
Research Article
Austin J Nucl Med Radiother. 2015; 2(1): 1012.
Development of Radiolanthanide-Labeled-Bis- Alendronate Complexes for Bone Pain Palliation Therapy
Fakhari A1, Jalilian AR2*, Yousefnia H2, Shafiee- Ardestani M1, Johari-Daha F2, Mazidi M2 and Khalaj A1
1Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
2Nuclear Science and Technology Research Institute (NSTRI), Tehran, Iran
*Corresponding author: Amir R. Jalilian, Nuclear Science and Technology Research Institute (NSTRI), Tehran, Iran
Received: September 03, 2015; Accepted: September 03, 2015; Published: February 16, 2015
Abstract
The search for the development of new ligands with higher stability, better pharmacokinetics and lower unwanted tissue uptakes (liver and GI) is still ongoing. In this work, synthesis, purification and structure characterization of DTPA-bis-ALN conjugate is reported followed by evaluation in model animals. A DTPA-conjugated bis-alendronate analog (DTPA-bis-ALN) 3, was prepared for possible bone pain palliation therapy after radiolabeling with Ho-166 and Sm-153. Radiolanthanide-DTPA-bis-ALN complexes were prepared starting excess amounts radiolanthanide chloride and DTPA-bis-ALN in 60-90 min at 50-60oC in phosphate buffer followed by solid phase purification on C18 Sep Pak column. RTLC was used for radiochemical purity followed by log P determination, stability studies and biodistribution studies in normal mice. The purified radiolabled complexes were prepared in high radiochemical purity (>98%, RTLC) and significant specific activity (7-10 GBq/mmol). The log P for the complex was calculated as -0.594 and -0.43, consistent with water soluble complexes. The complexes were stable in final solutions (25ºC) and presence of human serum (37ºC). The biodistribution of the labeled compounds in normal mice demonstrated unwanted activity uptake in lungs, spleen and liver in case of 166Ho-DTPA-bis-ALN and liver lung and kidney in case of 153Sm-DTPA-bis-ALN. Very limited bone uptake in both cases demonstrates complex instability or loss of bone avidity due to change of structure-activity relationship and/or anionic property of poly-dentate complex leading to renal excretion.
Keywords: Alendronate; Sm-153; Ho-166; Solid phase extraction; biodistribution; DTPA-conjugate
Introduction
Bone pain arises in more than 50% of bone metastases usually an outcome of various tumors metastases such as prostate (80%), breast and lung carcinoma (50%) [1,2]. A possible modality in bone pain management in these patients is the application of a systemic bone avid radiopharmaceutical for with potential benefits [3]. Although new alpha emitters have been introduced to the medical society for increasing the effectiveness of bone pain palliation, however, inaccessibility, high prices and limited vendors have negative effect on their vast applications [4].
Radiolabeled bis-phosphonates such as 153Sm-EDTMP, 188/186Re- HEDP, 177Lu-EDTMP and also 166Ho-EDTMP have been approved by well-known pharmaceutical legal bodies, however, the research and development of bone pain palliation compounds is an ongoing research area around the world. Recently many bone-seeking agent such as DO2A2P have been prepared and evaluated for their stereoisomer studies [5], and still the application of radiolanthanides is preferred [6] and they are entering faster and more efficient in the clinical trials in developing countries [7].
The uni-elemental abundance of natural holmium, makes holmium-166 (Eβ- max = 1.84 MeV, T1/2 = 26.8 h), an accessible, inexpensive radionuclide with enough specific activity for radiolabeling and targeted therapy modalities.
Possible therapeutic 166Ho bone-seeking bis-phosphonate agents including aliphatic chains as well as macrocycles have been reported such as 166Ho-DOTMP [8,9], 166Ho-EDTMP [10,11], 166Ho-APDDMP [12], as well as 166Dy/166Ho-EDTMP [12] for management of bone metastasis and pain prevention in breast cancer and multiple myeloma [13].
