. Cancer Res. 1989; 49: 1843-1849.
- El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006; 8: 234-243.
- Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007; 13: 108-116.
- Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001; 61: 4766-4772.
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004; 10: 942-949.
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005; 102: 18538-18543.
- Ling KL, Pratap SE, Bates GJ, Singh B, Mortensen NJ, George BD, et al. Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun. 2007; 7: 7.
- Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, et al. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One. 2006; 1: e129.
- Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003; 9: 4404-4408.
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002; 169: 2756-2761.
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006; 24: 5373-5380.
- Slavina EG, Chertkova AI, Zabotina TN, Gan'shina IP, Lichinitser MR. Variations in the number of regulatory T cells (CD4+CD25+) in patients with breast cancer during herceptin therapy. Bull Exp Biol Med. 2006; 141: 361-363.
- Hueman MT, Stojadinovic A, Storrer CE, Foley RJ, Gurney JM, Shriver CD, et al. Levels of circulating regulatory CD4+CD25+ T cells are decreased in breast cancer patients after vaccination with a HER2/neu peptide (E75) and GM-CSF vaccine. Breast Cancer Res Tr. 2006; 98: 17-29.
- Okita R, Saeki T, Takashima S, Yamaguchi Y, Toge T. CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncol Rep. 2005; 14: 1269-1273.
- Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28- T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006; 67: 1-12.
- Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002; 168: 4272-4276.
- Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Gastineau DA, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010; 12: 631-644.
- Mahaley MS Jr., Brooks WH, Roszman TL, Bigner DD, Dudka L, Richardson S. Immunobiology of primary intracranial tumors. Part 1: studies of the cellular and humoral general immune competence of brain-tumor patients. J Neurosurg. 1977; 46: 467-476.
- Hao C, Parney IF, Roa WH, Turner J, Petruk KC, Ramsay DA. Cytokine and cytokine receptor mRNA expression in human glioblastomas: evidence of Th, Th2 and Th3 cytokine dysregulation. Acta Neuropathol. 2002; 103: 171-178.
- Sawamura Y, Diserens AC, de Tribolet N. In vitro prostaglandin E2 production by glioblastoma cells and its effect on interleukin-2 activation of oncolytic lymphocytes. J Neurooncol. 1990; 9: 125-130.
- Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010; 12: 351-365.
- Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, et al. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004; 53: 64-72.
- Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005; 174: 636-645.
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007; 13: 828-835.
- El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006; 105: 430-437.
- Fecci PE, Sweeney AE, Grossi PM, Nair SK, Learn CA, Mitchell DA, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006; 12: 4294-4305.
- Grauer OM, Nierkens S, Bennink E, Toonen LW, Boon L, Wesseling P, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007; 121: 95-105.
- El Andaloussi A, Sonabend AM, Han Y, Lesniak MS. Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia. 2006; 54: 526-535.
- Du YC, Lin P, Zhang J, Lu YR, Ning QZ, Wang Q. Fusion of CpG-ODN-stimulating dendritic cells with Lewis lung cancer cells can enhance anti-tumor immune responses. Tissue Antigens. 2006; 67: 368-376.
- Friedberg JW, Kim H, McCauley M, Hessel EM, Sims P, Fisher DC, et al. Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-alpha/beta-inducible gene expression, without significant toxicity. Blood. 2005; 105: 489-495.
- Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006; 29: 558-568.
- Lubaroff DM, Karan D, Andrews MP, Acosta A, Abouassaly C, Sharma M, et al. Decreased cytotoxic T cell activity generated by co-administration of PSA vaccine and CpG ODN is associated with increased tumor protection in a mouse model of prostate cancer. Vaccine. 2006; 24: 6155-6162.
- Meng Y, Carpentier AF, Chen L, Boisserie G, Simon JM, Mazeron JJ, et al. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int J Cancer. 2005; 116: 992-997.
- Dietrich PY, Dutoit V, Tran Thang NN, Walker PR. T-cell immunotherapy for malignant glioma: toward a combined approach. Curr Opin Oncol. 2010; 22: 604-610.
- Masson F, Calzascia T, Di Berardino-Besson W, de Tribolet N, Dietrich PY, Walker PR. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol. 2007; 179: 845-853.
