
Special Article - Antioxidants in Foods
Austin J Nutri Food Sci. 2018; 6(3): 1107.
Physicochemical and Functional Properties of Ethiopian (Roba Variety) Peanut (Arachishypogaea L.) for Industrial Use
Gebremedhin G1,2*, Shimelis A², Tarekegn B³ and Cernak M4
¹Department of Food Process Engineering and Postharvest Technology, Ambo University, Ethiopia
²School of Chemical and Bioengineering, Addis Ababa Institute of Technology, Ethiopia
³Addis Ababa Science and Technology University, Ethiopia
4Department of Physical Electronics, Masaryk University, Czech Republic
*Corresponding author: Gebremedhin Gebremariam, Department of Food Process Engineering and Postharvest Technology, Ambo University, Ethiopia
Received: May 28, 2018; Accepted: June 27, 2018; Published: July 04, 2018
Abstract
Peanut (Arachishypogaea L.) is considered as a highly nutritious and as a functional food has been growing. Target tests and experiment were carried out to ascertain the physiochemical and functional property of peanut. Rova variety, Ethiopian peanut was analyzed for its oil content, fatty acid profiles, total phenolic content, along with antioxidant scavenging activity, peroxide value, unsaturated fatty acid, saturated fatty acid, acid value, refractive index, saponification and un saponification matter, microstructure, hardness, moisture, vitamin E (alpha), contact angle, iodine value, density, dynamic viscosity, flash, fire points, color (L*, a* and b*) and sensory evaluation. The mean value for the peanut analyzed were, oil: 53.73%, O/L ratio: 1.335, saturated fatty acid: 77.41%, unsaturated fatty acid: 22.05%, peroxide value: 1.56, iodine value: 89.23, Saponification value: 182.37, Refractive index: 1.45, density: 0.91, dynamic viscosity: 55.72mPa.s, flashpoint: 230.0, fire point: 245, total polyphenols: 200.23, and hardness: 109.90N. This indicates the peanut with high oil content, high antioxidant capacity, with the desired composition of fatty acids and vitamin E were identified which would be useful for the industrial purpose to develop nutritional superior peanut products.
Keywords: Peanut; Fatty acid profile; Functional properties; Microstructure; Physical properties
Introduction
The peanut (Arachis Hypogea L.) is an annual legume grown in the tropics and temperate regions around the globe [1] and widely consumed throughout the world and is one among the five widely grown oil crops in Ethiopia [2]. It is widely used as an economic food enhancement to counter malnutrition owing to its high nutritional value [3]. It is a globally important oilseed valued as a source of high-quality cooking oil, crude protein, crude fat, crude fiber, water, ash, total sugar, amino acids, fatty acids, vitamins, minerals, phytosterol, resveratrol, squalene, and other anti-nutritional factors [4] and appreciated worldwide as an affordable, flavorful, serving as a primary ingredient for peanut butter, confections, and nutritional bars, among other finished products.
The most attractive and impressive oilseed crop contains so many bio-constituents for human betterment such as protein, oil (linoleic acid and oleic acid, also a good source of Omega-6 fatty acids and Omega-3 fatty acids) and vitamin E are the most important, and they act on reactive oxygen species, as an anti-oxidant as a curative for early ageing of human being [5]. To use for the different purpose the local variety of peanut (Roba), the physicochemical and functional characteristics of the oil and the peanut seed should be quantified. The behavior of the peanut seed and oil helps to know dietary use, quality and shelf life determination. The peanut seed (Rova variety), no compositional data in terms of being dietary, physical, and function properties have been reported, and no studies have been conducted on its potential as a new variety. Therefore, the present study analyzed the physicochemical characteristics and functional properties of the peanut seed based on recent techniques and methods available, finally, the generated information use for processors, the exporters, breeders, as well as by the researchers engaged in improving the quality of Ethiopia peanut and in other world.
Materials and Methods
Chemicals and samples
n-Hexane, methanol, 95% ethanol, potassium hydroxide, sodium thiosulphate, potassium iodide, chloroform, glacial acetic acid, 2,2-Diphenyl-1-picryhydrzyl radical (DPPH), Folin-Ciocalteu, sodium carbonate, starch, BHT, ascorbic acid, petroleum ether, ethanol, acetone, Gallic acid standard, and vitamin E standard (a). A domestic, commercial peanut (Roba variety) was obtained from the Were Research Center, Oromia, Ethiopia.
Extraction methods
The extraction was performed in duplicate, with solvent, n-hexane (99% purity). An automated Soxhlet set (The Soxhlet extractor SXT- 06, Shaanxi, China) was used to extract peanut oil. To achieve this, 5g of sample was packed in a cartridge placed inside a 250ml extractor device. The sample was extracted for 8h at the solvent’s boiling point temperature. Then extra solvent form sample oil was removed by rotary vacuum evaporator. The extracted was stored in brown bottle at refrigerator for further analysis.
The total oil was calculated using the following;
Analysis of peanut oil
The Acid value, peroxide value, iodine number, saponification value and un saponification matter oil were determined according to standard methods (AOAC, 2010). Density and dynamic viscosity of peanut oil were measured at 20°C based on method [6]. The refractive index of seed oil was measured at 20°C using an Abbemat 550 refractometer and droplets of oil were added to the measuring prism using a disposable pipette. The Flash and Fire was measured using in frary gun thermometer, open cup, torch nozzle and heater based on ASTM method.