On the other hand, samarium-153 has favorable radiation characteristics, such as medium-energy beta particle emissions (Emax = 810 keV, range of about 3.0 mm), medium-energy gamma photon (103 keV, 28%) in addition to particle emissions which make it suitable for monitoring the therapy with imaging, widely used in pain palliation radiopharmaceutical in form of EDTMP complex [10].
The search for the development of new ligands with higher stability, better pharmacokinetics and lower unwanted tissue uptakes (liver and GI) is still ongoing.
Considering the inhibitory binding affinity constant (Ki) of bisphosphonates used in clinics alendronic acid and the idea of developing bone avid agents based on alendronic acid is of great interest. In recent studies, using simple bis-phosphonate –radiolanthanide complexes such as 177Lu-zoledronate [14], 166Ho-pamidronate [15], 177Lu-pamidronate; and 177Lu-alendronate [16] have not shown any improved properties in their bone avidity compared to their clinical rivals. The resulting complexes were not stable in vivo and/or showed lower bone uptake compared to other therapeutic bisphosphonates.
An interesting novel approach that has been aimed in this work is developing bis phosphonate ligands including metal chelating agents including DTPA moiety. The incorporation of two bis-phosphonate moiety in a single molecule can lead to better targeting of bone tissue. The multi dentate poly-amino carboxylic acid containing bis-phosphonate ligand, presumably to form stable chelates with many metals including lanthanides was developed by conjugation of cyclic DTPA dianhydride and [4-amino-1-hydroxy-1-(hydroxyoxido- phosphoryl)- butyl]phosphonic acid (Alendronic acid), as a possible carrier moiety, for the development as beta emitter-based radiopharmaceuticals for bone pain palliation (Figure 1).
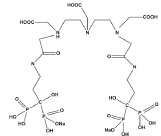
Figure 1: Chemical structure for DTPA-bis-ALN.
In this work, synthesis, purification and structure characterization of DTPA-bis-ALN conjugate is reported followed by the preparation, quality control and preliminary evaluation of related Ho-166 and Sm- 153 complexes.
Experimental
Production of 166Ho was performed at the Tehran Research Reactor (TRR) using 165Ho (n, γ) 166Ho nuclear reaction. Natural holmium nitrate with purity of >99.99% was obtained from ISOTEC Inc. Samarium-152 Oxide with purity of >98% was obtained from ISOTEC Inc., USA and used in radionuclide production. Whatman No. 2 was obtained from Whatman (Maidstone, UK). Radio-chromatography was performed by using a Bioscan AR-2000 radio TLC scanner instrument (Bioscan, Paris, France). A high purity Germanium (HPGe) detector coupled with a Canberra™ (model GC1020-7500SL) multichannel analyzer and a dose calibrator ISOMED 1010 (Dresden, Germany) were used for counting distributed activity in mice organs. All other chemical reagents were purchased from Merck (Darmstadt, Germany). Calculations were based on the 80.6 keV peak for 166Ho and 103 keV for 153Sm. The approval of NSTRI Ethics Committee was obtained for conducting this research. The wild-type mice (NMRI) were purchased from Pasteur Institute of Iran, Karaj, and all weighing 20-25 g and were acclimatized at proper rodent diet and 12h/12h day/ night light/darkness.
Production and quality control of radiolanthanide solutions
Holmium-166 and samarium-153 were produced by neutron irradiation of 100 μg of natural 165Ho(NO3)3 (165Ho, 99.99% from ISOTEC Inc.) and Sm2O3 (from ISOTEC Inc.) respectively according to reported procedures at the Tehran Research Reactor at a thermal neutron flux of 4×1013 n.cm-2.s-1[17].
Preparation of fresh cyclic DTPA dianhydride
Cyclic DTPA dianhydride was prepared according to the reported method with slight modifications [18]. Pyridine (1.2 mL) and acetonitrile (1.2 mL) warmed to 50°C were mixed with a solution of DTPA (490 mg, 1.37 mmol) in acetic anhydride (0.8 mL, 8.60 mmol) and heated at this temperature for 24 h. The resulting anhydride was insoluble in pyridine and acetonitrile. It was collected by filtration, purified by repeated washing with acetic anhydride, and with anhydrous ether at the end. The solid was dried under vacuum to remove the last trace of solvent. The melting point of white solid was 182oC-185oC and its 1H NMR and IR spectra were in accordance with the literature.
