
Research Article
Austin J Nutri Food Sci. 2018; 6(1): 1098.
Deep Inside Polyphenols of Hellenic Thyme Honey
?ousias P, Karabagias IK*, Riganakos KA
Department of Chemistry, University of Ioannina, Greece
*Corresponding author: ?oannis ? Karabagias, Laboratory of Food Chemistry, Department of Chemistry, University of Ioannina, Greece
Received: December 20, 2017; Accepted: February 20, 2018; Published: February 27, 2018
Abstract
The aim of the present study was to investigate the polyphenol fraction of thyme honeys collected from different regions by means of phenolic acids (caffeic, chlorogenic, para-coumaric, ferulic, gallic, syringic, vanillic) and flavonoids (apigenin, chrysin, galangin, kaempferol, luteolin, myricetin, quercetin). Thirty three honey samples were collected from 11 regions, belonging to different regional departments in the Hellenic zone. The analysis of polyphenols was carried out using High Performance Liquid Chromatography (HPLC). Results showed that the majority of thyme honey samples contained phenolic acids and flavonoids. Based on these findings, Hellenic thyme honey is suggested to serve as a good source of antioxidants in the daily diet.
Keywords: Thyme honey; Phenolic content; Food character; Geographical origin
Introduction
Honey comprises a real treasure of health and power and its nutritional value has been enhanced from Ancient Times. It is the product of Apis mellifera honeybees via collecting nectar from living parts of the plants or excretions of plant-sucking insects, which bees collect, transfer in the honeycomb, transform it by adding enzymes secreted by their hypopharyngeal glands and store it to mature [1]. Honey usually takes the name of the plant or tree from which bees collect nectar or honeydew. Therefore, numerous types of honey namely: thyme, citrus, pine, fir tree, chestnut, heather, etc. are grown, among other parts of the world, in the Hellenic zone.
Its major constituents are the simple sugars fructose and glucose along with some other disaccharides or trisaccharides of which the total content ranges between 75-85%. The second honey major component is water, which usually ranges between 15-20%. However, there are numerous micro-constituents such as proteins, enzymes, vitamins, phenolic acids, flavonoids, carotenoids, Maillard reaction products, etc. in much lower proportions. Despite the lesser amounts of honey micro-constituents compared to major components, what really impresses is not so much the individual components of honey, as their coexistence of all these substances in a mass in which hold a specific, naturally based ‘’optimal’’ proportions and the way these components act on the human body through the diet [2]. During the last 20 years the scientific community has been focused on research related to beneficial health effects after consumption of naturally antioxidant components called ‘phytochemicals’’. Among them flavonoids (flavones, flavonols, flavanones, is flavones, flavans, flavanols, anthocyanidins, etc.) and phenolic acids (benzoic and cinnamic acids derivatives) have gained numerous research, since epidemiological studies have confirmed their health benefits in relation to prevention of cancer, cardiovascular disorders or any other type of diseases related to weak immune system [3-6]. It should be stressed, that polyphenols are one of the most important groups of compounds occurring in plants, seeds, roots and pollen in the form of glycosides, comprising at least 8000-8500 different known structures [7,8]. However, the theoretical number of polyphenols is estimated to be ca. 8 million [7]. Given the fact that there is no extended research on Hellenic honey phytochemical compounds [9-12], the objective of the present study was to get through the polyphenol content of Hellenic thyme honey, in terms of its nutritional characterization, by identifying specific flavonoids and phenolic acids.
Materials and Methods
Honey samples
Thirty three (N=33) Thymus spp. honey samples were collected from local supermarkets from 11 different regions in Hellas: Arkadia (3 samples), Kefalonia (3 samples), Evia (3 samples), Psara (3 samples), Attiki (3 samples), Irakleio (3 samples), Hania (3 samples), Sfakia (3 samples), Kavala (3 samples), Paramythia (3 samples) and Igoumenitsa (3 samples). All honey samples were stored in glass containers, shipped to the laboratory and maintained at 4-5 °C until analysis.