- Nagasawa DT, Fong C, Yew A, Spasic M, Garcia HM, Kruse CA, et al. Passive immunotherapeutic strategies for the treatment of malignant gliomas. Neurosurg Clin N Am. 2012; 23: 481-495.
- Tsuboi K, Saijo K, Ishikawa E, Tsurushima H, Takano S, Morishita Y, et al. Effects of local injection of ex vivo expanded autologous tumor-specific T lymphocytes in cases with recurrent malignant gliomas. Clin Cancer Res. 2003; 9: 3294-3302.
- Kitahara T, Watanabe O, Yamaura A, Makino H, Watanabe T, Suzuki G, et al. Establishment of interleukin 2 dependent cytotoxic T lymphocyte cell line specific for autologous brain tumor and its intracranial administration for therapy of the tumor. J Neurooncol. 1987; 4: 329-336.
- Tsurushima H, Liu SQ, Tuboi K, Matsumura A, Yoshii Y, Nose T, et al. Reduction of end-stage malignant glioma by injection with autologous cytotoxic T lymphocytes. Jpn J Cancer Res. 1999; 90: 536-545.
- Kruse CA, Cepeda L, Owens B, Johnson SD, Stears J, Lillehei KO. Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin-2. Cancer Immunol Immunother. 1997; 45: 77-87.
- Holladay FP, Heitz-Turner T, Bayer WL, Wood GW. Autologous tumor cell vaccination combined with adoptive cellular immunotherapy in patients with grade III/IV astrocytoma. J Neurooncol. 1996; 27: 179-189.
- Plautz GE, Barnett GH, Miller DW, Cohen BH, Prayson RA, Krauss JC, et al. Systemic T cell adoptive immunotherapy of malignant gliomas. J Neurosurg. 1998; 89: 42-51.
- Plautz GE, Miller DW, Barnett GH, Stevens GH, Maffett S, Kim J, et al. T cell adoptive immunotherapy of newly diagnosed gliomas. Clin Cancer Res. 2000; 6: 2209-2218.
- Wood GW, Holladay FP, Turner T, Wang YY, Chiga M. A pilot study of autologous cancer cell vaccination and cellular immunotherapy using anti-CD3 stimulated lymphocytes in patients with recurrent grade III/IV astrocytoma. J Neuro-Oncol. 2000; 48: 113-120.
- Sloan AE, Dansey R, Zamorano L, Barger G, Hamm C, Diaz F, et al. Adoptive immunotherapy in patients with recurrent malignant glioma: preliminary results of using autologous whole-tumor vaccine plus granulocyte-macrophage colony-stimulating factor and adoptive transfer of anti-CD3-activated lymphocytes. Neurosurg Focus. 2000; 9: e9.
- Arca MJ, Mulé JJ, Chang AE. Genetic approaches to adoptive cellular therapy of malignancy. Semin Oncol. 1996; 23: 108-117.
- Lokhorst HM, Liebowitz D. Adoptive T-cell therapy. Semin Hematol. 1999; 36: 26-29.
- Hayes RL, Arbit E, Odaimi M, Pannullo S, Scheff R, Kravchinskiy D, et al. Adoptive cellular immunotherapy for the treatment of malignant gliomas. Crit Rev Oncol Hematol. 2001; 39: 31-42.
- Eberlein TJ, Rosenstein M, Spiess P, Wesley R, Rosenberg SA. Adoptive chemoimmunotherapy of a syngeneic murine lymphoma with long-term lymphoid cell lines expanded in T cell growth factor. Cancer Immunol Immunother. 1982; 13: 5-13.
- Rosenstein M, Yron I, Kaufmann Y, Rosenberg SA. Lymphokine-activated killer cells: lysis of fresh syngeneic natural killer-resistant murine tumor cells by lymphocytes cultured in interleukin 2. Cancer Res. 1984; 44: 1946-1953.
- Mulé JJ, Shu S, Schwarz SL, Rosenberg SA. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984; 225: 1487-1489.
- Mazumder A, Rosenberg SA. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J Exp Med. 1984; 159: 495-507.
