
Research Article
Austin J Nutri Food Sci. 2016; 4(1): 1073.
Preliminary Comparison of Fatty Acid Composition(s) of Selected Commercial Rice Brands Commonly Consumed in North America
Gines BR¹, Gray J² and Abugri DA²*
¹Department of Agricultural, Environmental Sciences, Tuskegee University, USA
²Department of Chemistry, Department of Biology, Tuskegee University, USA
*Corresponding author: Abugri DA, Department of Biology/Chemistry, College of Arts and Sciences, Tuskegee University, Tuskegee, AL, 36088, USA
Received: August 31, 2015; Accepted: December 23, 2015; Published: January 11, 2016
Abstract
There is growing interest in whole cereals consumption and research in recent years partly due to their rich sources of bioactive and nutritious compounds for human health. Many different brands of rice are widely cultivated and consumed in North America and Asia. Here, we investigated the fatty acid profile(s) of five selected rice brands commonly consumed in North America via gas chromatography analysis. All rice brands showed desirable quantities of nutritional fatty acids, with the essential fatty acid linoleic acid as a major fatty acid component. Linoleic (C18:2n6 cis) acid followed by linolenic (C18:3n3) acid were the predominant polyunsaturated fatty acids found in all of the rice brands. Linoleic acid was found to represent 34.8-38.1% of the identified fatty acids while linolenic represents 1.13-1.58%. Overall, the brown rice brands showed relatively higher amounts of unsaturated fatty acids than the white rice brands. The data presented in this article adds to the nutritional and potential health value of these rice cultivars.
Keywords: Rice; Nutrition; Health; Lipids; Fatty acids; North america
Introduction
Rice (Oryza sativa L.) is one of the major grains produced and consumed worldwide [1-3]. In continents such as North America and Asia, every part of the cereal has unique applications. Additionally, rice remains a staple food worldwide [3]. In US alone, it has been reported that about 72.6% to 77.2% of the human population consumes either white or brown rice [4]. The underline factors to this trend have been ethnicity, nationality, race, and socio-economic status level among others [4]. Besides its human consumption, it is directly or indirectly used as animal feeds especially its husks, stems and leaves [5]. For many years, it has been known that rice is an important cereal with great sources of essential fatty acids, amino acids, and other bioactive compounds such as vitamins and phenolic acids [1,6-10].
Wild (Zizania sp.) and regular rice (O. sativa L.) have been shown to contain high amounts of the essential fatty acid linoleic acid, which constitutes 27.3 – 41.0% of the total fatty acids present across a number of rice bran varieties [11]. Linoleic acid is found in the lipids of cell membranes and is used in the biosynthesis of many prostaglandins, proving to be a major regulator of many cellular processes. Additionally, derived indices from fatty acid composition, such as omega 6/omega 3 (n6/n3) ratios, help to evaluate the quality of lipid fraction for different brands or cultivar rice from a nutritional point of view [8,12,13].
Rice is often marketed based on their color and quality with little known about their nutritional compositions. However, little or no data has been published on different commercial rice brands commonly sold in the USA in regards their nutritional compositions, in particular the fatty acid profiles. Thus, the present study is aimed at characterizing and determining the fatty acid composition and derived nutritional indices to evaluate from a nutritional point of view the selected brands rice commonly sold and consumed in the USA.
Materials and Methods
Rice samples
Five varieties of rice, Oryza sativa L: were purchased from a supermarket in Tuskegee, AL, USA. Great Value® long grain (GV LG) enriched rice (white), Great Value® (GV) brown rice, and Mahatma® (M) brown rice were grown in the USA. Mahatma® Jasmine rice (white) is a product of Thailand, and Golden Star® Basmati rice (white) was grown in India. These grains were ground into small mesh size (< 2 mm) using a coffee grinder (Mr. Coffee® grinder, model IDS77). The Basmati rice was ground to < 2 mm using a Rival® 6-speed blender, model No. RV-928. The samples were stored for a week at room temperature (25°C) in sealed polyethylene zip-lock bags prior to the analyses. This method of storage was adopted to mimic normal household treatment of rice. All reagents were of HPLC grades purchased from Sigma-Aldrich and Fischer Scientific.