Reagents and solutions
All standard phenolic compounds, regarding flavonoids [apigenin (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran- 4-one), chrysin (5,7-dihydroxy-2-phenyl-4H-chromen-4-one), galangin (3,5,7-trihydroxy-2-phenylchromen-4-one), kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one), luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromenone), myricetin(3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4- chromenone), quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy- 4H-chromen-4-one)] and phenolic acids [caffeic (3,4-dihydroxycinnamic acid), gallic (3,4,5-trihydroxybenzoic acid) ,vanillic (4-hydroxy-3-methoxybenzoic acid), para-coumaric ((E)-3-(4- hydroxyphenyl)-2-propenoic acid), chlorogenic ((1S,3R,4R,5R)- 3-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-1,4,5 trihydroxycyclohexanecarboxylic acid), syringic (4-hydroxy-3,5- dimethoxybenzoic acid), and ferulic acid ((E)-3-(4-hydroxy-3- methoxy-phenyl)prop-2-enoic acid] were purchased from Fluka AG (Switzerland). Water and methanol were of analytical grade and purchased by A.R, Lab-Scan (Dubein, Ireland). Finally, formic acid 98-100% puriss p.a. was purchased from Riedel-de Haen (Germany).
Extraction of phenolic compounds
The extraction of phenolic compounds was carried out according to the methodology described in previous studies [13,14]. The obtained honey extracts (methanolic fraction) were stored at -18 oC prior analysis.
High Performance Liquid Chromatography (HPLC) instrumentation and analysis conditions
The chromatographic analysis of phenolic acids and flavonoids compounds was performed using LC 20AD HPLC system (SHIMADZU, Kioto, Japan) coupled to a diode array detector SPD M20A (SHIMADZU, Kioto, Japan). The methodology applied was a modified version of a previous work [15]. In particular, phenolic acids were detected at λ = 290 nm, whereas flavonoids at λ = 340 nm. Gradient elution was used at a flow rate of 1 mL/min using an aqueous solution of formic acid and methanol as the mobile phase. The respective gradient elution program is given in (Table 1). Separation of the phenolic compounds were carried out using a Hypersil ODS C18 reversed phase column (Jones Chromatography; 250 mm x4.6 mm x 5 μm) at room temperature Identification of phenolic compounds was achieved by comparing the retention times of individual chromatographic peaks with the retention times of standards. The injection volume used was 20 μL. The analysis of individual phenolic standards and honey samples were carried out in triplicate.
Time (min)
A (%, v/v)
B (%, v/v)
HCOOH: H2O (1:19)
MeOH
0
70
30
15
60
40
20
55
45
30
40
60
50
20
80
52
20
80
60
20
80
HCOOH: formic acid, H2O: HPLC water, MeOH: methanol of analytical grade
Table 1: Gradient elution program during HPLC analysis of phenolic compounds.
Results and Discussion
HPLC analysis showed that the majority of thyme honey samples contained flavonoids and phenolic acids (Tables 2 and 3) (Figures 1 and 2) recording a quite similar chromatographic profile. Polyphenols identified in the present study such as caffeic, paracoumaric and syringic acids, along with apigenin and luteolin, have been previously reported to dominate the polyphenol fraction of thyme plant [16]. Tsiapara et al. [9], Spilioti et al. [10], and Karabagias et al. [11,12], reported the presence of para-hydroxy benzoic acid, vanillic acid, protocatechuic acid, caffeic acid, para-coumaric acid and syringic acid, along with myricetin, kaempferol, chrysin and quercetin in monofloral thyme honeys produced in the Hellenic zone. Therefore, polyphenols combined in groups or by individual may be characteristic markers of honeys with the same botanical origin.
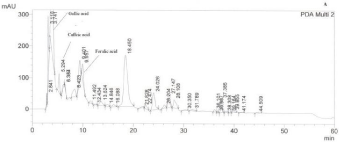
Figure 1: A typical HPLC chromatogram of thyme honey from Attiki. Phenolic acids are numbered according to retention time (min). PDA Multi 2: 290±4 nm.
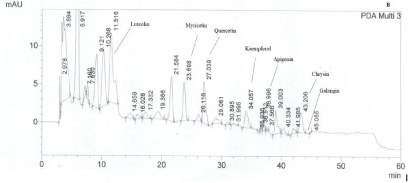
Figure 2: A typical HPLC chromatogram of thyme honey from Kefalonia. Flavonoids are numbered according to retention time (min). PDA Multi 3: 340±4 nm.
