
Review Article
Austin Neurosurg Open Access. 2015; 2(4): 1038.
Intraoperative Neurophysiological Monitoring Techniques for the Resection of Malignant Brain Tumors Located in Eloquent Cortical Areas
Lorena Vega-Zelaya and Jesús Pastor*
Clinical Neurophysiology, Hospital Universitario “La Princesa”, Spain
*Corresponding author: Jesús Pastor, Clinical Neurophysiology, Hospital Universitario “La Princesa”, C/Diego de León 62, 28006, Madrid, Spain
Received:August 10, 2015; Accepted: September 21, 2015Published: September 23, 2015
Abstract
Glioblastoma Multiforme (GBM) is the most common central nervous system tumor. Despite progress in both medical and surgical treatments for this disease, the life expectancy associated with GBM is short; only a limited number of patients survive more than three years following diagnosis. When tumors are located in eloquent areas, the achievement of Gross Tumor Resection (GTR) is limited by the risk of permanent neurological deficits, restricting patients’ quality of life. Mapping techniques have enabled clinicians to localize eloquent cortical and subcortical fibers and Intraoperative Neurophysiological Monitoring (IONM) allows to monitor the function of at-risk neurological structures during the surgery. We have identified several criteria that may provide us with both reliable and efficient means of monitoring said structures. The efficacy of such techniques has improved, as have their sensitivity, specificity and safety. Tumor resection is more successful when guided via fluorescence but carries the risk of permanent neurological deficits. IONM minimizes said risk without compromising the chances of a successful resection. Diligent monitoring may also enable clinicians to avoid performing a surgery in which the patient awake provided that the language areas of the brain are not involved. The achievement of maximum GTR is the most important prognostic factor with respect to patient survival in the setting of high-grade gliomas. IONM and monitoring techniques maximize the effectiveness of GTR and are associated with reduced rates of surgery-related deficits.
Keywords: Direct cortical stimulation, High-grade gliomas, Motor-evoked potentials, Somatosensory-evoked potentials, Visual-evoked potentials, Anesthetized craniotomy, 5-aminolevulenic acid
Introduction
Glioblastoma Multiforme (GBM) is the most common and lethal primary malignancy of the Central Nervous System (CNS). Several adjuvant therapies have been developed to improve progression-free survival, including surgical resection, local radiotherapy and systemic chemotherapy. Despite these innovations, the median survival time following diagnosis is only 14.6 months [1]. Nevertheless, 3–5% of patients survive more than 3 years; said patients are known as longterm survivors [2]. Younger age and good Karnofsky Performance Scores (KPSs) at the time of diagnosis are both associated with longer survival [3], but the first and most important step in the treatment of any primary malignant brain tumor is Gross Total Resection (GTR) [4]. To preserve patients’ quality of life, the primary goal of surgery is the achievement of GTR without compromising neurological function. Advances in surgical techniques such as Intraoperative Neurophysiological Monitoring (IONM), intraoperative Magnetic Resonance Imaging (MRI), Diffusion Tensor Imaging (DTI), stereotactic guidance, and fluorescence-guided resection have facilitated the delineation of tumor borders and may help to optimize maximal safe surgical resection [5-7].
In patients with tumors located in eloquent brain areas (areas responsible for carrying out basic neurological functions) such as the sensorimotor cortex and the language cortex and subcortical structures such as the basal ganglia and the internal capsule, the proper identification of relevant tracts is necessary to preserve adequate neurological function [8]. Tractographyvia Diffusion Tensor Imaging (DTI) and intraoperative Magnetic Resonance Imaging (MRI) have proven useful in the identification of Internal Capsule (IC) and Thalamocortical Fibers (ThCF); dynamic changes during surgery (loss of cerebrospinal fluid and low-grade brain edema) and a lack of reliability with respect to the identification of small and functional tracts pose potential risks to patients [9,10]. IONM enables clinicians to access the function of patients’ motor and sensory systems during surgery to preserve neurological function and increases the success of radical tumor resection.
This article encompasses a review of the most commonly used techniques available for the mapping and monitoring of neural function in the setting of glioma surgery involving eloquent brain areas, the warning criteria for each modality, and the intrinsic technical limitations of each technique.