Fatty acid determination
The lipid fraction of peanut seed oil samples was extracted and fatty acids methyl esters were prepared according to [7] and the fatty acid profile was determined by gas chromatography with mass spectrophotometer (GC-MS).
Fatty acid methylation
In a 50mL round bottom flask fitted with reflux condenser, the Soxhet extracted oil (1gm) was placed and dissolved in 2% methanolic potassium hydroxide (10mL) prepared by mixing KOH with methanol. The mixture was heated on a water bath at 50°C for 1h. The reaction mixture was allowed to cool down to room temperature and saturated NaCl (3mL) was added to the reaction mixture and the solution was swirled gently several times. N-Hexane (20mL) was added into the solution and then the mixture was transferred to a separator funnel. The organic layer (upper layer) was separated, dried over anhydrous sodium sulfate and filtered through a man No.1 filter paper. The solvent was removed by rotary evaporation. The transesterified sample was prepared at 10ppm concentration in triplicate for GC-MS analysis.
GC–MS instrument
Oils extracted from peanut was be analyzed by gas chromatography (Agilent technologies 7820A GC system coupled with Agilent technologies 5977E MSD, USA). The chromatographic capillary column (HP-5) 30m long and 0.25mm in internal diameter was used. Ultra-high purity (99.999%) helium gas, as the carrier gas, was used at constant flow mode. An Agilent 7820A auto sampler was used to inject 1μL of the sample with a split less injection mode into the inlet heated to 275°C. Oven temperature was programmed with the initial column temperature of 60°C held for 2 min, and then, the temperature was increased at a rate of 10°C/min until the column temperature was reached 200°C, and then heated at the rate of 3°C/min till the temperature reached 240°C. No mass spectra were collected during the first 4 min of the solvent delay. The transfer line and the ion source temperature will 280°C and 230°C, respectively. The detector voltage wills 1600 V, and the electron energy will 70 eV. Mass spectra were collected from 40–600 m/z. The parameters, such as the quality, and probability values of peaks identified was made through a library search using NIST 2014. 1μL of oils peanut were injected into gas chromatography coupled with mass spectrometry (GC-MS) and each component of essential oils were identified by comparing its mass spectrum with reference data from the equipment database (NIST 2014 Mass Spectral Library).
Measurements of physical properties of peanut seed
Moisture content of whole peanut was dried in a forced air oven at 130°C for 6 hours based on the method of Young, Whitaker [8]. The weight differences before and after oven drying was used to calculate moisture content (MC; % dry weight).
Color measurement of peanut seeds
Surface color of peanut was measured using colorimeter, model of CM-700d/600d (Konica Minolta, INC, Japan) and recorded in L* (lightness/darkness), a* (redness/greenness), and b* (yellowness/ blueness) color values. The polycarbonate measuring dish was filled peanut samples to determine the average value of 10 replications. The illuminant was D65 and the standard observer was 10°. The colorimeter was calibrated using black and white.
Hardness of peanut seeds
The hardness of peanut sample was analyzed using Texture Analyzer model (TA plus machine, AMENTEK Lloyd Instruments Ltd, Forum House, UK) installed with the Nexygen Plus software. The compression was applied on a peanut sample placed on the plate using a cylindrical probe. The mean value of the maximum peak of the first compression (N) in the force-time curves were considered to evaluate hardness of peanut seeds. Five measurements were performed at each plasma experiments.
Contact angle measurement
The contact angle measurement was performed according to the method described by [9]. The surface wet ability of the peanut samples was evaluated at room temperature using DSA 30 (Kruss, Germany) software controlled system for quick measuring of static and dynamic contact angles sessile drop technique. A drop of ultra- pure water with a volume of 0.5μl was placed on the flat horizontal sample surface with a micro syringe and was immediately (within 1 s) and automatically photographed with a CCD camera, 61 fps (780 × 580px) or 311fps (780 × 60px). The software controlled x, y, z-axes of the sample with a resolution of 0.1°and contact angle ranges from1 to 180°. The contact-angle was computationally determined from the captured images and reported the average of at least five measurements placed on different peanut samples and at different positions on the peanut.
Microstructure of peanut seeds
Acording the [9], the peanut samples were placed on SEM stubs and were coated with a thin layer of platinum (Pt) before sputter (Q150R). After sputtering, the surface morphology of peanuts was examined by Scanning electron microscope (MIRA3 made by Tuscan, Brno, and is fully PC controlled SEM equipped with a Schottky Field Emission electron).
Analysis of Functional Properties of Peanut Seeds
Total phenol and antioxidant activities
Sample preparation for extraction: Peanut seeds were milled (High Speed Universal Disintegrator (FW100) Grinder, China) with a speed of rotating knife (2400rpm) and passed through a mesh size 16 sieve to obtain identically sized particles then, was retained in a sealed bag in a refrigerator (1-2°C) until use. Milled peanut seed particle size is important to facilitate analyses of mass transfer during the extraction of oil and antioxidant. Peanut seeds were defatted first with n-hexane (10% w/v) using a soxhlet extraction unit for 8h. The defatted samples were then air-dried and extracted with methanol (100mL) using an incubator shaker (Thermo Shaker Incubator, Model, THZ-103B, China). All suspensions were then filtered through a Whatman No.1 filter paper and the residues re extracted twice, each time with additional 100ml of the same solvent. The filtrates were combined and the solvent evaporated under reduced pressure using a rotary evaporator (Eyela, Model N-1000) at 40°C. The methanolic extracts were used for the determination of total polyphenol and antioxidant activity.