Region
Caffeic acid
Chlorogenic acid
para-Coumaric acid
Ferulic acid
Gallic acid
Syringic acid
Vanillic acid
Arkadia (N=3)
I
I
I
I
I
I
I
Kefalonia(N=3)
I
I
I
I
I
I
I
Evia (N=3)
I
I
I
I
I
I
I
Psara (N=3)
I
I
I
I
I
I
I
Attiki (N=3)
I
NI
NI
I
I
NI
NI
Irakleio (N=3)
I
I
I
I
I
I
I
Hania (N=3)
I
I
I
I
I
I
I
Sfakia (N=3)
I
I
I
I
I
I
I
Kavala (N=3)
I
I
I
I
I
I
I
Paramythia (N=3)
I
I
I
I
I
I
I
Igoumenitsa (N=3)
I
I
I
I
I
I
I
N: number of honey samples. I: identified. NI: not identified. Each sample was run in triplicate (n=3). Phenolic acids are listed alphabetically.
Table 2: Phenolic acids identified in Hellenic thyme honeys according to geographical origin.
Region
Apigenin
Chrysin
Galangin
Kaempferol
Luteolin
Myricetin
Quercetin
Arkadia (N=3)
I
I
NI
I
I
I
I
Kefalonia(N=3)
I
I
I
I
I
I
I
Evia (N=3)
I
I
I
I
I
I
I
Psara (N=3)
I
I
I
I
I
I
I
Attiki (N=3)
I
I
I
I
I
I
I
Irakleio (N=3)
I
I
I
I
I
I
I
Hania (N=3)
I
I
I
I
I
I
I
Sfakia(N=3)
I
I
I
I
I
I
I
Kavala(N=3)
I
I
I
I
I
I
I
Paramythia (N=3)
I
I
NI
I
I
I
I
Igoumenitsa (N=3)
I
I
NI
I
I
I
I
N: number of honey samples. NI: not identified. Each sample was run in triplicate (n=3). Flavonoids are listed alphabetically.
Table 3: Flavonoids identified in Hellenic thyme honeys according to geographical origin.
At this point let’’s take a typical look inside some similar works in the international literature. Phenolic acids such as caffeic, gallic, chlorogenic, ferulic, syringic, and para-coumaric and flavonoids such as myricetin, tricetin (5,7,3,4,5´,-pentahydroxyflavone), quercetin, kaempferol, luteolin, galangin, isorhamnetin, hesperetin etc. have been previously reported in European and American honeys [17-21]. In particular, citrus honey contains significant amounts of hesperetin [S)-2,3-dihydro-5,7-dihydroxy-2-(3- hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one]; helianthus honey contains quercetin [17], whereas that of rosemary contains kaempferol [22], Italian, Spanish, and Portuguese eucalyptus honeys contained pinocembrin (5,7-dihydroxy-2-phenyl-2,3-dihydro-4Hchromen- 4-one), pinobanksin [(2S,3R)-3,5,7-trihydroxy-2-phenylchroman- 4-one] and chrysin, which are constituents of propolis [18]. The flavonoids quercetin, kaempferol, isorhamnetin and their glucosinolates were identified in native Argentinean Diplotaxis honeys [19]. Twelve monofloral and 5 multifloral honeys from Florida were characterized by the presence of two plant hormones (2-cis, 4-trans-abscisic acid and 2-trans, 4-trans-abscisic acid) in combination with para-coumaric acid, rutin, chrysin, pinocembrin, quercetin, luteolin and kaempferol [20]. Five phenolic acids (ferulic, syringic, trans-cinnamic, chlorogenic and para-hydroxycinnamic) and nine flavonoids (catechin, kaempferol, rutin, quercetin, luteolin, apigenin, galangin, pinocembrin and pinobanksin) were identified in monofloral Sardinian honeys including, among them, thyme honeys [21]. Based on the aforementioned, present results are in agreement with previous work in the literature and point out the significance of phytochemical compounds in botanical origin identification and nutritional characterization of honey. However, beekeeping practices along with the overall environmental conditions in a specific geographical area should be also considered, since these parameters may affect the polyphenol content of honey. What is also of great importance, is that polyphenol content of Hellenic thyme honeys has been associated with antioxidant, anticancer and antiatherogenic activities [9-12,14] enhancing, thus, its nutritional value.
