
Research Article
Austin Neurosurg Open Access. 2016; 3(2): 1053.
Prognostic Analysis in Malignant Gliomas: Relationship between Brain Perfusion Magnetic Resonance and Methylation Analysis of MGMT Gene Promoter
Gepp RA¹*, Rocque AL², Martins BJFA³, Batista GR4, Farage L4 and Pratesi R5
¹Neurosurgery Department, SARAH Network of Rehabilitation Hospitals, Brazil
²Molecular Genetics Laboratory, SARAH Network of Rehabilitation Hospitals, Brazil
³Radiology Department, SARAH Network of Rehabilitation Hospitals, Brazil
4Radiology Department, Medical School, University of Brasilia, Brazil
5University of Brasilia, Brazil
*Corresponding author: Ricardo A. Gepp, Neurosurgery Department, SARAH Network of Rehabilitation Hospitals,SQSW 300 Bloco D, apt 104 Brasília, DF, Brazil
Received: July 11, 2016; Accepted: October 11, 2016; Published: October 14, 2016
Abstract
Objective: MRI is the current standard in the diagnostic of brain tumors, but images are insufficient for the prognostic assessment. MGMT promoter methylation is an important factor in the prognosis. The aim of the study was to examine the relationship between perfusion MRI and MGMT promoter methylation.
Methods: A total of 39 patients were evaluated. Statistical analysis and a study of the survival of the methylated and unmethylated groups were performed. Perfusion MRI for each group and the survival curves were constructed.
Results: Survival of patients negative for MGMT promoter methylation was 17.9 months, while in the methylation-positive was 29.2 (p<0.05). Survival of MGMT promoter methylation positive, in relation to perfusion, showed that variations between high and low perfusion values were not significant (p=0.944). Patients with no methylation and with high perfusion had poorer survival (p=0.038).
Conclusion: The presence of MGMT promoter methylation in conjunction with low perfusion are factors of better prognosis in gliomas.
Keywords: Glioma; Magnetic resonance imaging; Comparative study; Prognosis
Introduction
Malignant gliomas are the most frequent primary tumor of the Central Nervous System (CNS) in adults. The cornerstone treatment in CNS tumors is the triad: surgery, chemotherapy and radiation therapy. Despite all technological progress, survival is still poor [1]. Magnetic resonance is the current standard imaging diagnostic tool, providing anatomical and functional images. Anatomical findings have been proven insufficient for the prognostic assessment of brain tumors; therefore, other methods with functional assessment are necessary [2]. Studies like Dynamic Susceptibility-Weighted Contrast-Enhanced (DSC) perfusion Magnetic Resonance Imaging (MRI) with the measurement of Relative Cerebral Blood Volume (rCBV) shows a good correlation with the presence of Vascular Endothelial Growth Factor (VEGF) [3,4].
The imaging-based criteria in assessing tumor recurrence has been changing with the introduction of new drugs such as Temozolomide and Bevacizumab: the MacDonald criteria (1990) are becoming less relevant, while the Revised Assessment in Neuro-Oncology (RANO) criteria are being increasingly used in the radiologic evaluation of response to treatment and tumor recurrence [5,6]. Temozolomide is an alkylating agent that methylates the DNA, thereby leading to an increase in the number of DNA strand breaks, that induces cellular apoptosis.
O6-Methylguanine-DNA-Methyltransferase (MGMT) promoter methylation testing has been shown to be an important prognostic factor [7,8]. The methylation of the MGMT promoter inhibits the repair of DNA damaged by temozolomide; thus, the lack of methylation may render tumor cells resistant to temozolamide [7]. Tumor biology has a key role in the resistance to drugs, hence in patient survival. The detection of imaging features that could identify tumor molecular biology characteristics has been analyzed in a number of recent studies, mostly to indicate the susceptibility of neoplasms to the available treatments. The importance of evaluating prognostic factors in cerebral glioma patients lies in the possibility of determining individualized treatment strategies [9-11].
The objective of this study was to assess the prognostic factors in malignant gliomas relying on the investigation of MGMT promoter methylation and establish a correlation with rCBV values obtained in the Perfusion-Weighted Image (PWI).