Intraoperartive Neurophysiological Monitoring Modalities
Mapping to localize eloquent areas
Functional areas: Intraoperative electroencephalographic recordings obtained directly from the cortical surface and Electrocorticography (ECoG) are used to monitor stimulationtriggered epileptiform discharges and after discharges before and during electrophysiologic functional mapping in the setting of glioma surgery (Figure 1). Said procedures are performed before electrical stimulation of the cortex is using either grid or strip electrodes with bandwidths of 1.5–1000 Hz. Weutilize spectral analyses using a Fast Fourier Transform (FFT), using non-overlapping windows of 8 s in length, for each ECoG recording. These results are depicted as a Density Spectral Array (DSA) for frequencies ranging from 1 to 50 Hz, which allows us to define three functional areas as follows: i) an activity loss area, defined based on decreases in the appearances of all frequencies, particularly the faster alpha and beta bands; ii) an irritative area, defined based on the presence of interictal epileptiform discharges appearing either as spikes (<80 ms) or as sharp waves (80- 200 ms) with amplitudes greater than 3 standard deviations above basal activity; and iii) normal cortex, a region devoid of either irritative elements or the abnormal loss of bioelectrical activity [11]. The identification of complex spectral changes over time enables clinicians to observe local cortical excitability [12], which is important for the following reason: said identification allows clinicians to determine the risk of stimulation-triggered seizure activity and to determine the locations of the irritative zones relative to the site of stimulation, which makes it possible to estimate the risk of triggering Ads [13]. It is also important to identify cortical areas with larger amounts of lesions (denoted by the loss of cortical rhythm) to preserve cortical areas with fewer lesions.
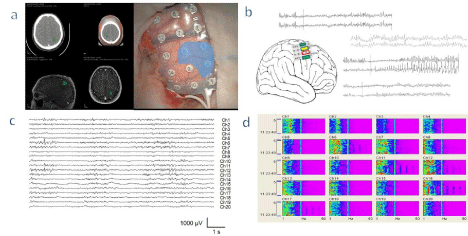
Figure 1: Electrocorticography and mapping in a patient with a peri-rolandic tumor. a - Neuronavigational images of a tumor located at the central sulcus (left) and
grid with 20 electrodes onto the cortex (right). Electrodes shaded in gray show the region with loss of activity. b - Examples of pairs of electrode records, the yellow
ones show the basal activity before direct cortical stimulation. The green electrodes display normal response after stimulation (records at the top and bottom).
When the stimulation was performed at the electrodes framed in red square we obtained an after-discharge showing an irritative and seizure-like morphology.
c - Illustration of a raw record of an electrocorticography. d - A Density Spectral Array (DSA) used to help identify different functional areas. For each electrode, we
have a plot showing the power spectral density in a color code, for every frequency in the abscissa’s axis, along the time shown in the ordinate axis. It can be noted
electrodes 3 to 5, where the power of all frequencies is clearly lower than for the rest of the grid.
Motor and sensory mapping: Following the identification of functional areas via ECoG, the locations of the pre- and postcentral gyri and the Central Sulcus (CS) are determined via somatosensory evoked potential phase reversal (Figure 2a). However, it is not unusual to discover that the presumed location of the CS as determined via neuronavigation is erroneous [14]. This maybe the result of inter individual anatomic variability, as the tumor itself may cause anatomic distortions, dissociations between the anatomy and physiology of the region and imprecise calibrations of the neuronavigational system. Therefore, the “correlated” presumed location of the CS as determined via neuronavigation must be confirmed via neurophysiology. The accurate identification of the CS is extremely important because it makes possible the identification of the Primary Motor Cortex (PMC); although there are many instances in which the identification may not be successful on a first attempt, instances including the repositioning of the recording electrode because of a small surgical field or distorted pericentral anatomy, there is evidence that somatosensory evoked potential phase reversal increases both the efficiency and the safety of PMC identification [13].
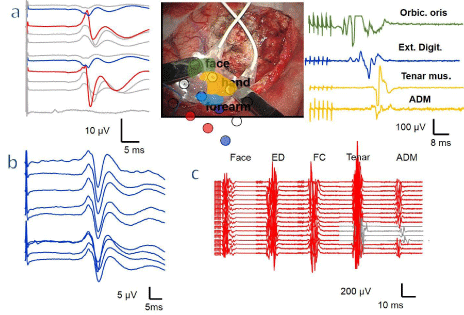
Figure 2: Mapping and monitoring in cortical surgery in a patient previously operated. a - Placement of the grid near the tumor (middle picture). The colored areas
show the motor parts of the face, hand, and forearm and they correspond to its MEP colored with the same color (right). The red discs show the area of the postrolandic
zone, with a phase reversal illustrated in the figure on the left, and the blue discs, the pre-rolandic cortex zone. b - After the mapping y during resection, the
SSEP was monitoring through the grid. c - The MEP elicited by the DCS through the grid and was used for monitoring of motor system during the entire surgery. A
reversal and selective alteration of motor response was observed (grey traces of tenar and ADM muscle).