Extraction of peanut for analysis of the antioxidant and polyphenols: Samples were extracted based on the procedures used by Bishi, Lokesh [10]. Briefly, five gram of dried groundnut powder was extracted by stirring with 50ml of methanol at 25°C at 150rmp for 24hrs using temperature shaker incubator (ZHWY-103B) and then filtered through what man No. 4 paper. The residue was then extracted two wise with addition of 50ml methanol as the above procedure. The combined methanol extracts were evaporated at 40°C to dryness using rotary evaporator (Stuart R3300). The crude extracts were weighed to calculate the yield and re-dissolved in methanol at the concentration of 30mg/ml and stored in a refrigerator (-4°C), until used for further work.
Measurement of antioxidant activities and total polyphenol
Total polyphones contents (TPC) determination: A modified Folin-Ciocalteu procedure as described by [11] was used for the determination of total polyphenol contents. Samples (0.1mL) were mixed with 1.0mL of the Folin- Ciocalteu reagent (previously diluted with distilled water 1:10 v/v), and the reaction was terminated using 1mL of 7.5% sodium carbonate. The mixture was vortexed for 15sec for color development. After 30 min incubation at room temperature (28±1°C), the absorbance was measured at 765nm using a UV-VIS spectrophotometer (Perkin Elmer Lamda 950 UV/Vis/NIR). The standard curve was prepared Figures 1 & 2 using gallic acid standard solutions of known concentrations, a linear calibration graph was constructed with gallic acid concentrations of 20, 50, 100, 150, 200, and 250μg/ml and the results were expressed as mg gallic acid equivalent/100g sample.
C= total polyphenol content(mg/gm); c- concentration of gallic acid (mg/ml); V- volume of extract in assay (ml) M- mass of pure plant methanolic extract(gm).
Free Radical Scavenging Assay (DPPH): The effect of methanol extracts on DPPH radical was estimated according to Win, Abdul- Hamid [12]. A 0.004% freshly prepared solution of DPPH radical solution in methanol was prepared and then 4ml of this solution was mixed with methanol extract of sample. Finally, the samples were incubated for 30 min in the dark at room temperature. Scavenging capacity was read by spectrophotometric (Perkin Elmer Lamda 950 UV/Vis/NIR) by monitoring the decrease in absorbance at 517nm. This absorption maximum was first verified by scanning freshly prepared DPPH from 200-800 nm using the scan mode of the spectrophotometer. Free Radical Scavenging activity DPPH in percent (%) was then calculated below and the scavenging activity as shown in Figure 3.
where Ao is the absorbance of the control and A1 is the absorbance of the sample.
Vitamin E analysis (a): Vitamin E(a) concentration of the sample was measured according to [13]. For the separation of alpha vitamin E, the sample was saponified in the following way; 5.0g of sample was mixed with 10ml of 50% KOH in 40ml of ethanol at 95°C for 45 min and was added 2 drops of pyrogallol crystal (to prevent oxidation and serves as and antioxidant). Mix and agitate with 10min intervals. This solution was subsequently transferred to a separating funnel along with 40ml distilled water and 20mL ethanol successively in order to get rid of the aqueous phase. 40ml deionized water was added to the sample and transferred to the digested sample into the separating funnel. Next 20ml ethanol and 70ml petroleum ether was added to each sample and shake the sample following by mechanical shaker for 5minutes. After shaking wait for few minutes and separate the pure solution and transferred into another separating funnel. Then, 70ml hexane was added to the remaining digested sample and shakes again using the mechanical shaker for 5 minute wait to allow separating the pure solution from the separating funnel and transferred to the other separating funnel. 30ml hexane and 30ml petroleum ether was added to the sample to the remaining digested and shake as the previous principle and separated as previously procedure. Next, 100ml distilled water was added to the pure collected sample on the separating funnel for washing and separate the bottom solution and repeat 3 times. Checked using phenolphthalein indicator until colorless was occurring. The sample was filtered using what man No.540, 125mm only the bottom portion to remove if water remains. The filtered sample was transferred and collected to the round flask and evaporated using rotary vacuum evaporator (773mbar and 40°C), dried the remaining sample using nitrogen, reconstituted using the addition of 10ml methanol, sonicated with ultrasonic for two minutes and finally, transferred to brown vial glass for further analysis.
Quantification of Vitamin E (alpha): Vitamin E content was measured by reversed phase HPLC and DAD -detection at 292nm wavelength. 10μL of sample was injected into a reversed-phase C18 column (SBC18, 250mm ×3, 5μm i.d.). 98% methanol and 2% distilled water was used as a mobile phase during analysis with a flow rate of 1ml/min. The column was purged with methanol for 10 min after each sample analysis to remove the traces of impurities and residues. The vitamin E was identified by comparison with the standard. Standards of a vitamin E were diluted with methanol. The stock solutions of vitamin E for the procedure were 10, 20, 30, 40, 60 and 80 PPm respectively. An equal volume of vitamin E was mixed, diluted with methanol, and placed in an auto sampler vial. The mixture was vortexes before use. Its concentration was determined by the absorbance maximums of the solutions using according to Beer’s Law. Calculations of the unknown were done by comparison of peak areas and the calculated concentrations of the standard solutions as indicated in Figure 1.