Materials and Methods
Patients
The study comprised 45 patients with grade III and IV gliomas based on World Health Organization (WHO) classification, treated from January 2010 through December 2013. All of the studied patients were initially treated with Temozolomide, in addition to surgery and radiation therapy. The study was approved by the research ethics committee and the patients signed informed consent forms.
The inclusion criteria were the following: age >18 years, preoperative perfusion MRI, histological assessment with mitotic index (Ki-67), MGMT promoter methylation evaluation, and a minimum follow-up of 12 months or until death. Surgical resection more than 90% estimated by two neuroradiologists was important inclusion criteria.
The exclusion criteria were the following: patients with severe comorbidities and short follow-up. Six patients were excluded for inadequate follow-up and histological profile. Patients undergoing biopsy or surgical resection of the primary lesion that were less than 90% were excluded from this study. Two patients with severe heart disease and another one patient with kidney problems were excluded from this study.
The clinical variables analyzed were age, sex, Karnofsky Performance Scale (KPS) score at the inception of treatment, occurrence of death, and survival time in months. The endpoint was death or the termination of the assessment when the time of follow-up was completed. Survival time was calculated in months. All patients were operated by the same neurosurgeon and gross total resection was the objective of the surgery.
Imaging studies
Patients underwent baseline MRI. New evaluations were performed on 1.5 and 3T GE imaging units (Milwaukee, WI) and included at least: sagittal T1-weighted, axial T2-weighted and FLAIR images; post gadolinium sagittal and axial T1-weighted images and DSC perfusion MRI study (Echo-planar GRE TR 2100ms, TE 80ms with 5-mm-thick sections, no gap, total of 17 sections, 35 repetitions). The contrast agent dose was 0.05 mmol/kg; infusion was performed using a power injector at the rate of 4 mL/s. The dynamic infusion was at all times preceded by one-fourth of the gadolinium dose in order to reduce the leakage effect. The perfusion maps were generated at GE AW Workstation 4.5 (Milwaukee, WI). The studies were reviewed by two Board-certified radiologists with more than five years of experience. The radiologists were blinded to the results of the MGMT promoter methylation and the histological assessment. The different types of MRI scanner did not affect the performance of perfusion.
The following variables were evaluated: lesion location, lesion size, pattern of contrast enhancement, tumor necrosis, rCBV measurement in the region of interest and dissemination of the lesion to the contralateral hemisphere or along the tracts. A threshold above 1.75 for rCBV was established for this sample to denote high perfusion [12].
Histological and MGMT promoter methylation status assessment
Tumor histology was analyzed by a team of qualified, independent pathologists. The following variables were described: degree of anaplasia according to the WHO criteria; mitotic index Ki- 67; presence, numerical values and classification of MGMT promoter methylation.
The assessment of the MGMT promoter methylation status was conducted using genomic DNA isolated from paraffin-embedded sections of tumor tissue. The details of these techniques have been published by Riemenscheneider et al. [13]. In order to estimate the percentage of MGMT promoter methylation in each sample, three separate readings were performed, and the mean was calculated. A minimum of five normal DNA samples collected from the blood of healthy volunteers were used as controls in each assessment [11].
Statistical analysis
Univariate analysis was performed in order to determine the initial relevance of the variables with respect to the prognosis of survival based on the endpoint death. The data were submitted to multivariate Cox regression analysis to establish the significant prognostic factors. The relative risk of death was determined for each significant variable in the multivariate analysis. The survival analysis of the methylated and unmethylated MGMT promoter groups was performed and evaluated by the log-rank test. A survival analysis relating MGMT promoter methylation to perfusion values was also performed, and the MGMT promoter methylation status was analyzed in relation to high or low levels of cerebral perfusion. The analysis of these data was conducted using the log-rank test. The second part of the statistical analysis was the assessment of the correlation between the imaging features defined as important according to the study design and the MGMT promoter methylation status. This analysis was performed using the chi-square test, with a p-value < 0.05 to denote statistical significance.
Results
The assessment of MGMT promoter methylation was performed for 45 patients with malignant CNS gliomas who underwent surgery during the study period. Four were excluded from the study because they had a final histological diagnosis of oligodendroglioma, which changes the treatment response. Two other patients were excluded due to a short follow-up period. The mean age of the patients was 47 years old, with a predominance of male (Table 1). The mean KPS score of the patients was 70. The primary lesions were predominantly found in the frontal lobe, with 19 cases (Table 1). The tumor lesions were predominantly cortical in 29 patients. All patients studied in this series showed a resection of more than 90% of the primary lesion.