Direct Cortical Stimulation (DCS) for identifying the PMC is accomplished using paired electrodes. Stimulation is performed using 4-6 pulse trains at 500 Hz (the reason we denote this paradigm as high frequency; this technique is also known as multipulse, which is misleading), with bi-phasic pulses of 150-200 μs in duration/phase. Motor evoked potentials are assessed using pairs of subdermal needles (12/18 mm SGM®, Ljubiceva, Croatia) spaced approximately 2 cm apart that are inserted into the contralateral muscles. Depending on tumor location, we utilize the following muscles: the orbicularis oculi, orbicularis oris, deltoid, brachial biceps, Extensor Digitorum (ED) carpal flexor, Abductor Pollicis Brevis (APB), Abductor Digitiminimi (ADM), quadriceps, Tibialis Anterior (TA) and Abductor Hallucis (AH). Stimulation begins at 4 mA and increases continuously in increments of 1-2 mA, until a Compound Muscle Action Potential (CMAP) is recorded, at minimum amplitude of 30 μV, or until an upper limit of 30 mA is achieved without eliciting a CMAP (Figure 2a) [7,11].
An alternative strategy entails the use of Ojemann’s stimulation or low-frequency stimulation, which consists of a 50-60 Hz train, 3-5 seconds in length, with a pulse-width as high as 400 μs [14,15].
The best evidence to date that stimulation mapping techniques impact post-surgical outcomes is as follows: using DCS mapping during surgery results in the development of severe post-surgical neurological deficits in 3.4% of patients. However, as many as 8.2% of patients develop severe neurological deficits following resections performed without DCS mapping. However, DCS mapping not only reduces the percentage of neurological deficits but also allows for more successful GTR [15].
Subcortical electrical stimulation during glioma surgery is useful for distinguishing tumors from the corticospinal tract [16,17]. Subcortical stimulation is undertaken in a manner similar to that used for DCS, although subcortical stimulation involves the use of cathodal (negative-current) stimulation.
A systematic comparison of the 50-Hz stimulation with the multipulse stimulation technique combined with both monopolar and bipolar probes with respect to the identification of the corticospinal tract was performed for Szelényi et al. [18]. They concluded that monopolarcathodal stimulation is more effective with respect to subcortical activation with a lower motor threshold (MT) compared with all other stimulation modalities.
In most of cases, a linear relationship exists among the five monopolar 0.2–0.5 ms pulses and 3–4 ms ISI, and a threshold of 1 mA of stimulation, which equals approximately 1 mm of distance to the CST [9]. Many studies have challenged this theory and attempted to determine the lowest intensity of stimulation allowed before the resection should be stopped to prevent injury to the CST. This safety margin has not been standardized and has been defined as 6 mA in some studies, whereas other studies have suggested that both significant signal changes in MEP monitoring and permanent motor deficits do not occur before an MT of 1-3 mA [19]. Therefore, it is important to emphasize that these MT safety margins may be followed as long as any alterations in MEP continuous motor monitoring are not observed; otherwise, the resection must be stopped immediately.
In a recent study, it was noted that continuous and dynamic mapping by integrating the mapping probe at the tip of a suction device improves mapping accuracy, particularly at low stimulation intensities, with greater repercussions in cases involving the resection of infiltrative tumors [20].
Diffusion Tensor Imaging Fiber Tracking (DTI-FT) is the only technique available for the noninvasive depiction of subcortical white matter tracts and is widely used for preoperative mapping [16,21,22]. Nevertheless, various studies have investigated the accuracy of intraoperative DTI-FT compared with intraoperative subcortical electrical stimulation mapping of the motor pathways and noted good sensitivity and specificity [23-25]. However, DTI-FT is an anatomical imaging analysis and does not include true electrophysiological functional data. Furthermore, other studies have observed CST shifts within a range of -8 to 15 mm and noted that the direction of the shift was not predictable [26]. Therefore, DTI-FT is an effective additional tool for preoperative planning; however, when it is used intraoperatively for the resection of tumors adjacent to the CST, it requires neurophysiological confirmation.