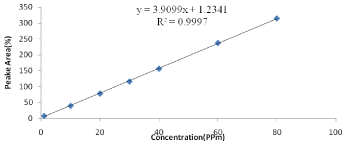
Figure 1: Calibration curve for standard solutions of Vitamin E (alpha).
Sensory evaluation
Randomly, peanut sample (five peanuts) was served in dishes withthree-digit coded and panelists were asked to evaluate samples for sensory acceptance based on different sensory attributes viz, color, odor, texture-touch, and overall appearance, then after were evaluated by 25 semi-trained panelists, using a 1-9 hedonic scale according to [14].
Analysis methods
All analytical measurements were conducted minimum in triplicate. Values of each parameter are expressed as mean ± standard deviation.
Results and Discussion
Chemical analysis of peanut oil
Surface oxidation and development of undesirable changes may occur in food from extreme doses of processing technologies that may modify the functions of fatty acids, inducing lipid oxidation. Table 1 shows fatty acid compositions of peanut oils were obtained. The fatty acids identified from peanut oil were 13.34% palmitic acid (C16:0), 4.47% stearic acid (18:0), 43.46% oleic acid (C18:1), 32.56% linoleic acid (18:2), 1.35% arachidic acid (20:0), 1.39% gadoleic acid (C20:1), and 2.89% behenic acid (22:0). This is in agreement with previously reported data [4,15]. The major fatty acids of the unsaturated fatty acids suggest that the peanuts oil is highly nutrient. The ratio of oleicto- linoleic acid (O/L) is a quality index employed to decide peanut shelf-life and oil stability. Ranging from 1 to 1.5, 1.5 to 9.0 and above 9.0, classified as normal, mid and high-oleic type, respectively [16]. The present study was carried out with normal oleic peanuts (O/L = 1.335). The total saturated fatty acids and unsaturated fatty acids in oil extracted from samples of peanut seed oil was 21.92% and 77.41%, respectively. As the Codex Alimentarius suggested that the archaic and higher fatty acid content of a rachis peanut oil should not exceed 48 g/kg [17].
Properties of peanut oil
Mean±SD
Palmitic (16:0)
13.34±0.85
Stearic (18:0)
4.47±0.32
Oleic (18:1)
43.46±0.72
Linoleic (18:2)
32.56±1.13
Arachidic (20:0)
1.35±.01
Gadoleic (20:1)
1.39±0.49
Behenic (22:0)
2.89±0.27
Unsaturated Acid in%
77.41
Saturated Fatty Acid %
22.05
USFA/SFA
3.510657596
O/L
1.335
Saponification value, mgKOH/g oil
192.37±1.23
Iodine value (Wijs), g/100g
89.23±2.12
Unsaponifiable matter, % (m/m), max
0.80±0.21
Oil content (%)
53.73±2.12
Free fatty acid in terms of oleic acid
0.262±0.02
Vitamin E(alpha),µg/kg
33.78±0.05
Peroxide value milli equivalents peroxide Oxygen/kg
1.56±0.20
Refractive index at 20?
1.45±0.01
specific gravity
0.911±0.01
Flash point ?
Fire point in ?
230.0±2.51
245.00±2.31
Density at 20?
0.910±0.91
Dynamic Viscosity, mPa.s at 20?
55.72±1.31
Table 1: Physicochemical properties of peanut oil.
Acidity value is an indicator for edibility of oil and suitability for industrial use and any extreme change could lead to an unwanted in influence on the sensory acceptability and shelf life of the treated food product. Peanut is a high oil content product (50–55%), with high unsaturated fatty acids, which are susceptible to oxidation [18,19]. The experimental result shows an acid value of 0.82mgKOH/g Table 1 in the peanut oil sample which means that there is a very low level of hydrolytic activity in the oil, and which is already in use for edible purpose and this falls within the recommended by Alimentarius [20] codex. Results obtained from this work indicated that the acid value of the peanut oil corresponds to low levels of free fatty acids present in the oil, which suggested low levels of hydrolytic and lipolytic activities in the oil.
Lipid oxidation is a complex process involving free radical chain mechanisms forming fatty per oxidation products [21] and Peroxide Value (PV) is important parameters for elucidating the peanut oil quality and assessing the oxidation extent. As Table 1 indicated, the PV produced from peanut oil was 1.56 mEqO2 kg-1 oil and it is low as the Codex Alimentarius Commission stipulated permitted maximum peroxide levels of 10 mEqO2 kg-1 oil [20]. This result shows that low content of peroxide value indicates that resist lipolytichydrolysis and deterioration.
Saponification value of oil extracted from peanut seed was 182.37mg KOH/g peanut oil. According to [22], saponification value is used in checking adulteration and high saponification value recorded for the oilseed suggests that the oils contain high molecular weight fatty acids and low level of impurities. The unsaponifiable matter of peanut oil contains 0.15–0.90% hydrocarbon sterol esters and 0.59–1.22% free sterols and in this experiment, unsaponification mater was 0.8% in Table 1.