- Ettinghausen SE, Lipford EH 3rd, Mulé JJ, Rosenberg SA. Recombinant interleukin 2 stimulates in vivo proliferation of adoptively transferred Lymphokine-Activated Killer (LAK) cells. J Immunol. 1985; 135: 3623-3635.
- Mulé JJ, Shu S, Rosenberg SA. The anti-tumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo. J Immunol. 1985; 135: 646-652.
- Lafreniere R, Rosenberg SA. Successful immunotherapy of murine experimental hepatic metastases with lymphokine-activated killer cells and recombinant interleukin 2. Cancer Res. 1985; 45: 3735-3741.
- Quan WD Jr, Palackdharry CS. Common cancers--immunotherapy and multidisciplinary therapy: Parts III and IV. Dis Mon. 1997; 43: 745-808.
- Vauleon E, Avril T, Collet B, Mosser J, Quillien V. Overview of cellular immunotherapy for patients with glioblastoma. Clin Dev Immunol. 2010; 2010.
- Lotze MT, Grimm EA, Mazumder A, Strausser JL, Rosenberg SA. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981; 41: 4420-4425.
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982; 155: 1823-1841.
- Kaaijk P, Troost D, Dast PK, van den Berg F, Leenstra S, Bosch DA. Cytolytic effects of autologous lymphokine-activated killer cells on organotypic multicellular spheroids of gliomas in vitro. Neuropath App Neuro. 1995; 21: 392-398.
- George RE, Loudon WG, Moser RP, Bruner JM, Steck PA, Grimm EA. In vitro cytolysis of primitive neuroectodermal tumors of the posterior fossa (medulloblastoma) by lymphokine-activated killer cells. J Neurosurg. 1988; 69: 403-409.
- Dillman RO, Duma CM, Ellis RA, Cornforth AN, Schiltz PM, Sharp SL, et al. Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J Immunother. 2009; 32: 914-919.
- Jacobs SK, Wilson DJ, Kornblith PL, Grimm EA. Interleukin-2 or autologous lymphokine-activated killer cell treatment of malignant glioma: phase I trial. Cancer Res. 1986; 46: 2101-2104.
- Lillehei KO, Mitchell DH, Johnson SD, McCleary EL, Kruse CA. Long-term follow-up of patients with recurrent malignant gliomas treated with adjuvant adoptive immunotherapy. Neurosurgery. 1991; 28: 16-23.
- Dillman RO, Duma CM, Schiltz PM, DePriest C, Ellis RA, Okamoto K, et al. Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother. 2004; 27: 398-404.
- Sankhla SK, Nadkarni JS, Bhagwati SN. Adoptive immunotherapy using lymphokine-activated killer (LAK) cells and interleukin-2 for recurrent malignant primary brain tumors. J Neuro-Oncol. 1996 ;27: 133-140.
- Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ, et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995; 76: 840-852.
- Boiardi A, Silvani A, Ruffini PA, Rivoltini L, Parmiani G, Broggi G, et al. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol Immunother. 1994; 39: 193-197.
- Blancher A, Roubinet F, Grancher AS, Tremoulet M, Bonaté A, Delisle MB, et al. Local immunotherapy of recurrent glioblastoma multiforme by intracerebral perfusion of interleukin-2 and LAK cells. Eur Cytokine Netw. 1993; 4: 331-341.
- Jeffes EW 3rd, Beamer YB, Jacques S, Silberman RS, Vayuvegula B, Gupta S, et al. Therapy of recurrent high grade gliomas with surgery, and autologous mitogen activated IL-2 stimulated killer (MAK) lymphocytes: I. Enhancement of MAK lytic activity and cytokine production by PHA and clinical use of PHA. J Neuro-Oncol. 1993; 15: 141-155.
- Barba D, Saris SC, Holder C, Rosenberg SA, Oldfield EH. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg. 1989; 70: 175-182.
- Curran CS, Bertics PJ. Eosinophils in glioblastoma biology. J Neuroinflammation. 2012; 9: 11.
- Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, et al. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS One. 2011; 6: e22029.
- Kita H1. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011; 242: 161-177.
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006; 24: 147-174.
- Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008; 38: 1254-1263.