FAME preparation: Fatty Acid Methyl Esters (FAMEs) were extracted using approximately 0.5 g of rice flour using a direct methylation method [14]. Briefly, rice samples were placed into a 16 x 125 mm screw-cap Pyrex culture tube. One milliliter of BF3 in MeOH (14%, wt/vol) was added to the Pyrex tubes containing the samples. The tubes were vortexes for 15 seconds to enhance complete mixture of the solvent and sample matrix, then incubated at 100- 110°C using a heating block for 30 minutes. Following incubation at 100-110°C , the samples were put on ice for 5 min to completely cool to room temperature [14]. One ml of deionizer water and 2 mL of n-hexane (HPLC grade, Sigma Aldrich) were then added, and the tubes were vortexes for 3 minutes. After centrifugation at 2000 rpm for 5 min, rpm using a clinical tabletop centrifuge (model number IEC Centra® CL 2, International Equipment Company, Needham Heights, MA, USA). The hexane layer containing the FAMEs was collected into a gas chromatography (GC) vial. The vial was capped and placed at -20°C until GC analysis. FAMEs were not dried.
Gas liquid chromatography (GLC) analysis of FAMEs
Individual fatty acids were separated and quantified using previous procedures reported by Abugri et al. [15]. Briefly, an Agilent GLC 6890N equipped with flame ionization detector was used for the analysis. The rice sample FAMEs were injected into the column via an automated split injector with a split ratio of 20:1. Separations of individual fatty acids were done using an Agilent DB23 capillary column (model no.122-2362, 60.0 m×250μm× 0.25μm, J and W Scientific). The set up conditions were; initial oven temperature was set at 130 Co, held for 1 min, subsequently rammed to 170 Co at a rate of 6.50 Co/min. Then the oven temperature was moved to 215 °C at a flow rate of 2.75 °C /min and held for 12 min then increased to 230 °C at 40 °C/ min. Helium gas was used as a carrier gas with a flow rate of 2.6 mL/min with an average velocity of 40 cm/s. Both the injector and the detector were set at 250 °C throughout the analysis [15]. Individual fatty acids were identified by comparison of their retention times with external standards (GLC463 and GLC 68F) retention times. The amounts of individual fatty acid methyl esters identified were expressed in % of the total fatty acid areas chromatograms identified.
Indices of lipid quality
Some lipid health indices were calculated from fatty acid composition including polyunsaturated fatty acids (PUFA)/ Saturated Fatty Acids (SFA), monounsaturated fatty acid (MUFA)/ saturated fatty acid (SFA), unsaturated fatty acid (UFA) /saturated fatty acid (SFA), and omega 6/omega 3 PUFAs , PUFA/SFA, MUFA/SFA, UFA/SFA, n6/n3, IA and TI. PUFA/SFA, MUFA/SFA, and UFA/ SFA ratios show the relationship between health-benefitting Polyand Mono-Unsaturated Fatty Acids (PUFA & MUFA) and healthrisk- contributing Saturated Fatty Acids (SFA). Omega 6/ omega 3 PUFAs indices are commonly used to assess the health benefits of diet; foods higher in omega 3s are considered to have better healthbenefitting properties. Additionally, the index of atherogenicity (IA) and thrombogenicity (IT) shows the relationship between pro- and anti-atherogenic/thrombogenic fatty acids. Atherogenicity favors the adhesion of lipids to cells, while thrombogenicity shows the tendency to form clots in blood vessels. First proposed by Ulbricht & Southgate [16] to characterize the atherogenic and thrombogenic potential of diet, we used the following equations to determine the following indices:
IA = [(a*12:0) + (b*14:0) + (c*16:0)] / [d*(PUFA - (n6+n3)) + e*(MUFA) + f *(MUFA-18:1c)]
IT = [g*(14:0 +16:0 + (18:0)] / [(h *MUFA) + (m*n6) + (n*n3) + (n3/n6)]
Where a, c, d, e, f=1, b=4, g=1, h, m=0.5 and n=3
In this study, we assessed the thrombogenic and atherogenic indices of different rice varieties, however, the C12:0 value was excluded from the atherogenic index equation because C12:0 was not determined in our analyses.
Statistical analysis
All extractions and analyses were carried out in triplicates. The means (with standard deviations or standard error of the mean) are reported. Two-way ANOVA followed by Turkey’s multiple comparisons test was performed using GraphPad Prism version 6.05 for Windows, Graph Pad Software, La Jolla California USA, (www. graphpad.com). Significant difference was determined at p < 0.05.