Conjugation of cyclic DTPA di-anhydride with alendronate
The DTPA conjugation to alendronate was performed based on reported similar methods with slight modifications [19]. To a magnetically stirred solution of cyclic DTPA dianhydride (108 mg, 0.3 mmol) in anhydrous DMF (2 mL) was added triethylamine (0.16 mL, 1.2 mmol) under nitrogen (final pH. 8). After 15 min a solution of alendronic acid (32.7 mg, 0.08 mmol) in DMF (1 mL) was added dropwise to the reaction mixture at room temperature for 30 min. The reaction mixture was then allowed to stir for 24h at 60ºC. A few drops of water were added to quench the reaction. To the reaction mixture diethyl ether was added and the precipitate was recovered and further purified using flash chromatography. Yield: 48% (32.7 mg). The structure of compound was determined using spectroscopic methods.
Radiolabeling of DTPA-bis-ALN with radiolanthanides
A stock solution of DTPA-bis-ALN in pure ethanol was prepared (20mg.mL-1). For labeling, an appropriate amount of the radiolanthanide solution containing the required activity (0.1 mL, 4 mCi) was added to the desired amount of DTPA-bis-ALN solution (60μL). The pH adjusted using phosphate buffer to 5. The complex solutions were kept at room temperature for 1-6 h. Also another set of experiment was performed at 60ºC warm bath for 1-6 h. The radiochemical purity was determined using ITLC and HPLC. The 80-88% radiochemical purity was obtained. For further purification the reaction mixture was passed through a freshly activated C18 Sep-Pak column (pre-washed by ethanol and water) and fractions were collected by washing with water: methanol mixture. The final solution was passed through a 0.22-μm membrane filter and pH was adjusted to 7-8.5 with 0.05 molL-1 phosphate buffer (pH 5.5). For Radiochemical purity of the complexes, instant thin layer chromatography was used. A 5μl sample of the final fraction was spotted on a chromatography Whatman No. 3, paper, and developed in Whatman 3 MM chromatography paper or ITLC-SG eluted with NH4OH (56%): MeOH (%100): H2O (%100) (0.2:2:4; v/v/v) as mobile phase mixture.
Sterility and apyrogenicity of the radiopharmaceutical
Sterility was controlled on a random sampling following decay of radioactivity. The Limulus Amoebocyte Lysate (LAL) test was used for validation of radiopharmaceutical production according to the European protocol [20].
Stability of radiolanthanide-DTPA-bis-ALN complexes in final formulation
For serum stability studies, 300μL of freshly prepared healthy human serum was added to 7.4MBq (200 μCi, 100 μL) of radiolabeled complex final solution and the resulting mixture was incubated at 37ºCfor 48 h. Every 12 h to a portion of the 50 μL of the mixture, trichloroacetic acid (10%, 100 μL) was added and the mixture was centrifuged at 3000 rpm for 5min followed by decanting the supernatant from the debris. The stability was determined by paper chromatography analysis of supernatant using Whatman 3 MM chromatography paper or ITLC-SG eluted with NH4OH (56%): MeOH (%100): H2O (%100) (0.2:2:4; v/v/v).
In vitro protein binding of radiolanthanide-DTPA- bis -ALN in presence of human serum: In vitro protein binding of radiolanthanide-DTPA- bis -ALN was carried out in human blood by protein precipitation according to published procedure [21]. To 3 mL fresh human plasma, 1mL of the labeled complex was mixed and incubated for 1h at 37ºC. Contents of the tube were centrifuged at 3000 rpm for 10 min for separation of serum and blood cells. After mixing approximately equal volume of 10% Trichloroacetic Acid (TCA), the mixture was centrifuged at 3000 rpm for 10 min. Residue was separated from supernatant and both layers were counted for radioactivity in a well type gamma counter. Protein binding of the complex was expressed as the fraction of radioactivity bound to protein, in percentage of the total radioactivity.