The dynamic viscosity of a peanut oil sample was operated at 20°C. The result experimentally showed that at 20°C, the dynamic viscosity was 55.72mPa.s, as shown in Table 1. This type of viscosity affects by temperature [23-25]. A viscosity measurement helps to understand the stabilization of the viscosity of peanut oil at lower and higher temperature. Viscosity is a function of molecular size and structure with increases typically occurring for larger and/or more extended types of molecules [26]. It also a function of intermolecular fascinations between molecules, with stronger attractions properties of liquids with higher viscosities [27] and varies with differing degrees of unsaturation as Rodrigues, Cardoso [27] reported.
Iodine Value (IV) of the Rova variety was observed to be 89.23g/100g and the IV of peanut oil is the range of 86 -107 [17]. The result shows that peanut oil shears the highest value of unsaturated fatty acid which causes oxidation stability because of IV, which is a measure of unsaturation amount. As reported by [28] low IV have been associated with greatly enhanced shelf life and decreased rancidity The density of the peanut oil sample was obtained to check the level of peanut oil compatibility with water which means the ability of the oil to separate from water and result in the density at 20°C was 0.91g/ml which will help in the case of contamination caused by bacteria due to its mixture with water. The density of oil depends on fatty acid content and temperature of that oil [29].
As shown in Table 1, refractive index of Roba variety peanut oil was 1.45 and this result agreed with [30], and Refractive Index (RI) plays an important role in many branches of physics, biology and chemistry and knowledge of RI of oil is one of the crucial importance in applications of adulteration of oil and purity. This physical property has been used extensively in many applications to identify and characterize materials, including lipids. Relationships between RI and fatty acid, including chain length and degree of unsaturation have been observed for many years. Within creasing concentrations of unsaturated fatty acids, as commonly expressed by the iodine value, the RI of given oil will increase. The flashpoint in the experiment conducted was observed to be 230°C and the fire point of the peanut oil sample was observed to be 245°C. These properties measure the thermal stability of oil [31], based on this the oil sample obtained from peanut (Rova variety) shows a great response to the flame at elevated temperature.
Peanut is rich in vitamins including vitamin E and others. Vitamin E is the natural antioxidant and contains three isomers (a, ? and d) [4]. It is reported that the natural vitamin E (especially alpha) has the effects to enhance immunity, delay senescence, and reduce, the incidence of cardiovascular disease and cancer, and it has a significant impact on the stability (oxidative stability) of the oil. The Vitamin E (alpha), expressed as mg/100 g is presented in Table 1, was 33.78 and this result was similar with [32].
Physical and functional properties of peanut seeds
Polyphenols are common constituents in plant products and important antioxidants, which are contained, in large amounts, in peanut [33] and used as antifungal infections in peanuts. Polyphenols play a role in the prevention of degenerative diseases, mainly cardiovascular diseases, antiplatelet, anti-inflammatory, anticancer, antimutagenic, and antifungal properties and along with other properties also a potent antioxidant (reactive oxygen species scavenger) and metal chelators [34,35] and also are used as indicators to assess the degree of oxidation. The total polyphenol content of peanut seeds was 200.23mg Gallic acid/100g. This amount similar to kinds of literatures [36-38].
The DPPH free radical was used as substrate to evaluate the antioxidant activity of the peanut and activity has been studied in the present study as shown in (Figure 3). Although antioxidant activity is not a direct quality attribute used in the food industries, it is a close indicator of various polyphenols present in the food products. Radicle scavenging effects of phenolic compounds might be due to their redox characteristics, which includes possible mechanisms such as freeradical scavenging activity, transition metal-chelating activity and singlet-oxygen quenching capacity [39]. The synthetic antioxidants such as BHT and butylated hydroxyanisole are commonly used in processed foods. However, there has been growing concern over their safety and toxicity. Therefore, research on dietary intake of antioxidative substances and the assay of the natural antioxidant source as peanut and peanut product has attracted much attention. The activity of peanut sample was higher than that of synthetic antioxidant, BHA but lower than ascorbic acids. IC50 is half maximal inhibitory concentration and it is a measure of the effectiveness of a scavenging activity and the peanut sample has an ability to scavenge even at a lower concentration. This indicates peanut has good radical scavenging activity.
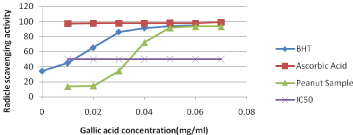
Figure 3: Free radical scavenging activity (DPPH).
Attree, Du [36] reported the moisture content of raw peanut seed ranged from 5 to 6% and our result was 5.38% as indicated in Table 2. The moisture content of peanut is a critical factor to be measured and controlled in its marketing, processing, and storage [40]. Additionally, it has apro found effect on its characteristics, texture, palatability, and consumer preferably, and preservation time, and related study indicated that moisture content accelerated the process of oxidative rancidity reactions, and further affected the product taste when the moisture is too high or too low. The optimum moisture content of peanut for storage (5.15%) according to [41] report.
Physical and functional properties peanut seed
Mean±SD
L*(lightness/darkness)
33.55±1.2
a*(redness/greenness)
13.67±0.23
b*(yellowness/blueness)
11.23±0.59
Moisture content(db, %)
5.38±0.10
Hardness(N)
109.90±0.78
Contact angle(%)
93.48±2.68
Total polyphenols
200.23±1.41
Sensory evaluation of peanut seed
Color
8.10±0.19
Odor
8.53±0.21
Texture-touch
8.01±0.11
Overall appearance
8.24±0.25
Table 2: Physical and functional properties peanut seed.