Results and Discussion
The percentages (%) of the total individual Fatty Acids (FA) identified in 5 different rice varieties are reported in (Tables 1 & 2). Additionally, the ratios Polyunsaturated Fatty Acid (PUFA)/ Saturated Fatty Acids (SFA), Monounsaturated Fatty Acid (MUFA)/ Saturated Fatty Acid (SFA), Unsaturated Fatty Acid (UFA) /Saturated Fatty Acid (SFA), and omega 6/omega 3 as well as the individual classifications are shown in (Figures 1 & 2). Altogether, we identified the total individual fatty acid number to be 21 for M. brown, GV brown, and M. Jasmine rice, 17 for GV LG rice and 14 for Basmati rice with chain lengths ranging from C13:0 to C20:5n3 (Tables 1 & 2). These chain length ranges agreed with previous studies of lipid content and fatty acid composition of brown rice cultivars found in the USA [17].
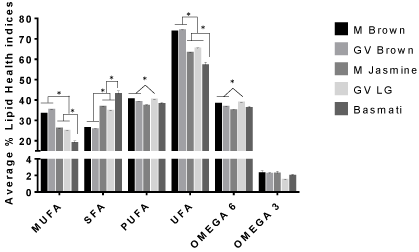
Figure 1: Mean percentage of lipid health indices within selected rice brands
presented as means plus SEM for triplicate independent determination.
*Represents significant difference between other rice brands for each health
index determined by 2-way Anova analysis using Graphpad Prism software.
p < 0.05.
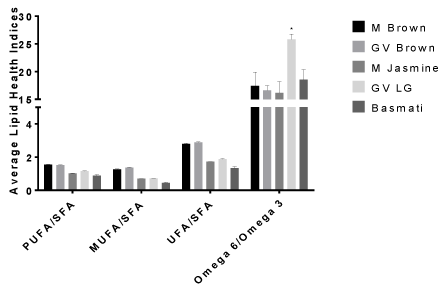
Figure 2: Mean percentage ratio of lipid health indices in selected rice brands
presented as means plus SEM for triplicate independent determinations.
* Represents significant difference between other rice brands for each health
index determined by 2-way Anova analysis using Graphpad Prism software.
p < 0.05.
There were significant differences in the amount of some of the predominant saturated and unsaturated fatty acids found in brown rice samples as well as white rice samples. Among saturated fatty acids, palmitic acid (C16:0) was found to be the major fatty acid in all rice samples. White rice samples (Jasmine, GV long grain, and Basmati) contained higher amounts of palmitic acid than the brown rice brands by ~7-16% (Table 1). This trend is unclear and may require further research. The most prominent monounsaturated fatty acid found in all rice brands was oleic acid. The amount oleic acid (C18:1n9 cis) was similar for Mahatma Brown and GV Brown, while the white rice varieties contained lower amounts of oleic acid by more than 7.4% with Basmati rice containing the least percentage of oleic acid (Table 2). Linoleic (C18:2n6 cis) followed by linolenic (C18:3n3) were the predominant polyunsaturated fatty acids found in all of the selected rice brands. The trend observed for the major fatty acids C16:0, C18:1n9, and C18:2n6 (Table 1) in all brands concurred with a previous studies with Saudi Hassawi Rice [18]. Also shown in (Table 1) is the Index of Thrombogenicity (IT). Comparing the rice varieties, higher IT values are associated with the polished, white rice varieties as opposed to the brown rice brands studied. This trend suggests that white rice may contribute to increased health risk such as coronary heart disease more so than brown rice varieties [19]. Additional studies should be carried out to correlate rice diet index values and disease in vivo.
Fatty Acids (%)
M Brown
GV Brown
M Jasmine
GV LG
Basmati
C13:0ns
0.23±0.07
0.22±0.04
0.08±0.00
0.07±0.01
0.16±0.07
C14:0
1.19±0.0 a
1.17±0.06 a
1.19±0.03a
2.12±0.03b
1.91±0.05
C15:0ns
0.06±0.01
0.09±0.04
0.08±0.03
-
-
C16:0
21.81±0.22a
20.71±0.2b
30.29±0.48c
28.17±0.36d
36.97±2.61e
C17:0ns
0.07±0.01
0.12±0.09
0.10±0.00
-
-
C18:0
2.04±0.10a
2.34±0.09a,b
2.94±0.04b
2.69±0.13
3.40±0.16c
C19:0
0.20±0.02a
0.21±0.06a
1.42±0.12b
1.31±0.07b
-
C20:0ns
0.56±0.25
0.80±0.29
0.43±0.07
0.35±0.05
0.25±0.11
C22:0ns
0.14±0.01
0.11±0.02
0.20±0.08
-
-
SFA
26.29±0.49a
25.77±0.35a
36.72±0.27b
34.72±0.46b
42.94±2.73c
M Brown: Mahatma Brown; GV Brown: Great Value Brown; GV LG: Great Value long grain Means within rows followed by the same letter are not significant different at p < 0.05.