Conclusion and Future Perspectives
Results of the present study showed that 14 polyphenols were identified in Hellenic commercial thyme honeys. Therefore, the presence of such bio-functional compounds along with minerals, vitamins, proteins, enzymes, organic acids, Maillard reaction products, etc. found in honey, creates an ideal natural antioxidant food companion, which may act in different ways and comprise a basic foodstuff in the daily diet of humans.
Hence, there is a great tendency and research challenge to explore the polyphenol content of all honey types produced in Hellas, so more representative results are to be obtained and ‘‘’honey dietary guidelines’’ may be then proposed.
References
- Council directive 2001/110/EC relating to honey. Official Journal of the European Communities, L 10/47-52. 2002; 01:12.
- Bikos A. All about Honey. Bee-shop.gr, Athens, Hellas. 1991; 147-150: 166- 169.
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary flavonoids and cancer risk in the Zutphen elderly study. Nutr Canc. 1994; 22: 175-184.
- Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001; 90: 157-177.
- Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008; 73: 117-124.
- Kassim M, Achoui M, Mustafa MR, Mohd MA, Yusoff KM. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr Res. 2010; 30: 650-659.
- Ikan R. Natural Products, a laboratory guide, Chapter: Acetogennins, 1-8, Academic Press Inc. Harcourt Brace Jovanovich, Publishers, 2nd edition. 1991.
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998; 56: 317-33.
- Tsiapara AV, Jaakkola M, Chinou I, Graikou K, Tolonen T, Virtanen V, Moutsatsou P, et al. Bioactivity of greek honey extracts on breast cancer (mcf-7), prostate cancer (pc-3) and endometrial cancer (ishikawa) cells: profile analysis of extracts. Food Chem. 2009; 116; 702-708.
- Karabagias IK, Vavoura MV, Nikolaou C, Badeka AV, Kontakos S, Kontominas MG, et al. Floral authentication of Greek unifloral honeys based on the combination of phenolic compounds, physicochemical parameters and chemometrics. Food Res Int. 2014; 62: 753-760.
- Spilioti E, Jaakkola M, Tolonen T, Lipponen M, Virtanen V, Chinou I, et al. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. Plos One. 2014; 9: 1-10.
- Karabagias IK, Dimitriou E, Kontakos S, Kontominas MG. Phenolic profile, colour intensity, and radical scavenging activity of Greek unifloral honeys. Eur Food Res Technol. 2016; 242: 1201-1210.
- Yao L, Datta N, Tomás-Barberán FA, Ferreres F, Martos I, Singanusong R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003; 81: 159-168.
- Nousias P, Karabagias IK, Kontakos S, Riganakos KA. Characterization and differentiation of Greek commercial thyme honeys according to geographical origin based on quality and some bioactivity parameters using chemometrics. J Food Process Pres. 2017.
- Tomás-Barberán FA, Martos I, Ferreres F, Radovic BS, Anklam E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J Sci Food Agr. 2001; 81: 485-496.
- Proestos C, Chorianopoulos N, Nychas E, Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J Agric Food Chem. 2005; 53: 1190-1195.
- Soler C, Gil MI, García-Viguera C, Tomás-Barberán FA. Flavonoid patterns of French honeys with different floral origin. Apidologie. 1995; 26: 53-60.
- Martos I, Ferreres F, Tomás-Barberán FA. Identification of flavonoid markers for the botanical origin of Eucalyptus honey. J Agr Food Chem. 2000; 48: 1498-1502.
- Truchado P, Tourn E, Gallez LM, Moreno DA, Ferreres F, Tomás-Barberán FA. Identification of botanical biomarkers in Argentinean Diplotaxis honeys: Flavonoids and Glucosinolates. J Agric Food Chem. 2010; 58: 12678-12685.
- Marshall SM, Schneider KR, Cisneros KV, Gu L. Determination of antioxidant capacities, a-dicarbonyls, and phenolic phytochemicals in Florida varietal honeys using HPLC-DAD-ESI-MSn. J Agric Food Chem. 2014; 62: 8623- 8631.
- Petretto GL, Cossu M, Alamanni MC. Phenolic content, antioxidant and physico-chemical properties of Sardinian monofloral honeys. Int J Food Sci Tech. 2015; 50: 482-491.
- Gil MI, Ferreres F, Ortiz A, Subra E, Tomás-Barberán FA. Phenolic compounds in rosemary honey and floral nectar. J Agric Food Chem. 1995; 43: 2833-2838.