In vitro stability of radiolanthanide-DTPA- bis -ALN in presence of human serum
Final solution (200 μCi, 50 μL) was incubated in the presence of freshly prepared human serum (300 μL) (Purchased from Iranian Blood Transfusion Organization, Tehran) and kept at 37ºC for 2 days. Every 30 min to a portion of the mixture (50 μl), trichloroacetic acid (10%, 100μl) was added and the mixture was centrifuged at 3000 rpm for 5 min followed by decanting the supernatant from the debris. The stability was determined by performing frequent ITLC analysis of supernatant using above mentioned ITLC system.
Hydroxyapatite binding assay
The hydroxyapatite binding assay was performed according to the procedure described previously [21], with only a slight modification. In brief, to vials containing 1.0, 2.0, 5.0, 10.0, 20.0 and 50.0 mg of solid hydroxyapatite, 2ml of saline solution of pH 7.4 were added and the mixtures were shaken for 1h. Then, 50 ml of the radioactive preparation was added and the mixtures were shaken for 24 h at room temperature. The suspensions were centrifuged, and two aliquots of the supernatant liquid were taken from each vial and the radioactivity was measured with a well-type counter. Test experiments were performed using a similar procedure, but in the absence of hydroxyapatite. The percentage binding of radiolanthanide to Hydroxyapatite (HA) was calculated according to HB=1-A/B×100, where A is the mean radioactivity value of the supernatant sample under study and B is the mean total value of whole activity used.
Biodistribution studies
The biodistribution of free radiolanthanide cations as well as of radiolanthanide-DTPA- bis -ALN were determined in normal mice. For each compound, 100 μL (150μCi) of radioactive solution was injected directly to normal mice through caudal vein. The animals (n=3) were sacrificed at selected times after injection and percentage of injected dose in the tissues was determined with a γ -ray scintillation or a dose calibrator.
Results and Discussion
Preparation and structure confirmation of DTPA cyclic dianhydride
In order to prepare the bi-functional ligand, DTPA cyclic dianhydride, which was not cost effective, we tried the general procedure for its preparation [14]. The reaction was performed in pyridine containing DTPA acid form and acetic anhydride. The filtered mass was washed with cold acetic anhydride to remove the residues of the reactant. The solid was dried in oven for a couple of hours and finally re-crystallized to get a high purity product, suitable for spectroscopic and radiolabeling steps (Figure 2). Washing/drying steps were very important in that more repetition of these steps afforded high-purity product with rather long shelf-life. Such samples can be stored at room temperature under a blanket of N2 for up to one year.
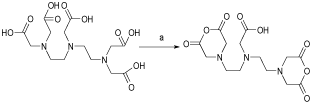
Figure 2: Schematic diagram of the synthesis of DTPA cyclic di-anhydride.
DTPA cyclic anhydride was characterized by IR spectroscopy. The formation of 1730 cm-1 peak indicated anhydride carbonyl group formation which is accompanied by a weaker 1695 cm-1 carboxylic acid peak of the untouched COOH. The IR spectrum of cyclic DTPAdianhydride is shown in (Figure 3).
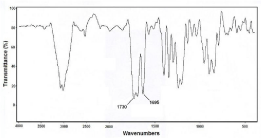
Figure 3: FT-IR spectrum of DTPA cyclic di-anhydride prepared in this study.
1H NMR spectrum of the above compound was recorded in DMSO at 25μC. The chemical shifts of CH2CO groups have the lowest field are very close so that a major singlet is observed around 3.76 ppm (a). The NCH2CH2N groups are more shielded and because of their similarity, a broad multiplet is observed at 2.6-2.56 ppm (b,c). The DMSO peak is observed at 2.5 ppm as a multiplet (d). The 1H NMR spectrum of cyclic DTPA-di-anhydride was in accordance with the literature.