Variables
Results
Average Age (years)
47
18 - 78
Gender
23
Male
16
Female
Tumor location
18
Frontal
5
Occipital
12
Parietal
4
Temporal
Tumor grade (WHO)
14
III
25
IV
MGMT
24
Methylated
15
Unmethylated
Table 1: Clinical and demograhic profile of the overall group of study patients.
The histological analysis revealed 25 cases of grade IV glioblastoma and 14 grade III anaplastic astrocytomas. The mean Ki-67 index was 26.4% (range, 1?67.4%). The presence of MGMT promoter methylation was quantified in numerical values and classified according to its expression; values ranged from 0.07 to 0.98. In total, 15 patients were negative for MGMT promoter methylation. Survival time ranged from 4 to 52 months (mean, 24.9 months). There were 17 deaths in the study sample, 12 of them in the unmethylated MGMT promoter group and 5 in the methylated MGMT promoter group of patients (Figure 1). The mean survival of the patients who were negative for MGMT promoter methylation was 17.9 months, while in the methylation-positive group survival reached 29.2 months (p<0.05). The relative risk for the patients with MGMT promoter methylation was 0.2604 (95% CI, 0.1147 to 0.5912), which shows that the presence of methylation was an important factor in the increased survival of the patients in the study sample.
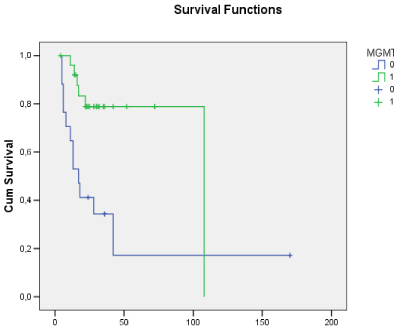
Figure 1: Overall survival curves showing that patients with methylated
MGMT promoter had longer survival compared with the unmethylated group.
The univariate analysis with the most significant variables was evaluated using the Cox regression model (Table 2). In the multivariate analysis, we obtained significant variables related to the endpoint death; high perfusion, with a relative risk of 7.9 (p<0.01), and the pattern of contrast enhancement, with a relative risk of 4.6 (p<0.04). The same analysis showed that MGMT promoter methylation was a protective factor, with a relative risk of 0.27 (p< 0.04), as well as a KPS score >70 with a relative risk reduction to 0.94 (p<0.04) (Table 2). Kaplan-Meier curves were constructed for both methylated and unmethylated MGMT promoter groups. Longer survival was found for the group with MGMT promoter methylation (p<0.01 by the logrank test). The survival curve of MGMT promoter methylation status in relation to perfusion demonstrated that variations in perfusion between high and low values were not significant (p=0.944) for the patients who were positive for MGMT promoter methylation. In the analysis of methylation-negative cases, the perfusion study yielded important data regarding survival time, as patients with high perfusion had poorer survival compared with those with low perfusion (p=0.038) (Figures 2 and 3). methylation was investigated. The relationships between MGMT promoter methylation and the following variables were examined: rCBV, contrast enhancement, pattern of contrast enhancement, and presence of necrosis on MRI. The presence of diffuse, strong and heterogeneous contrast enhancement was associated with unmethylated MGMT (p<0.05). Cerebral perfusion was different for the two MGMT promoter methylation status groups. However, the higher rCBV values in the unmethylated group were not statistically significant.
Variables
Relative risk
High Perfusion
7.9 (p<0.01)
High contrast enhancement
4.6 (p<0.04)
Methylated MGMT promoter
0.27 (p<0.04)
Karnofsky score >70
0.94 (p<0.04)
Table 2: Final result of the multivariate analysis for prognostic factors in anaplastic gliomas.
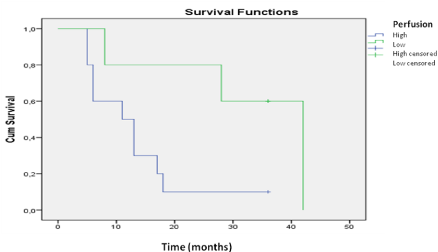
Figure 2: Survival analysis for the patients without MGMT promoter
methylation. A comparison of the groups with high and low perfusion reveals
that patients with lower perfusion levels had longer survival.
