Research Article
Austin J Nutri Food Sci. 2014;2(8): 1043.
Antioxidant Properties, Phenolic Profile and Nutritional Value for Sorbus umbellata Fruits from Turkey
Kivrak I1,2* and Kivrak S2,3
1Department of Chemistry and Chemical Treatment Techniques, Mugla Sitki Koçman University, Turkey
2Research Laboratory Center, Mugla Sitki Koçman University, Turkey
3Department of Nutrition and Dietetics, Mugla Sitki Koçman University, Turkey
*Corresponding author: : Kivrak I, Department of Chemistry and Chemical Treatment Techniques, Mugla Vocational School of Higher Education, Mugla Sitki Koçman University, 48000, Mugla, Turkey
Received: August 08, 2014; Accepted: September 26, 2014; Published: September 26, 2014
Abstract
Sorbus umbellata fruits are a wild edible natural food source with a special taste and aroma. S. umbellata fruits were particularly consumed by human and birds, and other livings. An analysis of S. umbellata fruits was performed by means of ultra-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry (UPLC-ESI-MS/MS). In the present study, total phenolic and flavonoid contents, individual phenolic profiles, related antioxidant activities (β-carotene linoleic acid, DPPH. and ABTS+. radical scavenging) and nutritional value were examined. S. umbellata fruits contained gentisic acid, protocatechuic acid as a major constituent; the others were ferulic acid, chlorogenic acid, caffeic acid, syringic acid, vanillic acid, 3,4-dihydroxybenzaldehyde. Total phenolic and flavonoid contents of S. umbellata fruits were evaluated and the results were in accordance with LC individual phenolic content. S. umbellata fruits, picking by gatherers, can be consumed as traditional-handmade jam, juice, ice-cream and liquor. Thus, the natural wild edible S. umbellata fruits may show up a value and importance in commercial food product and a source of natural healthy product with significant ingredients.
Keywords: Sorbus umbellata; Phenolic compounds; Antioxidants; Nutrients; UPLC-ESI-MS/MS
Introduction
Sorbus umbellata is a species in the genus Sorbus which includes approximately 99 to 207 species and belongs to the family of the Rosaceae. In the various region of Turkey, S. umbellata fruits were grown as a natural food source for hunter-gatherers at the autumn. In addition to fruit ingestion as a nutritional source, from the health point of view, the consumption of fruits in a healthy diet provides the reduction of cardiovascular diseases and degenerative pathologies [1-4].
Although there were studies devoted to the investigation of phenolic acids, sugars and minerals in berries, apples, peaches, papayas and avacados [4-7], while, to our knowledge, no data are reported in literature on phenolic compounds and antioxidant or antiradical activity in S. umbellata fruits from Turkey.
Phenolic compounds protect the plants against insects and animal pests. Thousands of phenolic compounds are found in plants and their products, because of remarkable structural differences of phenolic compounds. Phenolic compounds can contribute the taste and aroma of plant origin foods. Phenolic compounds also serve as natural antioxidants. They prevent diseases such as cancer, heart and lung diseases by stopping or inhibiting reactions caused by free radicals. A large group of flavonoids are responsible for colors of the foods. Anthocyanins, a group of the flavonoids, are natural coloring materials and they are the reason of pink, red and purple colors of vegetables, fruits, fruit juice and vine [8].
The phenolic compounds in medicinal pills and foods are in the main parts of secondary metabolites that are derived from phenylalanine or tyrosine of plant tissues [9,10]. Chemically, phenolics are defined as the compounds which have one or more hydroxyl groups and aromatic ring and their functional derivatives. Their existence in animal tissues and non-plant materials generally depends on digestion of the plant origin foods. Some of phenolic compounds are effective in forming flavor of fruit and vegetables. Especially, the reason of bitterness and astringency feelings in the mouth is phenolic compounds. The main reason of evaluation of fruits as functional foods is their health promoting effects. These effects are caused by antioxidant and antimicrobial properties of phenolic compounds [11].
Phenolic compounds are natural antioxidants and considered to have a preventive role in the development of cancer and heart disease [12]. Investigations related to biological and pharmacological features have also been reported for phenolic compounds, including free radicals scavenging, apoptosis of cancer cells [13,14] anti-herpetic, anti-Human Immunodeficiency Virus (HIV) reverse transcriptase and anti-HIV activity [15,16].