* Values are reported as mean ± SD from triplicate measurements (n=3).
nsno significant difference.
Table 1: Saturated fatty acid composition in selected rice*.
Fatty Acids (%)
M Brown
GV Brown
M Jasmine
GV LG
Basmati
C16:1tns
0.11±0.02
0.11±0.02
0.11±0.01
0.10±0.01
-
C16:1ns
0.18±0.01
0.19±0.03
0.18±0.00
0.14±0.00
0.12±0.02
C17:1ns
0.05±0.00
-
0.09±0.03
-
-
C18:1n9tns
0.20±0.04
0.17±0.03
0.34±0.05
0.23±0.04
-
C18:1n9c
31.66±0.46a
33.70±0.25b
24.27±0.52c
23.45±0.32d
17.89±1.73e
C18:1n7ns
0.84±0.01
0.84±0.02
0.69±0.02
0.74±0.01
0.58±0.02
C18:2n6c
38.09±0.61a
36.34±0.34b
34.84±0.59c
37.55±0.86a
35.83±1.30b
C18:3n6ns
0.11±0.02
0.11±0.02
0.19±0.05
0.17±0.02
-
C18:3n3ns
1.58±0.04
1.56±0.03
1.13±0.09
1.40±0.03
1.51±0.07
C20:1ns
0.20±0.08
0.23±0.03
0.31±0.06
0.27±0.03
0.36±0.09
C20:4n6ns
-
0.34±0.08
-
1.13±0.70
0.32±0.01
C20:3n3ns
0.79±0.33
0.46±0.18
0.96±0.49
-
0.34±0.07
C20:5n3ns
0.17±0.02
0.22±0.06
0.16±0.05
0.16±0.06
0.24±0.12
MUFA
33.23±0.36a
35.24±0.17a
26.00±0.43b
24.92±0.37b
18.95±1.66c
PUFA
40.74±0.28a
39.02±0.19
37.28±0.70b
40.41±0.20a
38.23±1.01b
IT
0.59±0.02a
0.57±0.01a
0.92±0.04b
0.90±0.01b
1.27±0.11c
IA
0.76±0.01a
0.69±0.01a
1.26±0.01b
1.29±0.03b
2.26±0.29c
M Brown: Mahatma Brown; GV Brown: Great Value brown; GV LG: Great Value long grain Means within rows followed by the same letter are not significant different at p < 0.05.
*Values are reported as mean ± SD from triplicate measurements (n=3).
nsno significant difference.
Table 2: Mono- and polyunsaturated fatty acid composition of selected rice*.
In agreement with our observations, palmitic acid was higher in the rice grains than other cereal grains such as corn (~11%) and rye grains (~16%) [6,20]. However, the polyunsaturated fatty acid, linoleic acid, is lower than those found in other standard cereal grains [6,20]. Nutritionally, PUFAs play an important role in human physiology. Additionally, omega 6 to omega 3 fatty acids may also play an important role in human health. As shown in (Figure 2), PUFA/ SFA and n6/n3 ratios indicate that both brown rice brands contain similar amounts of polyunsaturated fatty acids to saturated fatty acids (1.5:1) as well as omega 6/omega 3 ratios (15-16.5:1). Although, the omega 6/omega 3 ratios were higher than a typical rich food with well balanced omega 6 to omega 3 ratios of 2:1, 3:1, 5:1, and 10:1 which has been reported to have health benefits such as membrane structural development, fight against neurodegenerative disorders, promotes normal brain and nerves development [6,22,23]. Our data was above the recommended ratios [24]. This may have negative implication on human health such as chronic pro-inflammatory as attested to by many authors that consumption of diets rich in omega 6 alone is not well balanced enough for reduction of cardiovascular diseases [24- 27].
However, caution must be exercised in that the sample size were small, so we suggest that large studies on different rice brands nationwide need to be conducted. Great Value long grain rice had higher PUFA/SFA content (1.2:1) than the other white rice brands; however, GV long grain rice also showed a significantly higher omega 6 to omega 3 ratio (24.9:1) than Jasmine and Basmati rice with ratios of 15.62:1 and 17.31:1, respectively, indicating greater proinflammatory potential than the other rice samples.
Conclusion
Conclusively, there were significant differences between individual fatty acids and lipid health indices among different brands of rice. This study demonstrates that all 5 selected rice brands consumed among North America as well as other parts of the world, contained relatively high levels of unsaturated fatty acids as opposed to saturated fatty acids with linoleic acid as the chief unsaturated fatty acid and palmitic acid as the prominent saturated fatty acid. Comparatively, both brown rice brands analyzed exhibited unique fatty acid profiles than the white rice brands studied.