Production of the precursor
Various reaction conditions were tried for DTPA- bis -ALN conjugation. The conjugation was performed in DMF, DMSO as well as aqueous mixtures as the best reaction solvent showed to be DMF since the reaction work-up seemed feasible due to the precipitation of the final compound followed by solid washing using various solvents. For better yields the alendronate free base (1) was added to the ccDTPA (2) and not vice versa. Nitrogen atmosphere was also mandatory for the conjugation reaction since the presence of water can reduce the conjugation yields. The reaction scheme is shown in (Figure 4).
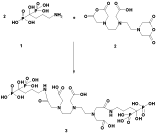
Figure 4: Reaction steps for preparation of DTPA-bis-ALN conjugate.
The 1H NMR study of the conjugate demonstrated the 1:2 conjugation ratio for DTPA: alendronate with the presence of all expected peaks for both alendronate and DTPA moieties (Figure 5). Due to superimposition of many signals at close chemical shifts, the exact peak determination for all CH2 groups for alendronate and DTPA was not possible.
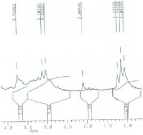
Figure 5: 1H NMR spectrum of DTPA-bis-ALN conjugate.
IR data for the conjugate demonstrated 3470 cm-1 consistent with carboxylic acid O-H and N-H stretch, 1634 cm-1 related to amide C=O stretch due to successful amide bond formation, also the presence of P-O bond (at 1180 cm-1), P-O-H functional group at 2362 cm-1 as described for phosphoric acid derivatives as reported [21].
The radiochemical purity in 10 mmol.L-1 DTPA aq. solution (solvent 1), free radiolanthanide cation is complexed to more lipophilic radiolanthanide -DTPA form and migrates to higher Rf. Small radioactive fraction remaining at the origin could be related to other La ionic species, not forming radiolanthanide-DTPA complex, such as LaCl4-, etc. and/or colloids. On the other hand, 10% ammonium acetate: methanol mixture (1:1) (solvent 2) was also used for the determination of radiochemical purity. The fast eluting species was radiolanthanide cation. Other ionic forms of radiolanthanide such as LaCl4- as well as colloids remained at the origin (Rf= 0) (Figure 6).
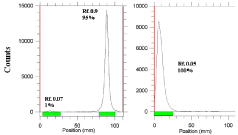
Figure 6: ITLC chromatograms of radiolanthanide solutions in 10 mM DTPA
solution (pH~ 4) (left) and in 10% ammonium acetate: methanol (1:1) (right)
on Whatman No. 1 Paper.
Labeling optimization studies
In order to obtain maximum complexation yields, several experiments were carried out varying different reaction parameters such as ligand concentration, pH, reaction time and temperature. Ligand concentration was varied between a wide range starting from 10 to 50mg/ml for DTPA-bis- ALN. It was observed that at room temperature 99% complexation was achieved with 15 mg/ml of DTPA-bis-ALN. The best ITLC mobile phase was considered by whatman No.2 paper using NH4OH: MeOH: H2O (0.2:2:4) as shown in (Figure 7).
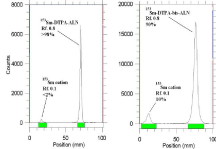
Figure 7: ITLC chromatograms of 153Sm-DTPA-bis-ALN solutions prior
to solid phase purification (right) and after solid phase purification (left) in
NH4OH: MeOH: H2O (0.2:2:4) as mobile phase on Whatman No. 1 Paper.
In order to achieve higher specific activities for the final labeled compounds, excess amounts of lanthanides were used in the radiolabeling procedure; however a solid phase purification step using C18 SepPak was designed and applied to the final mixtures in order to get higher radiochemical purities by removing excess amounts of unlabeled cations. By fraction eluting of the loaded cartridges applying the same solvent system used in the radio chromatography of the labeling procedure, the radiolabeled complex was eluted in the first 1-3 ml fractions. (Figure 8). Demonstrates the elution of the unlabeled cations from the column.

Figure 8: Cationic radiolanthanide content of solid phase elution’s for 153Sm-
DTPA-bis-ALN solution (right) and 166Ho-DTPA-bis-ALN solution eluted by
NH4OH: MeOH: H2O (0.2:2:4) mixture.