In this study authors aimed to reveal the total phenolic and flavonoid content with individual phenolic ingredients in terms of the antioxidant activity by means of oxidative inhibition of S. umbellata fruits from Turkey.
Materials and Methods
Sample
Sorbus umbellata fruits originating from southwestern Turkey were collected in 2013. Taxonomic identification was provided from Department of Biology, Faculty of Science, Mugla Sitki Koçman University, Mugla (Turkey). Samples were divided into two portions: one portion was lyophilized (Christ Freeze Dryer Alpha 1-4 LD plus, Germany) and reduced to a fine dried powder, other portion was stored at -18°C without the application of any process.
Standards and reagents
Phenolic standards (pyrogallol, homogentisic acid, 3,4-dihydroxybenzoic acid (protocatechuic acid), gentisic acid, pyrocatechol, galanthamine, p-hydroxy benzoic acid, 3,4-dihydroxybenzaldehyde, catechin hydrate, vanillic acid, caffeic acid, syringic acid, vanillin, epicatechin, catechin gallate, p-coumaric acid, ferulic acid, rutin, trans-2-hydroxy cinnamic acid, myricetin, resveratrol, trans- cinnamic acid, luteolin, quercetin, naringenin, genistein, apigenin, kaempferol, hesperetin, and chrysin) were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany).
Folin&Ciocalteu's phenol reagent (FCR), β-carotene, tween-40, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid) diammonium salt (ABTS), potassium persulfate (K2S2O8), neocuproine, ferrin, 5,5'-Dithiobis(2-Nitrobenzoic acid (DTNB), Acetylcholinesterase (AChE), Butyrylcholinesterase (BChE), Acetylcholine Iodine (AcI), Butyrylcoline Iodine (BuI), potassium acetate, aluminum nitrate, ammonium formate, ammonium acetate, Butylated Hydroxytoluene (BHT), α-tocopherol, Butylated Hydroxyanisole (BHA) were obtained from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). All other chemicals and solvents were of analytical grade and purchased from usual suppliers. Water used in the studies was treated in a Milli-Q water purification system (Advantage A10 Millipore, Pure Water Systems, Molsheim, France).
Nutritional value
The samples were analyzed for chemical composition (moisture, proteins, fat, carbohydrates and ash) using the AOAC procedures [17]. The crude protein content (N x 4.38) of the samples was estimated by the macro-Kjeldahl method; the crude fat was determined by extracting a known weight of powdered sample with petroleum ether, using a Soxhlet apparatus; the ash amount was determined by burning at 650 ± 15°C. Total carbohydrates were calculated by difference. Energy was calculated according [18] to Eq. (1).
Energy (kcal) = 4 x (g protein + g carbohydrate) + 9 x (g fat). Eq. (1)
Phenolic compound extraction, identification and quantification
Phenolic compounds were analyzed according to previously described method with slight modifications [19]. Briefly, samples were frozen -first with liquid nitrogen then lyophilized for 12 hours. 3 g of dried sample was extracted by 30 mL of 80% acetone at 25°C for 6 h. Then it was placed in ultrasonic bath for 15 min and the sample was centrifuged at 4000 rpm for 10 min at 20°C, and filtered using Whatman No 4. The residue was then extracted with three additional 30 mL portions of 80% (v/v) acetone, as described earlier. The solvent in the combined extracts were evaporated at 40°C and redissolved in 80% (v/v) methanol and filtered through a PTFE-20/25 filter disk for LC analysis. Phenolic compounds were analyzed by UPLC-MS/MS (Waters Acquity Ultra Performance LC, Xevo TQ-S MS/MS, Waters Co., Milford, MA, USA). The chromatographic and mass spectrometry conditions were previously given elsewhere [19]. Detection was carried out with a tandem mass spectrometry, using multiple reactions monitoring (MRM) mode. Mass spectra were acquired in positive Electrospray Ionization (ESI) mode. Data analysis and quantitation was executed using the Waters MassLynx and TargetLynx software (Waters Corp.). The MRM was applied to monitor the transitions of quantifier ion to qualifier ions (the parent > daughter ions transitions, m/z). Confirmation of compounds was achieved through two or more daughter ions. The phenolic compounds present in the samples were characterized according to their mass to charge (m/z) ratio with those of commercial standards. For the quantitative analysis of phenolic compounds, a calibration curve was obtained by injection of different concentration of standards.