Cortical and subcortical language mapping: Locating the functional cortical regions related to language is the goal of intraoperative language mapping stimulation, which is performed during awake surgery. For this type of mapping, the patient’s compliance is important, as a series of language tasks is performed during surgery by a trained neuropsychologist. Said tasks include systematic counting, naming, and reading; repetition and semantic tasks may also be used, depending on the primary tumor’s location.
Penfield introduced DCS to assess motor function in the clinical setting in 1961 [27]. DCS is the technique of choice when performing language mapping. A constant current stimulator delivers biphasic trains of 50 Hz with a maximum duration of 4 seconds using onemillimeter bipolar electrodes positioned 5 mm apart, beginning with a low stimulus at a constant current with 1.5-mA square-wave pulses and increasing to a maximum stimulus of 6 mA. The cortex is mapped every 5–10 mm, and the positive stimulation sites at which the language impairment was caused are marked; the same technique is utilized during the resection of the tumor via subcortical stimulation. Continuous monitoring of language function via language task explorations is performed throughout the resection.
The identification of the language areas and their fibers is not as successful as the localization of the cortical white matter in the PMC and the CST. Sanai et al. [28] successfully identified the language areas in 145 (58%) of 250 patients with gliomas. Temporary language deficits were observed in 22% of patients, whereas permanent language deficits were observed in only1.6% of patients.
The limitations of awake surgery must be considered. During such surgeries, the patient is awake with the head fixed and covered with cloth; the patient may be kept awake for up to 2 hours. Hence, patients must have both adequate cognitive function and the emotional maturity necessary to withstand such an environment. In fact, the Japan Society for Awake Surgery Guidelines limit the target patient population to patients ranging from 15-65 years of age.
Continuous neurophysiological monitoring techniques
Motor and sensory function: Following the identification of the PMC, as well as the determination of its relationship with the tumor, continuous motor monitoring is performed via stimulation of the motor cortex using pairs of grid electrodes and employing high frequency stimulation (Figure 2c) [7,11].
The primary criterion for the monitoring of Motor Evoked Potentials (MEP) in the setting of supratentorial surgery is an amplitude reduction of >50% [22,28,29]. Although previous authors have suggested that a threshold increase greater than 4 mA is necessary to maintain appropriate amplitude [19], these data are ambiguous [29]. Temporary motor deficits have been linked to reversible MEP amplitude declines of > 50%, whereas irreversible MEP declines and MEP losses are predictors of permanent motor deficits [30]. In a recently published study involving 34 patients who underwent glioma surgical resection guided using 5-Aminolevulinic Acid (5-ALA), no false-negative results were obtained [7]. However, the possibility of post operative neurological deficits without having alterations in MEPs following surgery must be taken into account. This scenario, which has been described as an untrue false-negative, may be explained by secondary events such as postoperative edema, hemorrhage and tumors resected from the Supplementary Motor Area (SMA) [22].
Another powerful tool for motor function monitoring is transcranial electrical stimulation, although it depends on the surgical approach to perform the scalp montage required. Common TES montages include hemispheric, inter-hemispheric or midline. The hemispheric montages usually use C3–Cz and C4–Cz [18,31,32]. They are recommended for facial and arm MEP but not leg MEP. They produce less movement in the patient. The inter-hemispheric montages are C1/C2 and C3/C4 [31,32]. They evoke arm, leg and sphincter MEP but are inadvisable for facial MEPs because of confounding facial nerve excitation [29]. The disadvantage of interhemispheric montages is that the increasing intensity allows for the progressive stimulation of caudal structures, which stimulates the midbrain or related areas. Therefore, during surgical interventions involving supratentorial structures, it is theoretically possible to stimulate the Corticospinal Tract (CST) within the region being treated, thereby obtaining false negative results, which may have disastrous consequences for the patient [31,33]. The hemispheric montage has demonstrated excellent sensitivity with respect to the detection of a new injury, as well as excellent specificity (Figure 3) [34].
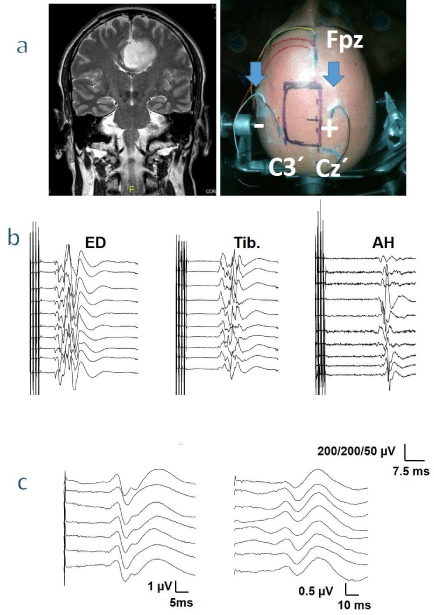
Figure 3: Monitoring of a patient harboring a tumor in the left frontal parasagittal region. a - Frontal MRI before the surgery (left), and an image indicating the
placement of the electrodes and area of the incision (right). Note that the surgical field is located between the TES electrodes (arrows). The electrodes for the
SSEP are indicated. b - Monitoring of MEP of upper and lower limbs. c - Response in the upper (left) and lower limb (right) SSEP. ED extensor digitorum, Tib.