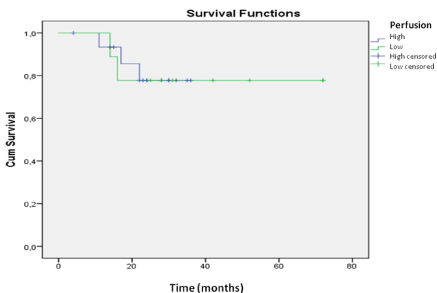
Figure 3: Survival analysis for the patients with MGMT promoter methylation.
The assessment of perfusion was not shown to be an important prognostic
factor for methylation-positive patients.
Discussion
No consensus exists on this field, yet several epidemiological studies show a progressive increase in the incidence of malignant brain tumors, especially among the elderly [2,14]. The prognosis of patients with malignant gliomas is generally poor. Age at diagnosis and the Karnofsky score are two well-established prognostic factors [10,14]. Patients with massive and deep lesions, or extending to the contralateral hemisphere, also have a poor prognosis. Imaging studies have been increasingly used as a prognostic tool and especially as means to discriminate patients who could respond to some modalities of treatment [10,15]. Law and co-workers analyzed the sensitivity and specificity of rCBV to determine the degree of malignancy of the brain tumor in relation to conventional imaging [16].
The presence of MGMT promoter methylation is associated with improved prognosis in malignant cerebral gliomas [7,8,13]. In a study by Hegi and co-workers, the independent analysis of the treatment group demonstrated that Polymerase Chain Reaction (PCR) determination of the methylation status of the MGMT promoter represented an important prognostic factor [13]. In the same study, it was found that, from 573 patients with glioblastoma, only 307 cases could be evaluated, 206 of which were amenable to analysis. This illustrates the technical difficulty in obtaining an adequate characterization of MGMT promoter methylation in some tissue specimens [13].
The evaluation of conventional MRI shows that the presence of necrosis and contrast enhancement correlates with CNS tumor malignancy. The absence of gadolinium enhancement may be related to malignant lesions in up to 30% of cases (Figure 4) [9,17]. For that reason, novel MRI techniques are being used with the purpose of better grading tumor features and prognosticating more effectively [1].
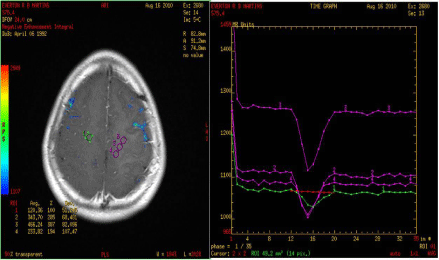
Figure 4: Photo of MRI perfusion imaging of patients with malignant glioma. Beside are curves showing the high perfusion blood flow in the tumor. A simple MRI
of this patient did not show typical signs of high-grade lesion, but high infusion was consistent with the findings of postoperative histology.
MRI-assessed cerebral perfusion is a good method for grading tumors and an important prognostic factor (Figure 4). A study conducted by Geer et al. demonstrated that PWI were instrumental in the evaluation of patients with brain tumors and aided in the decision-making process [12,18]. The measurement of rCBV shows a strong correlation with histological grading, as demonstrated by Law et al. [16]. These authors evaluated 73 patients with gliomas and demonstrated that rCBV varied according to the degree of malignancy of tumors [16].