Determination of the antioxidant activity with the β-carotene bleaching method
The total antioxidant activity was evaluated using β-carotene-linoleic acid test system based on the detection of inhibition of conjugated dien hydroperoxides due to oxidation of linoleic acid with slight modifications [20,21]. This method is based on bleaching of β-Carotene. β-Carotene (0.5 mg) dissolved in 1 mL of chloroform was added into mixture of 20 μL of linoleic acid and 200 mg of Tween 40 emulsifier. After evaporation of chloroform under vacuum, 100 mL of distilled water saturated with oxygen was added by vigorous shaking. As soon as the emulsion was added to each tube, the zero time absorbance was measured at 470 nm using a 96-well microplate reader. The absorbance of the emulsion was read again at the same wavelength after the incubation of the plate for 120 min at 50°C. Methanol was used as a control. The extract concentration providing 50% antioxidant activity (EC50) was calculated from the graph of antioxidant activity percentage against extract concentration. BHT, BHA and α-tocopherol were used as antioxidant standards for comparison of the activity [21]. The bleaching rate (R) of β;-carotene was calculated according to Eq. (2).
R = [ln(a/b)]/t Eq. (2)
where: ln = natural log, a = Absorbance at time zero, b = absorbance at time t (120 min). The antioxidant activity was calculated in terms of percent inhibition relative to the control, using eq. (3)
Antioxidant activity (%) = (RControl - RSample) / RControl x 100 Eq. (3)
DPPH free radical scavenging activity
The free radical scavenging activity of fruit extract was determined using DPPH free radical with slight modification [22]. In its radical form, DPPH free radical absorbs at 517 nm. Briefly, the reaction was initiated by the addition of 4 mL of DPPH free radical (0.4 mM) prepared in ethanol into 1 mL sample solution. After thirty minutes at room temperature, the absorbance was measured at 517 nm. Ethanol was used as a control. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. The capability of scavenging the DPPH free radical was calculated by using Eq. (4).
DPPH radical scavenging effect (%) = (AControl - ASample)/AControl x 100 Eq. (4)
where AControl is the initial concentration of the DPPH• and ASample is the absorbance of the remaining concentration of DPPH free radical in the presence of the extract and positive controls. The extract concentration providing 50% radical scavenging activity (EC50) was calculated from the graph of DPPH radical scavenging effect percentage against extract concentration. BHT, BHA and α-tocopherol were used as antioxidant standards for comparison of the activity [12].
ABTS cation radical decolorization assay
The spectrophotometric analysis of ABTS•+ scavenging activity was determined according to the previously described method, with slight modifications [12,23]. The ABTS•+ was obtained by the reaction between 7 mM ABTS in water and 2.45 mM potassium persulfate, stored in the dark at room temperature for 12 h. Oxidation of ABTS commenced immediately, but the absorbance was not maximal and stable until more than 6 h had elapsed. The radical cation was stable in this form for more than 2 days in storage in the dark at room temperature. Before usage, the ABTS•+ solution was diluted to get an absorbance of approximately 0.700 at 734 nm with ethanol. Then, 160 μL of ABTS•+ solution was added to 40 μL of sample solution in ethanol at different concentrations. After 10 min the absorbance was measured at 734 nm by using a 96-well microplate reader. The percentage inhibitions were calculated for each concentration relative to a blank absorbance (ethanol). The scavenging capability of ABTS cation radical was calculated using Eq. (5).
ABTS scavenging effect (%) = (AControl - ASample)/AControl x 100 Eq. (5)
where AControl is the initial concentration of the ABTS cation radical and ASample is the absorbance of the remaining concentration of ABTS cation radical in the presence of sample. The extract concentration providing 50% radical scavenging activity (EC50) was calculated from the graph of ABTS cation radical scavenging effect percentage against extract concentration. BHT, BHA and α-tocopherol were used as antioxidant standards for comparison of the activity [12].