Labeling yield increased with increasing molar ratio Ho: DTPAbis- ALN (from 1:5 to 1:50) and reached more than 99% in 60 minutes followed by C18 Sep Pak purification. The stability of prepared 166Ho/153Sm-DTPA-bis-ALN complex was checked up to 24 hours after preparation. The complex was stable in final pharmaceutical sample and its radiochemical purity was above 99% even 24 hours after preparation using Whatman 2 MM eluted with NH4OH: MeOH: H2O (0.2:2:4).
Stability test was developed for the complex in presence of human serum at 37ºC using ITLC as mentioned above and all data within 48 were above 89% at all time intervals.
For determination of protein binding the data showed the 57% protein binding using ITLC of the serum-radiopharmaceutical mixture, while 43% is found in free form in the circulation. The protein binding for the DTPA-bis-ALN ligand has been reported in different references from 54% in free form [12]; however no protein biding for metal DTPA-bis-ALN complexes were available in the literature.
HA assay demonstrated low capacity binding for 166Ho/153Sm- DTPA-bis-ALN complexes to hydroxy apatite. Even at 50 mg amount of HA, less than 30% binding was observed (Table 1).
Ligand / Hydroxyapatite
5 mg
10 mg
15 mg
20 mg
25 mg
50 mg
166Ho-DTPA-bis-ALN
18±0.2%
21±0.3%
23±0.92%
26±0.09%
28±0.1%
29±0.03%
153Sm-DTPA- bis -ALN
15±0.93%
22±0.23%
25±0.39%
26±0.32%
29±0.53%
30±0.32%
Table 1: Hydroxy apatite binding assay for complexes at 37ºC in 24 hr.
Biodistribution
For better comparison biodistribution study was performed for free Ho3+ and 153Sm3+ as well. The %ID/g data for free radiolanthanides are summarized in (Figure 9).
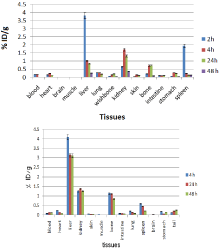
Figure 9: Biodistribution of 166HoCl3 (up) and 153SmCl3 (below) (1.85 MBq,
50μCi) in normal mice at various time intervals after iv injection via tail vein
(ID/g%: percentage of injected dose per gram of tissue) (n=3).
Holmium-166 cation: The liver radioactivity uptake of the cation is comparable to other radio-lanthanides such as Yb and Tb [21]. About 1 % of the cation radioactivity accumulates in the liver in 48 h. Low blood radioactivity content demonstrates the rapid removal of 166Ho from the circulation after injection. Lung, muscle and skin do not demonstrate significant 166Ho uptake while it is in accordance with other cations accumulation. A low bone uptake is observed for 166Ho which remains almost constant after 48 h (0.7%). Spleen also has significant 166Ho uptake possibly related to reticuloendothelial system. The free cation is soluble in water and it can be excreted via the urinary tract [20].
Samarium-153 cation: The liver uptake of the cation is comparable with many other radio-lanthanides mimicking calcium cation accumulation [22 ]; about 3-4% of the activity accumulates in the liver after 4 h and remains constant for 48 h. The blood content is low at all time intervals and this shows the rapid removal of activity from the circulation.
The lung, muscle and also skin do not demonstrate significant uptake which is in accordance with other cations accumulation [21]. A 1% bone uptake is observed for the cation which remains almost constant after 48 h. The spleen also has significant uptake possibly related to reticuloendothelial uptake. The kidney plays an important role in 153Sm cation excretion during 48 h (Figure 10).
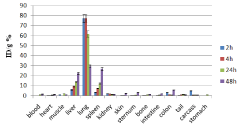
Figure 10: Percentage of injected dose per gram of 166Ho-DTPA-bis-ALN in
wild-type mice tissues at 2, 4, 24 and 48 h post injection (n=3)..