According to [42], the hardness is very important for nut processing and essential to optimize the conditions during roasting and other treatments. As a result, it is possible to evaluate the preferred physical quality characteristics of peanut during any treatment. Table 2 shows the hardness of peanut seed is 109.90 N. Understanding the hardness of the peanut helps to know the effect of processing operation parameters when subjected to different thermal and nonthermal treatments. During roasting, the hardness is the function of roasting temperature, moisture content, and strength of the peanuts, the air velocity and roasting time. Therefore based on the hardness of the initial peanut the processing operation parameters should be optimized without affecting the acceptable hardness of the peanut.

Figure 4: Microstructure of peanut.
Color is one of the most key physical appearances of food materials since it influences consumer preferences [43] and responsible for a final decision for purchasing by the consumer [44]. Color is one of the parameters that are used for process control during processing because the brown pigments will appear as the browning and caramelization reactions progress [45]. As indicated in Table 2, L*, a* and b* color values had an average of 33.55, 13.67 and 11.23, respectively.
According to [46], the contact angle formed between a water drop positioned on the surface of a material and its variation with time is related to the wet ability of the material. The possible reasons for contact angle measurement (a) the measure the degree of contamination, or clean surface, (b) surface roughness determination and (c)surface reconstruction measurement (the surface itself may change in the presence of the liquid).
The surface microstructure of peanut was investigated by Scanning Electron Microscope (SEM) at different magnification levels ×500, ×1000, ×2000, and ×5000, respectively as shown seen in Figure 2. Examination of the peanut seed microstructure is important in that physical changes at this level are associated with the maturity of peanut seed, the environmental conditions, well as the processing procedures [47]. Observing the microstructure changes during peanut processing aids in the understanding of the thermal modifications, such as cell wall rupture, protein and starch body distension, and cytoplasmic network disruption [47].
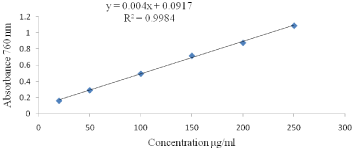
Figure 2: Gallic acid standard curve for the calculation of total polyphenols
content.
During processing of peanut, may change the natural surface morphology of peanuts resulting changes in surface color, weight loss, textural parameters and wet ability. Peanut with the surface quite smooth, with few pores, except for some occasional cracks, low weight loss and comparatively higher moisture content of peanuts, and this is responsible for structural compactness, more granules of larger size than peanut but adverse processing conditions change the surface topology from smooth to rough, the loss of cell structure and release of substances from damaged cell compartments, enhances the mass transfer and facilitates access and migration of vapor moisture into the peanut tissue and diffused out from the peanuts which caused the disintegration of larger globules and was observed and this might have facilitated the creation of microspores on the surface [48]. Different scholars have investigated the iner and the outer structure of peanut, such as, the effects of maturity and food processing [47,49,50], characteristics of the epidermal tissue is reported by [49], the shape and size of epidermal cells determined by [49], also the volume of the cotyledon were determined [47,49,51]. The network of protein bodies, starch grains, and lipid granules is of peanut also measured [49]. Therefore, the study of microstructure of peanut helps to know the change during processing, especially during roasting.
Sensory attributes were scored to assess the sensory quality the peanut seeds because the first quality judgment made by a consumer on food at the point of sale and consumer is its visual appearance. Physical appearance analyses of peanut (color, odor, texture and overall appearance) were used in the maintenance of peanut physical quality. The quality appearance peanuts almost was maintained and scored in the rage of like very much Table 2. Total acceptance is critical because of the specific quality attributes that attract people [52]. Flavor of the peanut that ultimately increases the overall palatability of the products [53,54]). Hardness uses to evaluate the hardness of solid foods, the food is placed between the molar teeth and the panelist bites down evenly, evaluating the force to compress the food [55]. Roasting process changes the internal microstructure of samples, resulting in a texture that is typically more brittle, crispy, and/or crunchy [56]. Reported that high roasting temperatures effect on carbohydrates, proteins and oil stability and ultimately decrease overall palatability of products.
Conclusion
With the ever-growing health-consciousness of the consumers and industrialization, the need for identifying best variety is also growing. Varieties with high oil content, high O/L ratio, and antioxidant capacity, with desired composition of fatty acids and others important compounds are required in the current time, and which would be useful for the future breeding programme, research and processing in order to develop nutritional superior peanut varieties/ and peanut food products. Further, the produced information about peanut variety will be a concern for health concern people; hence can be utilized by peanut traders, peanut processors, and consumers.
The current study result indicated that Ethiopian peanut variety (Roba) has good antioxidants activities, contains high mono and polyunsaturated fatty acids, promised vitamin E, with good total polyphenols with other chemical and physical properties which are acceptable for the consumer. Therefore, the utilization of Roba variety in diet will give best results in combating diseases like cancer, diabetes and cardiovascular diseases based on the results indicated. In addition, knowledge of the viscosity, density, refractive index, microstructure, iodine value, peanut seed surface color, moisture, hardness, contact angle, flash and fire point and sensory acceptance of the peanut variety will help to optimize the processing parameters during processing and handling of peanut seed and peanut oil without affecting the desired quality.
Acknowledgement
We are appreciates Center for Food Science and Nutrition, Addis Ababa University, Ethiopian-Conformity Assessment Enterprise and Department of Physical Electronics, Masaryk University for the use of their laboratory equipment and assistance given.