tibialis anterior, AH abductor hallucis.
The midline montage is Cz-1 cm to Cz+6 cm [18,31]. It evokes symmetric leg MEPs and offers the advantage of constrained patient movement, although it is less effective than inter-hemispheric stimuli [29].
Stimulation parameters have not been standardized; therefore, either train pulse number and ISI or frequency (ISI = 1000/ frequency) are typically used, based on the experience of each center. Using 5 pulses is considered reasonable, although the range may be 4-8 pulses [31,32,35]. Adding pulses reduces the MEP threshold and increases its amplitude and duration. However, short pulses are more efficient and require a load less than 35% to induce a motor response [34,36]. The same debate has occurred regarding ISI. In these cases, some authors prefer 2-ms pulses, whereas others have opted for 4-ms pulses [18,37].
When sensory function monitoring is also required, cortical Somatosensory-Evoked Potentials (cSSEP) are directly recorded from the grid using a reference electrode placed on the contralateral ear lobe. They are elicited via electrical stimulation (constant-current) of the contralateral median nerve at the wrist (upper limb) or the posterior tibial is at the ankle (lower limb) by 200–300 pulses per train at 7.1 Hz and 200 μs widths. Monitoring the electrode entails the selection of higher amplitude responses for N1/P1/N2 potentials (Figure 2b).
Visual function: The performance of tumor surgery adjacent to either the optic tract or the visual cortex is not uncommon. However, intraoperative monitoring in this setting is uncommon, the reasons for which all well-established, as follows: the instability of recording, the lack of correlation with postoperative visual function and the high susceptibility to anesthetic agents, each of which explains the questions regarding its efficacy.
The use of subdural strip electrodes to record cortical VEP (cVEP) has proven to be more reliable than scalp electrodes and may be useful in monitoring the functional integrity of the posterior visual pathway due to its superior spatial resolution, signal to noise ratio and temporal stability [38,39]. We described a case characterized by highly stable cVEP responses, with minor variations in amplitude and virtually no variations in the latencies of its primary components, as well as a strong correlation with postoperative visual function (Figure 4).
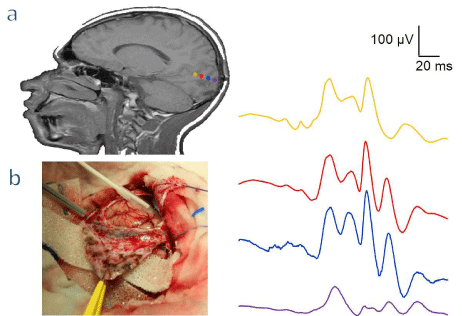
Figure 4: Patient with a tumor located near the primary visual cortex. a - The image on the left shows neuronavigational images with the position of the 4 electrodes
strip in the occipital cortex (colored discs), the left image depict the potentials recorded with response topographically different and of a very high amplitude (colors
correspond to the image of the left). b - A picture of the cortex after the opening of the dura, indicating the position of the strip.
We used flashing light-emitting diodes stimulated at 2.18 Hz, 100 pulses, widths of 10 μs, and a bandwidth of 10–1,000 Hz. We considered an increase in latency of 10% or a reduction in amplitude greater than 50% amplitude compared with baseline alarm criteria.
Some authors have proposed that increasing the frequency of the stimuli may allow for more stable responses [40]. However, other authors [38] have recorded cVEP with light flashes at 1 Hz in 17 patients undergoing tumor resections involving the parietal, posterior temporal and occipital lobes, as well as non-lesional posterior epileptic foci. The results have demonstrated stable recordings and correlated strongly with postoperative visual function.