- Moezzi J, Gopalswamy N, Haas RJ Jr, Markert RJ, Suryaprasad S, Bhutani MS. Stromal eosinophilia in colonic epithelial neoplasms. Am J Gastroenterol. 2000; 95: 520-523.
- Kural YB, Su O, Onsun N, Uras AR. Atopy, IgE and eosinophilic cationic protein concentration, specific IgE positivity, eosinophil count in cutaneous T Cell lymphoma. Int J Dermatol. 2010; 49: 390-395.
- Bethwaite PB, Holloway LJ, Yeong ML, Thornton A. Effect of tumour associated tissue eosinophilia on survival of women with stage IB carcinoma of the uterine cervix. J Clin Pathol. 1993; 46: 1016-1020.
- Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002; 41: 152-157.
- Goldsmith MM, Belchis DA, Cresson DH, Merritt WD 3rd, Askin FB. The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg. 1992; 106: 27-33.
- Goldsmith MM, Cresson DH, Askin FB. The prognostic significance of stromal eosinophilia in head and neck cancer. Otolaryngol Head Neck Surg. 1987; 96: 319-324.
- Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol. 1981; 34: 1343-1348.
- Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986; 58: 1321-1327.
- Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brünner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999; 189: 487-495.
- Ono Y, Ozawa M, Tamura Y, Suzuki T, Suzuki K, Kurokawa K, et al. Tumor-associated tissue eosinophilia of penile cancer. Int J Urol. 2002; 9: 82-87.
- Thompson AC, Bradley PJ, Griffin NR. Tumor-associated tissue eosinophilia and long-term prognosis for carcinoma of the larynx. Am J Surg. 1994; 168: 469-471.
- Wong DT, Bowen SM, Elovic A, Gallagher GT, Weller PF. Eosinophil ablation and tumor development. Oral Oncol. 1999; 35: 496-501.
- Said M, Wiseman S, Yang J, Alrawi S, Douglas W, Cheney R, et al. Tissue eosinophilia: a morphologic marker for assessing stromal invasion in laryngeal squamous neoplasms. BMC Clin Pathol. 2005; 5: 1.
- Tostes Oliveira D, Tjioe KC, Assao A, Sita Faustino SE, Lopes Carvalho A, Landman G, Kowalski LP. Tissue eosinophilia and its association with tumoral invasion of oral cancer. Int J Surg Pathol. 2009; 17: 244-249.
- von Wasielewski R, Seth S, Franklin J, Fischer R, Hübner K, Hansmann ML, et al. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin's disease, allowing for known prognostic factors. Blood. 2000; 95: 1207-1213.
- Verbik D, Joshi S. Immune cells and cytokines - their role in cancer-immunotherapy (review). Int J Oncol. 1995; 7: 205-223.
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454: 436-444.
- Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, et al. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006; 79: 1131-1139.
- Samoszuk M1. Eosinophils and human cancer. Histol Histopathol. 1997; 12: 807-812.
- Lu JQ, Rashidipour O, Wilson BA, Jack AS, Pugh J, Mehta V. Eosinophil infiltrates in pilocytic astrocytomas of children and young adults. Can J Neurol Sci. 2014; 41: 632-637.
- Dunn GP, Fecci PE, Curry WT. Cancer immunoediting in malignant glioma. Neurosurgery. 2012; 71: 201-222.
- Driggers L, Zhang JG, Newcomb EW, Ge L, Hoa N, Jadus MR. Immunotherapy of pediatric brain tumor patients should include an immunoprevention strategy: a medical hypothesis paper. J Neurooncol. 2010; 97: 159-169.
- Zhang JG, Kruse CA, Driggers L, Hoa N, Wisoff J, Allen JC, et al. Tumor antigen precursor protein profiles of adult and pediatric brain tumors identify potential targets for immunotherapy. J Neurooncol. 2008; 88: 65-76.
- Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008; 38: 709-750.
- Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007; 119: 1313-1320.
- Mossman BT. Introduction to serial reviews on the role of reactive oxygen and nitrogen species (ROS/RNS) in lung injury and diseases. Free Radic Biol Med. 2003; 34: 1115-1116.
- Li JJ, Dickson D, Hof PR, Vlassara H. Receptors for advanced glycosylation endproducts in human brain: role in brain homeostasis. Mol Med. 1998; 4: 46-60.