Determination of total phenolic compounds
The concentrations of total phenolic content in ethanol extract were expressed as microgrammes of Pyrocatechol Equivalents (PEs), determined with Folin-Ciocalteu Reagent (FCR), according to the method of Slinkard and Singleton [13]. One milliliter of the solution (contains 1 mg) of the extract in methanol was added to 46 mL of distilled water and 1 mL of FCR, and mixed thoroughly. After 3 min, 3 mL of sodium carbonate (2%) were added to the mixture and shaken intermittently for 2 h at room temperature. The absorbance was read at 760 nm. The concentration of total phenolic content was calculated according to Eq. (7) that was obtained from the standard pyrocatechol graph:
Absorbance = 0.0073 x X (μg) pyrocatechol - 0.1665 (r2 : 0.9976) Eq. (7)
Determination of total flavonoid concentration
Measurement of total flavonoid concentration of the extract was based on the method [24] with a slight modification and results were expressed as quercetin equivalents. An aliquot of 1 mL of the solution (contains 1 mg of extract in methanol) was added to test tubes containing 0.1 mL of 10% aluminum nitrate, 0.1 mL of 1 M potassium acetate and 4.8 mL of ethanol. After 40 min at room temperature, the absorbance was determined at 415 nm. Quercetin was used as a standard. The concentrations of flavonoid compounds were calculated according to Eq. (8) that was obtained from the standard quercetin graph:
Absorbance = 0.0082 x X (μg) quercetin - 0.0073 (r2 : 0:9998) Eq. (8)
Statistical analysis
Three replicates of sample were prepared for each analysis. The results are expressed as mean values ± standard deviation (SD). Analysis of variance was performed by ANOVA procedures. Significant differences between means were determined by Student's t-test. p<0.05 was regarded as significant.
Results and Discussion
Dietary patterns are thought to influence the onset and progression of chronic and degenerative diseases [25]. Increased consumption of fruits and vegetables containing high levels of phytochemicals has been recommended to prevent or reduce oxidative stress in the human body [25-28]. Fruits and vegetables are a primary food source providing essential nutrients for sustaining life; they also contain a variety of phytochemicals such as phenolics and flavonoids, which provide important health benefits [29].
In this study, the results of the nutritional composition and obtained energetic value of a wild edible fruits, S. umbellata, were shown in Table 1. S. umbellata fruits has carbohydrate and protein in high levels. However, the fruit studied in this paper had low fat content (2.02%), energetic value (350.82 kcal/100 g) was high. The average moisture content was 79.42% (value was not significantly different (p = 0.05)).
Parameter
S. umbellata
Moisture (g/100 g fw)
79.42 ± 2.47
Ash (g/100 g dw)
14.99 ± 1.08
Carbohydrate (g/100 g dw)
66.04 ± 2.87
Proteins (g/100 g dw)
17.12 ± 0.98
Fat (g/100 g dw)
2.02 ± 0.13
Energy (kcal/100 g dw)
350.82 ± 3.98
Table 1: Nutritional composition and obtained energetic value of S. umbellata.
Total phenolic and total flavonoid contents of S. umbellata fruits were displayed in Table 2. The determination of total phenolic content was included in this study because strong correlations between total phenolic content and antioxidant activity in various kinds of fruits were found in previous studies [30,31].
Total phenolic
μg PEs/mg extract
Total flavonoid
μg QEs/mg extract
S. umbellata
213.56±3.07
18.56±1.43
Table 2: Total phenolic and total flavonoid content of S. umbellata.
The results are in accordance with the individual phenolic ingredients. The fact is that phytochemicals occurring in food and natural products play a significant role in disease prevention and health promotion. This led to an ever-growing interest in nutraceutical products [32]. As it was previously reported in the literature that the total phenolic contents of blackberry, blueberry, strawberry and sweet cherry were also significantly high [33]. This work has clearly shown that the Turkish S. umbellata fruit have high phenolic and flavonoid contents.
Antioxidant activities of the extracts of S. umbellata fruit sample by β-Carotene-linoleic acid, DPPH. and ABTS.+ assays were evaluated in Table 3. Results were found to be statistically significant (p<0.05) when compared with the control. In the extract of β-carotene/ linoleic acid assay, S. umbellata possessed the inhibition value (IC50) 16.42±0.98 μg/ mL. Studied extract indicated lower lipid peroxidation inhibition activity than BHA, BHT, α-tocopherol.