Intraoperartive Neurophysiological Monitoring in Fluorescence Guided Surgery
5-ALA induced protoporphyrin IX (PpIX) has been used in the Fluorescence Guided Resection (FGR) of high-grade gliomas [41]. Administered in an oral form, PpIX accumulation is elevated in many malignant tissues, several tissue abnormalities, and the mucosa [42]. This accumulation may be visualized by irradiating the patient with a blue light (wavelength of 400 nm). As mentioned above, one of the primary goals of the treatment of GBM is to achieve a GTR; FGR increased GTR to >64% [4,43]. Additional studies have reported that FGR has a high sensitivity (82.6) and specificity (88.8%) for highgrade gliomas and reduced the risk of tumor progression (HR 0.73) [44]. This evidence indicates that FGR is an invaluable technique with respect to achieving maximum tumor resection [44]; however, radical tumor resection always carries the danger of causing neurological deficits, particularly during the removal of tumors in or near eloquent areas of the brain. Hence, an elevated degree of neurological postsurgical alterations [45] were observed due to difficulty in identifying functional areas and tracts. Therefore, in this type of surgery, the use of IONM is critical to ensure the maximum possible tumor resection without causing postoperative neurological deficits.
We have demonstrate that it is possible to obtain a significant percentage of GTR (66.7%), with a mean tumor resection of 90.4 ± 3.7% in patients harboring tumors in or near eloquent areas during FGR surgery with IONM without causing neurological deficits [7]. In this series, surgery was stopped in four patients due to warning criteria despite the use of fluorescence. In each of these cases, no permanent neurological injuries were observed, although transitory alterations were observed in two cases. The decision to stop the surgery was made in accordance with the criterion to preserve neurological function over the use of fluorescence.
Discussion
Neurophysiological monitoring techniques for surgery of supratentorial tumors located in or near eloquent areas have continued to evolve and improve. Although no standardized protocols exist, there is increasing evidence supporting the efficacy and safety of neurophysiological monitoring. Different techniques may be used for each patient in accordance with the location of each patient’s tumor and the surgical approach used for tumor resection.
In patients harboring cortical tumors, motor mapping may be performed either in an anesthetized patient or an awake patient. We prefer the use of general anesthesia. As we have demonstrated previously [7,46], it may be safely performed when intensive neurophysiological mapping and monitoring are utilized. Interest in the performance of awake craniotomies has increased in recent years [47,48], not only for tumors of the rolandic cortex but also for tumors involving other eloquent cortical areas [49]. No differences in the immediate postoperative motor statuses, the extent of resection or threshold intensity were observed when both methods were compared. In our opinion, surgery involving anesthetized patients represents a better scenario in the operating room. Moreover, anesthesia provides more time to either repeat or create new cortical and peripheral stimuli and perform a comprehensive neurophysiological study. Most importantly, anesthesia saves the patient from having an unpleasant experience and may be used in patients whose cooperation is problematic (such as children or individuals with cognitive deficits). Therefore, in the event that no differences in immediate postoperative motor status, the extent of resection, or threshold intensity are observed when both methods are compared [50], the above arguments are sufficient to justify the use of anesthesia as opposed to the performance of an awake craniotomy for tumors located within or near eloquent areas that do not involve either language or specific cognitive functions.
Achieving maximum GTR is an important prognostic factor with respect to the survival in the setting of a high-grade glioma, and FGR is powerful techniques that may enable neurosurgeons to improve the degree of tumor resection. However, is it extremely important to identify the functional limits of each resection to ensure patients an adequate quality of life; therefore, the use of this technique with IONM ensures maximum tumor resection without the development of new neurological deficits.
The techniques described in this review have significantly evolved within recent years, resulting in changes in the way gliomas involving eloquent brain regions are treated; overall survival has increased, and the incidence of surgery-related deficits has decreased. The most important task currently facing clinicians is to improve the accuracy of said techniques and to perform prospective studies to generate larger amounts of evidence supporting the performance of glioma resection in patients with tumors previously described as non-respectable.
Acknowledgment
We want to thank Dr. Rafael G. Sola and Dra. Paloma Pulido for his help during intraoperative recordings. This work was supported by a grant from the Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica (I+D+I), Instituto de Salud Carlos III, Subdirección General de Evaluación y Fomento de la Investigación PI12/02839 and partially supported by F.E.D.E.R. The authors report no conflict of interest concerning the material or methods used in this study or the findings specified in this paper.
References
- Stupp R, Mason WP, Van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. N Engl J Med. 2005; 352: 987-996.
- Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain. 2007; 130: 2596-2606.
- Curran WJ, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993; 85: 704-710.
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006; 7: 392-401.