Drabycz and co-workers carried out a study in which they analyzed image texture and tumor location and their association with MGMT promoter methylation in glioblastoma [19]. In the texture analysis, the authors examined tumor margin characteristics, contrast enhancement, presence of cysts, and whether T2 signal was homogeneous or heterogeneous. Contrast enhancement was the only significant variable in their study (p=0.0006). The features in T2-weighted images were also significant (p<0.05); however, inter observer accuracy was 71% [19]. Moon analyzed texture in MRI, and demonstrated that tumors with ill-defined margins are associated with the presence of MGMT promoter methylation (p=0.048) [10]. The study showed that, although high perfusion values and the absence of MGMT promoter methylation are associated with poorer prognosis, it was not possible to establish a direct relationship between the variations in perfusion found in the MRI data and MGMT promoter gene methylation status in the tissue specimens. The analysis of survival, however, revealed that when patients were negative for MGMT promoter methylation, the presence of high perfusion was associated with poorer prognosis (p= 0.038). This fact shows that for MGMT promoter methylation-negative patients, the assessment of perfusion becomes markedly relevant in the prognostic study. The study conducted by Moon and co-workers evaluated the possibility of Computed Tomography (CT) and MRI studies becoming parameters to assess MGMT promoter methylation status [10]. Their study reported that the presence of MGMT promoter methylation was related to increased Apparent Diffusion Coefficient (ADC) and greater attenuation in the contrast-enhanced CT images, yet it failed to show a relationship with perfusion [10]. The analysis of CT imaging revealed lower attenuation in the methylation-positive patients (p=.009) [10]. In contrast, rCBV measurements were not statistically different between the groups (p=0.380) [10]. In another clinical study, Carrilo and co-workers analyzed the relationship of IDH1 mutation and MGMT promoter methylation with radiologic features with a view to obtaining a noninvasive prognostic marker [11,20]. In that study with 202 patients, it was not possible to establish a correlation between MGMT promoter methylation status and imaging features. However, IDH1 gene mutation correlated with the aspect of the perilesional edema [11,20]. The assessment of MGMT promoter methylation status can also assist in the analysis of the likelihood of tumor pseudo progression after treatment with radiation and chemotherapy [21-23]. A study performed by Kong and co-workers demonstrated that patients who are positive for MGMT promoter methylation and treated with temozolomide are at a greater risk for developing radiologic findings of tumor pseudo progression [24].
Some limitations can be found in the present study. Patients received the same initial treatment and were evaluated as if they had received homogeneous therapeutic regimens, when in fact there were variations in patient treatment following tumor recurrence. Surgical treatment varied as well, since not all cases were treated with total lesion resection. The same problems occurred in similar studies evaluating prognostic markers without complete control of the treatments performed during the study [10,24]. The number of patients are small, but in our opinion this was not a problem because we found a statistical difference, avoiding methodological error. Probably this study should be complemented by further studies with larger numbers of cases and each different type of brain tumor. A stumbling block in the study was the need for adequate tissue samples for MGMT promoter methylation testing, which limited patient sample size in the present study as well as in studies reported in the literature [7,16,25].
Anaplastic gliomas (grade III) and glioblastomas were enrolled in this study. The purpose of such design was to analyze the initial radiologic appearance, similarly to the initial assessment in daily clinical practice [10,26]. Anaplastic astrocytomas exhibits different biological characteristics of glioblastomas. The present study investigates the initial aspects of image where it is not always possible to differentiate between these two types of tumors. Further studies should also include analysis of IDH mutation status. Low-grade gliomas and anaplastic oligodendrogliomas were not included due to the presence of different biological characteristics, although it is also possible to measure MGMT promoter methylation in those tumors [27,28].
MGMT methylation markers assessment, especially using imaging features, is recent, and has sparked a number of studies. The presence of MGMT promoter methylation is an important prognostic factor, and novel imaging techniques seek to relate the genetic status of patients to the imaging findings.
Conclusion
Radiologic assessment of high-grade gliomas has shown considerable prognostic value. In the present study, we found that radiologic features of tumors, especially brain perfusion, are associated with the degree of tumor malignancy and, consequently, with patient survival. The analysis of the MGMT promoter methylation status was also found to be a major prognostic factor related to survival, as noted in the present study and literature. Patients with high perfusion and negative to methylation of MGMT had poor survival. Techniques correlating imaging studies with histologic and genetic features of tumors should be improved and radio genomic could be a new field.
References
- Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from post treatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibilityweighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 2009; 30: 552-558.
- Hirai T, Murakami R, Nakamura H, Kitajima M, Fukuoka H, Sasao A, et al. Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol. 2008; 29: 1505-1510.
- Reardon DA, Galanis E, DeGroot JF, Cloughesy TF, Wefel JS, Lamborn KR, et al. Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol. 2011; 13: 353-361.
- Wang MY, Cheng JL, Han YH, Li YL, Dou SW, Yan FS, et al. Comparison of volumetric methods for tumor measurements on two and three dimensional MRI in adult glioblastoma. Neuroradiology. 2011; 53: 565-569.