Acknowledgement
We wish to express our gratitude to Tuskegee University’s Department of Chemistry for supporting us with instrumentation and chemicals for this project.
References
- Liu L, Waters DL, Rose TJ, Bao J, King GJ. Phospholipids in rice: significance in grain quality and health benefits: a review. Food Chem. 2013; 139: 1133-1145.
- Khush GS. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol. 2005; 59: 1-6.
- Batres-Marquez SP, Jensen HH, Upton J. Rice consumption in the United States: recent evidence from food consumption surveys. J Am Diet Assoc. 2009; 109: 1719-1727.
- Vichapong J, Sookserm M, Srijesdaruk V, Swatsitang P, Srijaranai S. High performance liquid chromatographic analysis of phenolic compounds and their antioxidant activities in rice varieties. LWT - Food Science and Technology. 2010; 43: 1325-1330.
- Weihrauch JL, Matthews RH. Lipid content of selected cereal grains and their milled and baked products. Cereal Chem. 1977; 54: 444-453.
- Tian S, Nakamura K, Kayahara H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J Agric Food Chem. 2004; 52: 4808-4813.
- Zhou Z, Blanchard C, Helliwell S, Robardsa K. Fatty Acid Composition of Three Rice Varieties following storage. Journal of Cereal Science. 2002a; 1-9.
- Przybylski R, Klensporf-Pawlik D, Anwar F, Rudzinska M. Lipid Components of North American Wild Rice (Zizania palustris). J Am Oil Chem Soc. 2009; 86: 553-559.
- Gani A, Wani SM, Masoodi FA, Hameed G. Whole-Grain Cereal Bioactive Compounds and Their Health Benefits: A Review. J Food Process Technol. 2012; 3:146.
- Goffman FD, Pinson S, Bergman C. Genetic diversity for lipid content and fatty acid profile in rice bran. Journal of the American Oil Chemists' Society. 2003; 80: 485-490.
- Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. The American journal of clinical nutrition. 2003; 77: 1146-1155.
- Zhou Z, Robards K, Helliwell S, Blanchard C. Composition and functional properties of rice. International Journal of food science & technology. 2002b; 37: 849-868.
- Abugri DA, Tiimob BJ, Apalangya VA, Pritchett G, McElhenney WH. Bioactive and nutritive compounds in Sorghum bicolor (Guinea corn) red leaves and their health implication. Food Chem. 2013; 138: 718-723.
- Abugri DA, McElhenney WHKR, William KR. Comparison of Transesterification Methods for Fatty Acid Analysis in Higher Fungi: Application to Mushrooms. Food Analytical Methods. 2012; 5: 1159-1166.
- Ulbricht TL, Southgate DA. Coronary heart disease: seven dietary factors. Lancet. 1991; 338: 985-992.
- Taira H, Itani T. Lipid content and fatty acid composition of brown rice of cultivars of United States. J Agric Food Chem. 1988; 36: 460-462.
- AL-Bahrany AM. Chemical composition and fatty acid analysis of Saudi Hassawi Rice Oryza sativa L. Pakistan Journal of Biological Science. 2002; 5: 212-214.
- Valfré F, Caprino F, Turchini GM. The health benefit of seafood. Vet Res Commun. 2003; 27: 507-512.
- Ryan E, Galvin K, O’Connor TP, Maguire AR, O’Brien NM. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods for Human Nutrition. 2007; 62: 85-91.
- Singh M. Essential fatty acids, DHA and human brain. Indian J Pediatr. 2005; 72: 239-242.
- Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, et al. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005; 25: 3032-3040.
- Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010; 304: 1903-1911.
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002; 56: 365-379.
- Norris PG, Jones CJ, Weston MJ. Effect of dietary supplementation with fish oil on systolic blood pressure in mild essential hypertension. Br Med J (Clin Res Ed). 1986; 293: 104-105.
- Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, et al. Dietary n-3 polyunsaturated fatty acids suppress synthesis of interleukin-1 and tumor necrosis factor. N Engl J Med. 1989; 320: 265-271.
- Lichtenstein AH, Chobanian AV. Effect of fish oil on atherogenesis in Watanabe heritable hyperlipidemic rabbit. Arteriosclerosis. 1990; 10: 597-606.
- Lichtenstein AH, Chobanian AV. Effect of fish oil on atherogenesis in Watanabe heritable hyperlipidemic rabbit. Arteriosclerosis. 1990; 10: 597- 606.