- Anton K, Baehring JM, Mayer T. Glioblastoma multiforme: overview of current treatment and future perspectives. Hematol Oncol Clin North Am. 2012; 26: 825-853.
- Perry J, Okamoto M, Guiou M, Shirai K, Errett A, Chakravarti A. Novel therapies in glioblastoma. Neurol Res Int. 2012; 2012: 428565.
- Pastor J, Vega-Zelaya L, Pulido P, Garnés-Camarena O, Abreu A, Sola RG. Role of intraoperative neurophysiological monitoring during fluorescence-guided resection surgery. Acta Neurochir (Wien). 2013; 155: 2201-2213.
- Feigl GC, Ritz R, Moraes M, Klein J, Ramina K, Gharabaghi A, et al. Resection of malignant brain tumors in eloquent cortical areas: a new multimodal approach combining 5-aminolevulinic acid and intraoperative monitoring. J Neurosurg. 2010; 113: 352-357.
- Kamada K, Todo T, Ota T, Ino K, Masutani Y, Aoki S, et al. The motor-evoked potential threshold evaluated by tractography and electrical stimulation. J Neurosurg. 2009; 111: 785-795.
- Ozawa N, Muragaki Y, Nakamura R, Hori T, Iseki H. Shift of the pyramidal tract during resection of the intraaxial brain tumors estimated by intraoperative diffusion-weighted imaging. Neurol Med Chir (Tokyo). 2009; 49: 51-56.
- Vega-Zelaya L, Pedrosa-Sánchez M, Pastor J. Cortical mapping and neurophysiological monitoring during resection of an arteriovenous malformation in the rolandic region. Rev Neurol, 2014; 59: 20-24.
- Vega-Zelaya L, Garnés-Camarena O, Ortega GJ, Pastor J. Fundamentos de EEG cuantificado. Rev Neurol. 2015.
- Simon MV, Sheth SA, Eckhardt CA, Kilbride RD, Braver D, Williams Z, et al. Phase reversal technique decreases cortical stimulation time during motor mapping. J Clin Neurosci, 2014; 21: 1011-1017.
- Chang EF, Clark A, Smith JS, Polley MY, Chang SM, Barbaro NM, et al. Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: improvement of long-term survival. J Neurosurg. 2011; 114: 566-573.
- De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012; 30: 2559-2565.
- Bello L, Castellano A, Fava E, Casaceli G, Riva M, Scotti G, et al. Intraoperative use of diffusion tensor imaging fiber tractography and subcortical mapping for resection of gliomas: technical considerations. Neurosurg Focus, 2010; 28: E6.
- Berger MS, Ojemann GA, Lettich E. Neurophysiological monitoring during astrocytoma surgery. Neurosurg Clin N Am. 1990; 1: 65-80.
- Szelényi A, Kothbauer KF, Deletis V. Transcranial electric stimulation for intraoperative motor evoked potential monitoring: Stimulation parameters and electrode montages. Clin Neurophysiol. 2007; 118: 1586-1595.
- Seidel K, Beck J, Stieglitz L, Schucht P, Raabe A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J Neurosurg. 2013; 118: 287-296.
- Raabe A, Beck J, Schucht P, Seidel K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. J Neurosurg. 2014; 120: 1015-1024.
- Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005; 56: 130-137.
- Krieg SM, Buchmann NH, Gempt J, Shiban E, Meyer B, Ringel F. Diffusion tensor imaging fiber tracking using navigated brain stimulation--a feasibility study. Acta Neurochir (Wien). 2012; 154: 555-563.
- Ohue S, Kohno S, Inoue A, Yamashita D, Harada H, Kumon Y, et al. Accuracy of diffusion tensor magnetic resonance imaging based tractography for surgery of gliomas near the pyramidal tract: a significant correlation between subcortical electrical stimulation and post operative tractography. Neurosurgery, 2012; 70: 283-293.
- OstrŶ S, Belšan T, Otăhal J, Beneš V, Netuka D. Is intraoperative diffusion tensor imaging at 3.0T comparable to subcortical corticospinal tract mapping? Neurosurgery. 2013; 73: 797-807.
- Zhu FP, Wu JS, Song YY, Yao CJ, Zhuang DX, Xu G, et al. Clinical application of motor pathway mapping using diffusion tensor imaging tractography and intraoperative direct subcortical stimulation in cerebral glioma surgery: a prospective cohort study. Neurosurgery. 2012; 71: 1170-1183.
- Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2007; 61: 178-185.