The distribution of injected dose in rat organs up to 48 h after injection of 166Ho-DTPA-bis-ALN (200 μCi/ 150μl) solution was determined. Based on these results, it was concluded that the major portion of the injected activity of 166Ho-DTPA- bis -ALN was extracted from blood circulation into other tissues (Figure 11).
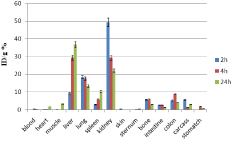
Figure 11: Percentage of injected dose per gram of 153Sm-DTPA-bis-ALN in
wild-type mice tissues at 2, 4, 24 and 48 h post injection (n=3).
The liver radioactivity uptake of the 166Ho-DTPA-bis-ALN was incomparable to other radio-lanthanide complexes. About 20 % of the radioactivity accumulates in the liver in 48 h. On the other hand high lung radioactivity was also demonstrates the unwanted accumulation in reticuloendothelial tissues. One other possible mechanism is the formation of larger complex structures due to phosphate groups as observed for 99mTc-MDP.
In case of 153Sm-DTPA-bis-ALN complex however the activity content was much lower in reticuloendothelial system compared to 166Ho-DTPA-bis-ALN. Instead, the main site of accumulation and excretion was shown to be kidneys. This might be a result of water solubility as well as m9ore anionic nature of complex compared to that of 166Ho-DTPA-bis-ALN.
Both complexes demonstrated poor/unacceptable behavior for a possible bone avid radiopharmaceutical compared to even their directly complexed La-alendronate complexes. Increased molecular size as well as presence of many chelating functional groups in the structure possibly led to the formation of non bone avid complexes with lower complex stability constants compared to La-bisphosphonates.
Conclusion
A comparative accumulation study for the complex was obtained in high radiochemical purity ITLC (>99%) and HPLC (>99.9%) and satisfactory stability in presence of human serum and final formulations were obtained. HA binding assay demonstrated >95% binding from 5-20 mg of HA in 24h at room temperature. The complex protein binding was about 55-58%. The biodistribution of the labeled compound in wild-rodents demonstrated unwanted activity uptake in lungs, spleen and liver in case of 166Ho-DTPA-bis- ALN and liver lung and kidney in case of 153Sm-DTPA-bis-ALN. Very limited bone uptake in both cases demonstrates complex instability or loss of bone avidity due to change of structure-activity relationship and/or anionic property of polydentate complex leading to renal excretion.
Acknowledgment
We acknowledge the financial support of Deputy of Research, Tehran University of Medical Sciences for conducting this research project. The authors wish to also to thank Pars Isotope Co., Tehran, Iran for providing animal facility services and Mr. M. Mazidi for performing animal tests.
References
- Campa JA, Rayne R. The management of intractable bone pain: a clinician’s perspective. Semin Nucl Med.1992; 22: 3-10.
- Serafini AN, Therapy of metastatic bone pain. J Nucl Med. 2001; 42: 895-906.
- Eary JF, Collin C, Stabin M, Vernon C, Petersdorf S, Baker M, et al. Samarium-153-EDTMP biodistribution and dosimetry estimation. J Nucl Med. 1993; 34: 1031-1036.
- Bagheri R, Jalilian AR, Bahrami-Samani A, Mazidi M, Ghannadi-Maragheh. M. Production of Holmium-166 DOTMP: A promising agent for bone marrow ablation in hematologic malignancies. Iran J Nucl Med. 2011; 19: 12-20.
- Breitz HB, Wendt III RE, Stabin MS, Shen S, Erwin WD, Rajendrann JG, et al. 166Ho-DOTMP Radiation-Absorbed Dose Estimation for Skeletal Targeted Radiotherapy, J Nucl Med. 2006; 47: 534–542.
- Bahrami-Samani A, Bagheri R, Jalilian AR, Shirvani-Arani S, Ghannadi-Maragheh M, et al. M. Production, Quality Control and Pharmacokinetic Studies of 166Ho-EDTMP for Therapeutic Applications. Sci Pharm. 2010; 78: 423-433.