- Pope WB, Chen JH, Dong J, Carlson MR, Perlina A, Cloughesy TF, et al. Relationship between gene expression and enhancement in glioblastoma multiforme: exploratory DNA microarray analysis. Radiology. 2008; 249: 268-277.
- Caseiras GB, Chheang S, Babb J, Rees JH, Pecerrelli N, Tozer DJ, et al. Relative cerebral blood volume measurements of low-grade gliomas predict patient outcome in a multi-institution setting. Eur J Radiol. 2010; 73: 215-220.
- Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009; 22: 633-638.
- Strik HM, Marosi C, Kaina B, Neyns B. Temozolomide dosing regimens for glioma patients. Curr Neurol Neurosci Rep. 2012; 12: 286-293.
- Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006; 66: 1258-1260.
- Moon WJ, Choi JW, Roh HG, Lim SD, Koh YC. Imaging parameters of high grade gliomas in relation to the MGMT promoter methylation status: the CT, diffusion tensor imaging, and perfusion MR imaging. Neuroradiology. 2012; 54: 555-563.
- Carrillo JA, Lai A, Nghiemphu PL, Kim HJ, Phillips HS, Kharbanda S, et al. Relationship between Tumor Enhancement, Edema, IDH1 Mutational Status, MGMT Promoter Methylation, and Survival in Glioblastoma. AJNR Am J Neuroradiol. 2012.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352: 987-996.
- Riemenschneider MJ, Hegi ME, Reifenberger G, Brandes AA, van den Bent MJ, Wick W, et al. MGMT promoter methylation in malignant gliomas. Target Oncol. 2010; 5: 161-165.
- Fabi A, Russillo M, Metro G, Vidiri A, Di Giovanni S, Cognetti F, et al. Pseudoprogression and MGMT status in glioblastoma patients: implications in clinical practice. Anticancer Res. 2009; 29: 2607-2610.
- Kapoor GS, Gocke TA, Chawla S, Whitmore RG, Nabavizadeh A, Krejza J, et al. Magnetic resonance perfusion-weighted imaging defines angiogenic subtypes of oligodendroglioma according to 1p19q and EGFR status. J Neurooncol. 2009; 92: 373-386.
- Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003; 24: 1989-1998.
- Drabycz S, Roldan G, de Robles P, Adler D, McIntyre JB, Magliocco AM, et al. An analysis of image texture, tumor location, and MGMT promoter methylation in glioblastoma using magnetic resonance imaging. Neuroimage. 2010; 49: 1398-1405.
- Geer CP, Simonds J, Anvery A, Chen MY, Burdette JH, Zapadka ME, et al. Does MR perfusion imaging impact management decisions for patients with brain tumors? A prospective study. AJNR Am J Neuroradiol. 2012; 33: 556-562.
- Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8: 1277-1280.
- Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2004; 25: 746-755.
- Arvinda HR, Kesavadas C, Sarma PS, Thomas B, Radhakrishnan VV, Gupta AK, et al. Glioma grading: sensitivity, specificity, positive and negative predictive values of diffusion and perfusion imaging. J Neurooncol. 2009; 94: 87-96.
- van den Bent MJ, Wefel JS, Schiff D, Jaeckle K, Junck L, Armstrong T, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011; 12: 583-593.
- Jeuken JW, Cornelissen SJ, Vriezen M, Dekkers MM, Errami A, Sijben A, et al. MS-MLPA: an attractive alternative laboratory assay for robust, reliable, and semiquantitative detection of MGMT promoter hypermethylation in gliomas. Lab Invest. 2007; 87: 1055-1065.
- Kong DS, Kim ST, Kim EH, Lim DH, Kim WS, Suh YL, et al. Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: the role of assessing relative cerebral blood flow volume and oxygen-6-methylguanine-DNA methyltransferase promoter methylation status. AJNR Am J Neuroradiol. 2011; 32: 382-387.
- Price SJ, Green HA, Dean AF, Joseph J, Hutchinson PJ, Gillard JH. Correlation of MR relative cerebral blood volume measurements with cellular density and proliferation in high-grade gliomas: an image-guided biopsy study. AJNR Am J Neuroradiol. 2011; 32: 501-506.
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352: 997-1003.
- Lacerda S, Law M. Magnetic resonance perfusion and permeability imaging in brain tumors. Neuroimaging Clin N Am. 2009; 19: 527-557.
- Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008; 247: 490-498.