References
- Bertioli DJ, Seijo G, Freitas FO, Valls JFM. An overview of peanut and its wild relatives. Plant Genetic Resources. 2011; 9: 134-149.
- Wijnands JHW, Biersteker J, Van Loo EN. Oilseeds business opportunities in Ethiopia. 2009.
- Sarvamangala C, Gowda MVC, Varshney R. Identification of quantitative trait loci for protein content, oil content and oil quality for groundnut (Arachis hypogaea L.). Field crops research. 2011; 122: 49-59.
- Wang Q. Peanut Processing Characteristics and Quality Evaluation. Springer. 2017.
- Quamruzzaman, Jafar Ullah, Jahedur Rahman, Rajesh Chakraborty, Mahfuzar Rahman, Golam Rasul. Organoleptic assessment of groundnut (Arachis hypogaea L.) as influenced by boron and artificial lightening at night. World Journal of Agricultural Sciences. 2016; 12: 1-6.
- Davis JP, Dean LN, Faircloth WH, Sanders TH. Physical and chemical characterizations of normal and high-oleic oils from nine commercial cultivars of peanut. Journal of the American Oil Chemists' Society. 2008; 85: 235-243.
- Li SS, Yuan RY, Chen LG, Wang LS, Hao XH, Wang LJ, et al. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC–MS. Food Chemistry. 2015; 173: 133-140.
- Young JH, Whitaker TB, Blankenship PD, Brusewitz GH, Troeger JM, Steele JL, Person NK. Effect of oven drying time on peanut moisture determination. Transactions of the ASAE. 1982; 25: 491-496.
- Štepánová V, Kelar J, Slavícek P, Chlupova S, Stupavska M, Jurmanova J, et al. Surface modification of natural leather using diffuse ambient air plasma. International Journal of Adhesion and Adhesives. 2017; 77: 198-203.
- Bishi SK, Lokesh K, Mahatma MK, Khatediya N, Chauhan SM, Misra JB. Quality traits of Indian peanut cultivars and their utility as nutritional and functional food. Food Chemistry. 2015; 167: 107-114.
- Singleton VL, JA Rossi. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American journal of Enology and Viticulture. 1965; 16: 144-158.
- Win MM, Hamid AA, Baharin BS, Dek MSP. Phenolic compounds and antioxidant activity of peanut’s skin, hull, raw kernel and roasted kernel flour. Pak J Bot. 2011; 43: 1635-1642.
- Zaman W, Akram M, Rehman R, Anwar J. Statistical Analysis and Quantification of alpha tocopherol in edible seeds and nuts of pakistan by reversed phase HPLC with UV/Visible Detector. J Chem Soc Pak. 2012; 34: 302-305.
- Basaran P, Basaran-Akgul N, Oksuz L. Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiology. 2008; 25: 626-632.
- Jubeen F, Bhatti IA, Maqbool U, Mehboob S. Fungal Incidence, Aflatoxin B 1, Tocopherols and Fatty Acids Dynamics in Ground and Tree Nuts during Storage at Two Moisture Levels. International Journal of Agriculture & Biology. 2012; 14.
- Shin EC, Craft BD, Pegg RB, Phillips RD, Eitenmiller RR. Chemometric approach to fatty acid profiles in Runner-type peanut cultivars by principal component analysis (PCA). Food Chemistry. 2010; 119: 1262-1270.
- Alimentarius C. Codex standard for named vegetable oils. Codex Stan. 1999; 210: 1-13.
- Nepote V, Mestrallet MG, Grosso NR. Oxidative stability in fried-salted peanuts elaborated with high-oleic and regular peanuts from Argentina. International journal of food science & technology. 2006; 41: 900-909.
- Olmedo R, Nepote V, Mestrallet MG, Grosso NR. Effect of the essential oil addition on the oxidative stability of fried–salted peanuts. International journal of food science & technology. 2008; 43: 1935-1944.
- Alimentarius C. Codex Alimentarius Standards for Fats and Oils from Vegetable Sources. Section 2. Codex Alimentarius. 1999.
- Ladikos D, Lougovois V. Lipid oxidation in muscle foods: a review. Food Chemistry. 1990; 35: 295-314.
- Al-Bachir M. Quality characteristics of oil extracted from gamma irradiated peanut (Arachis hypogea L.). Radiation Physics and Chemistry. 2014; 106: 56-60.
- Fasina OO, Hallman H, Craig-Schmidt M, Clements C. Predicting temperature-dependence viscosity of vegetable oils from fatty acid composition. Journal of the American Oil Chemists' Society. 2006; 83: 899.
- Santos JCO, Santos IMG, Souza AG. Effect of heating and cooling on rheological parameters of edible vegetable oils. Journal of Food Engineering, 2005; 67: 401-405.
- Rabelo J, Batista E, Cavaleri FVW, Meirelles AJA. Viscosity prediction for fatty systems. Journal of the American Oil Chemists' Society. 2000; 77: 1255-1262.
- Teeter HM, Cowan JC. Viscometric properties of higher fatty acids and their derivatives. Journal of the American Oil Chemists Society. 1956; 33: 163-169.
- Rodrigues JdA, Cardoso FdP, Lachter ER, Estevão LRM, Lima E, Nascimento RSV. Correlating chemical structure and physical properties of vegetable oil esters. Journal of the American Oil Chemists' Society. 2006; 83: 353-357.