- De Benedictis A, Moritz-Gasser S, Duffau H. Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas. Neurosurgery. 2010; 66: 1074-1084.
- Sanai N, Berger MS. Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg Focus. 2010; 28: E1.
- Macdonald DB, Skinner S, Shils J, Yingling C. American Society of Neurophysiological Monitoring. Intraoperative motor evoked potential monitoring - a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol. 2013; 124: 2291-316.
- Krieg SM, Schäffner M, Shiban E, Droese D, Obermüller T, Gempt J, et al. Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions. J Neurosurg. 2013; 118: 1269-1278.
- Deletis V, Shils JL. Neurophysiology in neurosurgery. Califormia: Academic Press. 2002.
- MacDonald DB, Al Zayed Z, Al Saddigi A. Four-limb muscle motor evoked potential and optimized somatosensory evoked potential monitoring with decussation assessment: results in 206 thoracolumbar spine surgeries. Eur Spine J. 2007; 16: S171–S187.
- Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol. 1994; 481: 243-250.
- Pastor Gómez J, Perla-Perla P, Pulido-Rivas P, Sola RG. [Hemispheric transcranial electrical stimulation: clinical results]. Rev Neurol. 2010; 51: 65-71.
- Quiñones-Hinojosa A, Lyon R, Zada G, Lamborn KR, Gupta N, Parsa AT, et al. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005; 56: 982-993.
- Hausmann ON, Min K, Boos N, Ruetsch YA, Erni T, Curt A. Transcranial electrical stimulation: significance of fast versus slow charge delivery for intra-operative monitoring. Clin Neurophysiol. 2002; 113: 1532-1535.
- Calancie B, Harris W, Brindle GF, Green BA, Landy HJ. Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg. 2001; 95: 161-168.
- Ota T, Kawai K, Kamada K, Kin T, Saito N. Intraoperative monitoring of cortically recorded visual response for posterior visual pathway. J Neurosurg. 2010; 112: 285-294.
- Torres CV, Pastor J, Rocío E, Sola RG. [Continuous monitoring of cortical visual evoked potentials by means of subdural electrodes in surgery on the posterior optic pathway. A case report and review of the literature]. Rev Neurol. 2012; 55: 343-348.
- Wiedemayer H, Fauser B, Armbruster W, Gasser T, Stolke D. Visual evoked potentials for intraoperative neurophysiologic monitoring using total intravenous anesthesia. J Neurosurg Anesthesiol. 2003; 15: 19-24.
- Stepp H, Beck T, Pongratz T, Meinel T, Kreth FW, Tonn JCh, et al. ALA and malignant glioma: fluorescence-guided resection and photodynamic treatment. J Environ Pathol Toxicol Oncol. 2007; 26: 157-164.
- Krammer B, Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci. 2008; 7: 283-289.
- Eljamel MS. Fluorescence image-guided surgery of brain tumors: explained step-by-step. Photodiagnosis Photodyn Ther. 2008; 5: 260-263.
- Su X, Huang QF, Chen HL, Chen J. Fluorescence-guided resection of high-grade gliomas: a systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2014; 11: 451-458.
- Díez Valle R, Tejada Solis S, Idoate Gastearena MA, García de Eulate R, Domínguez Echávarri P, AristuMendiroz J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma. Volumetric analysis of extent of resection in single-center experience. J Neurooncol. 2011; 102: 105-113.
- Pastor J, Pulido-Rivas P, Sola RG. [Neurophysiological assisted transsulcal approach to a high grade glioma without affect neither motor nor somatosensory function]. Rev Neurol. 2013; 56: 370-374.
- Duffau H, Capelle L, Sichez J, Faillot T, Abdennour L, Law Koune JD, et al. Intra-operative direct electrical stimulations of the central nervous system: the Salpêtrière experience with 60 patients. Acta Neurochir (Wien). 1999; 141: 1157-1167.
- Peruzzi P, Puente E, Bergese S, Chiocca EA. Intraoperative MRI (ioMRI) in the setting of awake craniotomies for supratentorial glioma resection. Acta Neurochir Suppl. 2011; 109: 43-48.
- Nguyen HS, Sundaram SV, Mosier KM, Cohen-Gadol AA. A method to map the visual cortex during an awake craniotomy. J Neurosurg. 2011; 114: 922-926.
- Sarubbo S, Latini F, Panajia A, Candela C, Quatrale R, Milani P, et al. Awake surgery in low-grade gliomas harboring eloquent areas: 3-year mean follow-up. Neurol Sci. 2011; 32: 801-810.