Antioxidant activity
β-Carotene-linoleic acid assay IC50 (μg mL-1)
DPPH • assay
IC50 (μg mL-1)ABTS•+ assay
IC50 (μg mL-1)S. umbellata
16.42±0.98
62.09±1.41
20.17±0.94
BHA
4.08±0.10
57.71±0.55
8.83±0.19
BHT
4.14±0.09
62.04±1.02
8.22±0.12
a-Tocopherol
6.67±0.17
9.77±0.28
7.57±0.56
IC50 values represent the means ± SD of three parallel measurements (p<0.05)
BHA Butylated hydroxyanisole, BHT Butylated hydroxytoluene
Table 3: Antioxidant activities of the extract of S. umbellata fruits sample by β-Carotene-linoleic acid, DPPH• and ABTS•+ assays.
However, in the DPPH•assay, the extract of S. umbellata exhibited the similar inhibition activity (IC50) 62.09±1.41 μg /mL with that of standards, BHA and BHT, and lower than α-tocopherol.
In the ABTS•+ assay, the extract of S. umbellata displayed lower cation radical scavenging activity with inhibition values (IC50) 20.17±0.94 μg/mL with that of standards, BHA, BHT and α-tocopherol. Thus, according to the antioxidant activity data presented in Table 3, the results obtained with the β-carotene/linoleic acid, the DPPH•, and the ABTS•+ assays supported each other.
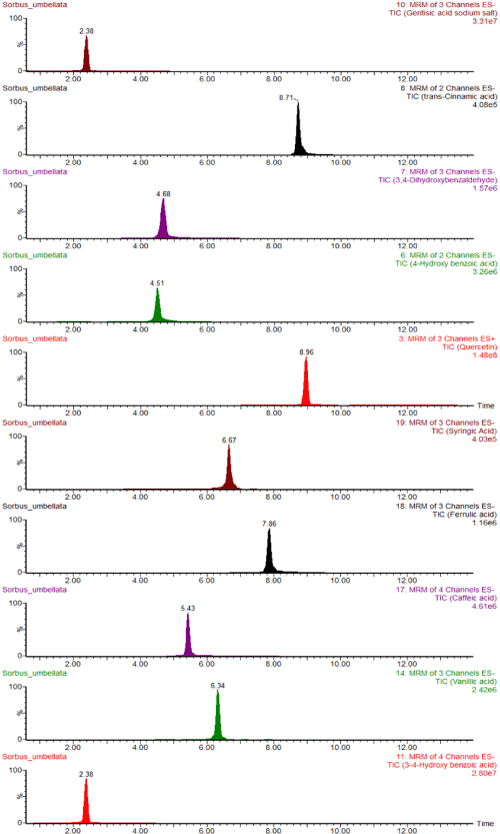
Identification of the phenolic compounds was carried out by comparing retention times and mass spectra with those of authentic standards. Individual phenolic results were explained in Table 4. S. umbellata fruits contained 102.87±1.29μg/g gentisic acid, 74.45±1.07μg/g protocatechuic acid as a major constituent; the others were 7.67±1.02μg/g ferulic acid, 7.51±0.99μg/g chlorogenic acid, 3.03±0.48μg/g caffeic acid, 2.91±0.78μg/g syringic acid, 2.52±0.56μg/g vanillic acid, 2.44±0.18μg/g 3,4-dihydroxybenzaldehyde. Total ion chromatograms of determined phenolic compounds in S. umbellata fruits using UPLC-ESI-MS/MS were displayed in Figure 1. These results agree with those reported by other authors in berries, chlorogenic, caffeic, p-hydroxybenzoic,ferulic, and p-coumaric acids were identified [34]. However, the results were correlated with total phenolic and flavonoid contents, and antioxidant activity of S. umbellata fruits.
Figure 1: UPLC-MS/MS Total Ion Chromatograms (TIC) of phenolic compounds in S. umbellata fruits.