- Mueller MM, Herold-Mende CC, Riede D, Lange M, Steiner HH, Fusenig NE. Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol. 1999; 155: 1557-1567.
- Curran CS, Evans MD, Bertics PJ. GM-CSF production by glioblastoma cells has a functional role in eosinophil survival, activation, and growth factor production for enhanced tumor cell proliferation. J Immunol. 2011; 187: 1254-1263.
- Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008; 205: 79-90.
- Grauer OM, Molling JW, Bennink E, Toonen LW, Sutmuller RP, Nierkens S, et al. TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol. 2008; 181: 6720-6729.
- Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009; 6: e10.
- Wiemels JL, Wiencke JK, Patoka J, Moghadassi M, Chew T, McMillan A, et al. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer Res. 2004; 64: 8468-8473.
- Wigertz A, Lonn S, Schwartzbaum J, Hall P, Auvinen A, Christensen HC, et al. Allergic conditions and brain tumor risk. Am J Epidemiol. 2007; 166: 941-950.
- Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007; 99: 1544-1550.
- Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000; 55: 688-697.
- Erkeller-Yuksel FM, Deneys V, Yuksel B, Hannet I, Hulstaert F, Hamilton C, et al. Age-related changes in human blood lymphocyte subpopulations. J Pediatr. 1992; 120: 216-222.
- Griesinger AM, Birks DK, Donson AM, Amani V, Hoffman LM, Waziri A, et al. Characterization of distinct immunophenotypes across pediatric brain tumor types. J Immunol. 2013; 191: 4880-4888.
- Griesinger AM, Donson AM, Foreman NK. Immunotherapeutic implications of the immunophenotype of pediatric brain tumors. Oncoimmunology. 2014; 3: e27256.
- Johnson LA, Sampson JH. Immunotherapy approaches for malignant glioma from 2007 to 2009. Curr Neurol Neurosci Rep. 2010; 10: 259-266.
- Okada H, Kohanbash G, Zhu X, Kastenhuber ER, Hoji A, Ueda R, et al. Immunotherapeutic approaches for glioma. Crit Rev Immunol. 2009; 29: 1-42.
- Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci. 2009; 16: 748-754.
- Heimberger AB, Archer GE, Crotty LE, McLendon RE, Friedman AH, Friedman HS, et al. Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective against murine intracerebral melanoma. Neurosurgery. 2002; 50: 158-164.
- Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010; 28: 4722-4729.
- Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011; 6: e23902.
- Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009; 57: 1458-1467.
- Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007; 67: 9630-9636.
- Dutta T, Spence A, Lampson LA. Robust ability of IFN-gamma to upregulate class II MHC antigen expression in tumor bearing rat brains. J Neurooncol. 2003; 64: 31-44.
- Lambert C, Ase AR, Séguéla P, Antel JP. Distinct migratory and cytokine responses of human microglia and macrophages to ATP. Brain Behav Immun. 2010; 24: 1241-1248.
- Deininger MH, Pater S, Strik H, Meyermann R. Macrophage/microglial cell subpopulations in glioblastoma multiforme relapses are differentially altered by radiochemotherapy. J Neuro-Oncol. 2001; 55: 141-147.
- Kim SS, Ye C, Kumar P, Chiu I, Subramanya S, Wu H, et al. Targeted delivery of siRNA to macrophages for anti-inflammatory treatment. Mol Ther. 2010; 18: 993-1001.
- Weingart JD, Sipos EP, Brem H. The role of minocycline in the treatment of intracranial 9L glioma. J Neurosurg. 1995; 82: 635-640.
- Ghosh A, Mukherjee J, Bhattacharjee M, Sarkar P, Acharya S, Chaudhuri S, et al. The other side of the coin: beneficiary effect of 'oxidative burst' upsurge with T11TS facilitates the elimination of glioma cells. Cell Mol Biol. 2007; 53: 53-62.
- Markovic DS, Vinnakota K, van Rooijen N, Kiwit J, Synowitz M, Glass R, et al. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav Immun. 2011; 25: 624-628.
- Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol. 2010; 225: 195-199.
- Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008; 57: 123-131.