- Louw WK. Dormehl IC, van Rensburg AJ, Hugo N, Alberts AS, Forsyth OE, et al. A. Evaluation of samarium-153 and holmium-166-EDTMP in the normal baboon model. Nucl Med Biol.1996; 23: 935-40.
- Zeevaart JR, Jarvis NV, Louw WK, Jackson, GE. Metal-ion speciation in blood plasma incorporating the tetraphosphonate,N,N-dimethylenephosphonate-1-hydroxy-4-aminopropilydenediphosphonate(APDDMP), in therapeutic radiopharmaceuticals. J Inorg Biochem. 2001; 83: 57-65.
- Pedraza-López M, Ferro-Flores G, de Murphy CA, Tendilla JI, Villanueva-Sánchez O. Preparation of (166)Dy/(166)Ho-EDTMP: a potential in vivo generator system for bone marrow ablation. Nucl Med Commun. 2004; 25: 615-21.
- Gano L, Marques F, Campello MP, Balbina M, Lacerda S, Santos I. et al. Radiolanthanide complexes with tetraazamacrocycles bearing methylphosphonate pendant arms as bone seeking agents. Q J Nucl Med Mol Imaging. 2007; 51: 6-15.
- Mirsaeed Nikzad, Amir R Jalilian, Simindokht Shirvani-Arani, Ali Bahrami-Samani, Hamid Golchoubian. Production, quality control and pharmacokinetic studies of 177Lu-zoledronate for bone pain palliation therapy, J Radioanal Nucl Chem. 2013; 298: 1273–1281
- Asma Fakhari, Amir R Jalilian, Hassan Yousefnia, Fariba Johari-Daha, Mohammad Mazidi, Ali Khalaj. et al. Development of 166Ho-pamidronate for bone pain palliation therapy, J Radioanal Nucl Chem. 2014.
- Asma Fakhari, Amir R. Jalilian, Hassan Yousefnia, Ali Bahrami-Samani, Fariba Johari-Daha, Ali Khalaj. Evaluation of two 177 Lu-labeled bis-phosphonates for bone pain palliation therapy. Iran J Nucl Med. 2014.
- Manual for reactor produced radioisotopes, IAEA, Vienna, 2003, IAEA-TECDOC-1340, ISBN 92–0–101103–2, ISSN 1011–4289, © IAEA. 2003; 71.
- Hnatowich DJ, Layne WW, Child RL. Radioactive labeling of antibody: A simple and efficient method. Science 1983; 220: 613-619.
- Wang S, Luo J, Lantrip DA, Waters DJ, Mathias CJ, Green MA, et al† Design and Synthesis of [111In]DTPA-Folate for Use as a Tumor-Targeted Radiopharmaceutical. Bioconjugate Chem. 1997; 8: 673-679.
- CGRPP-guidelines, version 2 March 2007, EANM Radiopharmacy Committee, guidelines on current good radiopharmacy, practice (cgrpp) in the preparation of radiopharmaceuticals.
- Dar UK, Khan IU, Javed M, Ahmad F, Ali M, Hyder SW,et al. Preparation and biodistribution in mice of a new radiopharmaceutical technetium-99m labeled methotrexate, as a tumor diagnostic agent. Hell J Nucl Med. 2012; 15: 120-124.
- Neves M, Gano L, Pereira N, Costa MC, Costa MR, Chandia M, et al. Synthesis, characterization and biodistribution of bispho- sphonates Sm-153 complexes: correlation with molecular modeling interac- tion studies. Nucl. Med. Biol.2002; 29: 329–338.
- L Daasch , D Smith. Infrared Spectra of Phosphorus Compounds, Anal. Chem. 1951; 23: 853–868.
- Du XL, Zhang TL, Yuan L, Zhao YY, Li RC, Wang K, et al. Complexation of ytterbium to human transferrin and its uptake by K562 cells. Eur J Biochem. 2002; 269: 6082-6090.
- Misra SN, Gagnani MA, Shukla RS. Biological and clinical aspects of lanthanide coordination compounds. Bioinorg Chem Appl. 2004; 2: 155–1592.