No
Compounds
tR (min)
T. aestivum
1
Pyrogallol
0.97
nd
2
Homogentisic acid
1.47
nd
3
Protocatechuic acid
2.38
74.45±1.07
4
Gentisic acid
2.38
102.87±1.29
5
Pyrocatechol
2.38
nd
6
Galantamine
2.68
nd
7
p-hydroxy benzoic acid
3.87
1.41±0.13
8
3,4-dihydroxybenzaldehyde
4.68
2.44±0.18
9
Catechin hydrate
3.45
nd
10
Vanillic acid
6.34
2.52±0.56
11
Caffeic acid
5.43
3.03±0.48
12
Syringic acid
6.67
2.91±0.78
13
Vanillin
4.50
nd
14
p-coumaric acid
4.65
nd
15
Ferulic acid
7.86
7.67±1.02
16
Epicatechin
6.97
0.38±0.06
17
Catechin gallate
5.91
nd
18
Rutin
8.36
1.81±0.38
19
trans-2-hydroxy cinnamic acid
6.32
nd
20
Myricetin
10.61
0.38±0.09
21
Resveratrol
7.23
nd
22
trans-cinnamic acid
8.71
0.16±0.04
23
Luteolin
10.65
0.59±0.07
24
Quercetin
8.96
1.30±0.10
25
Naringenin
9.29
0.26±0.03
26
Genistein
9.22
nd
27
Apigenin
10.95
0.11±0.05
28
Kaempferol
10.98
0.22±0.07
29
Hesperetin
10.62
0.14±0.04
30
Chrysin
11.39
0.12±0.01
31
Chlorogenic acid
5.44
7.51±0.99
32
Gallic acid
1.96
n.d.
Table 4: Phenolic contents (μg/g dry weight ± standard deviation) of S. umbellata fruits.
Conclusion
In the present study, mainly, the involvement of the studied fruit in the daily diet may provide health benefits, owing to its antioxidant properties and nutrients. Furthermore, the fruits could be used in nutraceutical or pharmaceutical industries.
The method used in the determination of individual phenolic compounds provides high accuracy and robustness owing to UPLC-ESI-MS/MS instrument. As a result, S. umbellata fruits are a highly valuable natural product containing phenolic compounds and antioxidant properties.
In the present study, individual phenolic composition obtained by UPLC-ESI-MS/MS analysis and nutritional parameters, antioxidant activities namely, β-carotene-linoleic acid, DPPH• and ABTS•+ assays were explored for S. umbellata fruits.
S. umbellata fruits have a rich content in terms of phenolic compounds, carbohydrate and protein. According to antioxidant activity data, fruits extract is a valuable natural antioxidant. S. umbellata fruits may be used in food industry as a jam, juice, ice-cream and liquor and health industry as a source of natural healthy product with significant ingredients.
References
- Knekt P, Järvinen R, Seppänen R, Hellövaara M, Teppo L, Pukkala E, et al. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am J Epidemiol. 1997; 146: 223-230.
- Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996; 312: 478-481.
- Yi W, Akoh CC, Fischer J, Krewer G. Effects of phenolic compounds un blueberries and muscadine grapes on HepG2 cell viability and apoptosis Food Res Int. 2006; 39: 628-638.
- Doumett S, Donatella F, Cincinelli A, Giordani E, Nin S, Bubba MD. Comparison of nutritional and nutraceutical properties in cultivated fruits of Fragaria vesca L. produced in Italy. Food Res Int. 2011; 44: 1209-1216.
- Piljac-Žegarac J, Šamec D. Antioxidant stability of small fruits in postharvest storage at room and refrigerator temperatures. Food Res Int. 2011; 44: 345-350.
- Corral-Aguayo RD, Yahia EM, Carrillo-Lopez A, González-Aguilar G. Correlation between some nutritional components and the total antioxidant capacity measured with six different assays in eight horticultural crops. J Agric Food Chem. 2008; 56: 10498-10504.
- Kumar M, Rawat V, Rawat JMS, Tomar YK. Effect of pruning intensity on peach yeald and fruit quality. Sci Hortic. 2010; 125: 218-221.
- Bilaloglu GV, Harmandar MF. lavonoidler, Aktif Press, Istanbul. 1999.
- Shahidi F. Antioxidants in food and food antioxidants. Nahrung. 2000; 44: 158-163.
- Shahidi F. Antioxidants in plants and oleaginous seeds, in Free Radicals in Food. Chemistry, Nutrition, and Health Effects. Morello MJ, Shahidi F, ve Ho C-T, editors. ACS Symposium Series 807, American Chemical Society, Washington DC. 2002: 162-175.
- Pehluvan M, Güleryüz M. Ahududu ve Bögürtlenlerin Insan Sagligi Açisindan Önemi, Bahçe. 2004; 33: 51 - 57.
- Öztürk M, Duru ME, Kivrak S, Mercan-Dogan N, Türkoglu A and Özler MA. In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: a comparative study on the three most edible mushrooms. Food Chem Toxicol. 2011; 49: 1353-1360.
- Slinkard K, Singleton VL. Total phenol analysis automation and comparison with manual methods. Am J Enol Vitic. 1977; 28: 49-55.
- Moreno MI, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000; 71: 109-114.
- Leal AR, Barros L, Barreira JCM, Sousa MJ, Martins A, Santos-Buelga C, et al. Portuguese wild mushrooms at the "pharma-nutrition" interface: Nutritional characterization and antioxidant properties. Food Res Int. 2013; 50: 1-9.
- Vracaric B. Nutrition in nature. In: Military publishing institute and People's book, Beograd. 1977.
- AOAC. Official methods of analysis. 16th edn. Arlington VA, USA: Association of Official Analytical Chemists. 1995.
- Heleno SA, Stojkovic D, Barros L, Glamoclija J, Sokovic M, Martins A, et al. A comparative study of chemical composition, antioxidant and antimicrobial properties of Morchella esculenta (L.) Pers. from Portugal and Serbia. Food Res Int. 2013; 51:236-243.
- Kivrak I, Kivrak S, Harmandar M, Çetintas Y. Phenolic Compounds of Pinus brutia Ten.: Chemical Investigation and Quantitative Analysis Using an Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry with Electrospray Ionization Source. Rec Nat Pro. 2013; 7: 313-319.
- Miller HE. A simplified method for the evaluation of antioxidants. J Am Oil Chem Soc. 1971; 48: 2-91.
- Kivrak I, Duru ME, Öztürk M, Mercan N, Harmandar M, Topçu G. Antioxidant, Anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia potentillifolia. Food Chem. 2009; 116: 470-479.
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995; 28: 25-30.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26: 1231-1237.
- Türkoglu A, Duru ME, Mercan N, Kivrak I, Gezer K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill Food Chem. 2007; 101: 267-273.
- Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004; 134: 3479S-3485S.
- Chu YF, Sun J, Wu X, Liu R. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002; 50: 6910-6916.
- León-Gonzáleza AJ, Truchado P, Tomás-Barberán FA, López-Lázaroa M, Barradas MCD, Martín-Cordero C. Phenolic acids, flavonols and anthocyanins in Corema album (L.) D. Don berries. J Food Comp Anal. 2013; 29: 58-63.
- Wolfe KL, Kang X, He X, Dong M, Zhang Q, Liu RH. Cellular antioxidant activity of common fruits. J Agric Food Chem. 2008; 56: 8418-8426.
- Yang J, Meyers KJ, van der Heide J, Liu RH. Varietal differences in phenolic content and antioxidant and antiproliferative activities of onions. J Agric Food Chem. 2004; 52: 6787-6793.
- Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH. Antioxidant and antiproliferative activities of raspberries. J Agric Food Chem. 2002; 50: 2926-2930.
- Almeida MB, Sousa PH, Arriaga AM, Prado GM, Magalh&aTilde;es CE, Maia GA, et al. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res Int. 2011; 44: 2155-2159.
- Roesler R, Malta LG, Carrasco LC, Pastore G. Evaluation of antioxidant properties of the Brazilian Cerrado fruit Annona crassiflora (Araticum). Food and Chemical Toxicology. 2006; 71: 102-107.
- Shahidi F, Naczk M. Food Phenolics, Technomic Publishing Company Book, Lanchester, USA. 1995.
- Heinonen IM, Lehtonen PJ, Hopia AI. Antioxidant Activity of Berry and Fruit Wines and Liquors. J Agric Food Chem. 1998; 46: 25-31.