- Wang Y, Sun D, Chen H, Qian L, Xu P. Fatty acid composition and antioxidant activity of tea (Camellia sinensis L.) seed oil extracted by optimized supercritical carbon dioxide. Int J Mol Sci. 2011; 12: 7708-7719.
- Noureddini H, Teoh BC, Clements LD. Densities of vegetable oils and fatty acids. Journal of the American Oil Chemists Society. 1992; 69: 1184-1188.
- Davis JP, Sweigart DS, Price KM, Dean LL, Sanders TH. Refractive index and density measurements of peanut oil for determining oleic and linoleic acid contents. Journal of the American Oil Chemists' Society. 2013; 90: 199-206.
- Bhuiyan TH, Chowdhury MN, Akter R, Khan M. Determination of Thermophysical Properties of Edible Oil at High Temperature Using Differential Scanning Calorimetry (DSC). Middle-East Journal of Scientific Research. 2016; 24: 3302-3306.
- Zhang J. Effects of fatty acid composition and endogenous antioxidants on the stability of different peanut oils [D]. Beijing: Chinese Academy of Agricultural Sciences. 2012.
- Tov SY, Badani H, Segev A, Hedvat I, Galili S, Hovav R. Determination of total polyphenol, flavonoid and anthocyanin contents and antioxidant capacities of skins from peanut (Arachis hypogaea) lines with different skin colors. Journal of Food Biochemistry. 2011; 36: 301-308.
- Chukwumah Y, Walker LT, Verghese M. Peanut skin color: a biomarker for total polyphenolic content and antioxidative capacities of peanut cultivars. Int J Mol Sci. 2009; 10: 4941-4952.
- Akhtar S, Khalid N, Ahmed I, Shahzad A, Suleria HA. Physicochemical characteristics, functional properties, and nutritional benefits of peanut oil: a review. Critical reviews in food science and nutrition. 2014; 54: 1562-1575.
- Attree R, B Du, B Xu. Distribution of phenolic compounds in seed coat and cotyledon, and their contribution to antioxidant capacities of red and black seed coat peanuts (Arachis hypogaea L.). Industrial Crops and Products. 2015; 67: 448-456.
- Kornsteiner M, Wagner KH, Elmadfa I. Tocopherols and total phenolics in 10 different nut types. Food Chemistry. 2006. 98: 381-387.
- Yang J, RH Liu, L Halim. Antioxidant and antiproliferative activities of common edible nut seeds. LWT-Food Science and Technology. 2009; 42: 1-8.
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005; 53: 7749-7759.
- Kandala CV, Sundaram J. Nondestructive moisture content determination of three different market type in-shell peanuts using near infrared reflectance spectroscopy. Journal of Food Measurement and Characterization. 2014; 8: 132-141.
- SHEN Xx, et al. Effects of Water Content on the Quality of Peanuts during Storage. Modern Food Science and Technology. 2011; 5.
- Eser E, Ekiz HI. Surface temperature a critical parameter to control peanut quality during far infrared pretreatment process. International Food Research Journal. 2016; 23: 2130-2137.
- Maskan M. Kinetics of color change of kiwifruits during hot air and microwave drying. Journal of Food Engineering. 2001; 48: 169-175.
- Lee HJ, Song HP, Jung HS, Jo C. Effect of atmospheric pressure plasma jet on inactivation of Listeria monocytogenes, quality, and genotoxicity of cooked egg white and yolk. Journal for Food Science of Animal Resources. 2012; 32: 561-570.
- Moss JR, Otten L. A relationship between colour development and moisture content during roasting of peanuts. Canadian Institute of food science and technology journal. 1989; 22: 34-39.
- Yan D, Jones J, Yuan XY, Xu XH, Sheng J, Lee JC, et al. Plasma treatment of electrospun PCL random nanofiber meshes (NFMs) for biological property improvement. J Biomed Mater Res A. 2013; 101: 963-972.
- Young CT, Schadel WE. Microstructure of peanut seed: a review. Food structure. 1990; 9: 3.
- Perren R, Escher F. Impact of roasting on nut quality, in Improving the safety and quality of nuts. 2013: 173-197.
- Young CT, Pattee HE, Schadel WE, Sanders TH. Microstructure of peanut (Arachis hypogaea L. cv.‘NC 7’) cotyledons during development. LWT-Food Science and Technology. 2004; 37: 439-445.
- Young CT, WE Schadel. A comparison of the effects of oven roasting and oil cooking on the microstructure of peanut (Arachis hypogaea L. cv. Florigiant) cotyledon. Food structure. 1993; 12: 7.
- Young CT, Schadel WE. Microstructure of peanut (Arachis hypogaea L. cv. Florigiant) cotyledons after oil cooking. Journal of food science. 1991; 56: 76-79.
- Mazumder P, Roopa BS, Bhattacharya S. Textural attributes of a model snack food at different moisture contents. Journal of Food Engineering. 2007; 79: 511-516.
- Mayerpotschak K. Infrared roasting of nuts, particularly hazelnuts. Confectionery production. 1985; 51: 313-313.
- Buckholz LL, Daun H, Stier E, Trout R. Influence of roasting time on sensory attributes of fresh roasted peanuts. Journal of Food Science. 1980; 45: 547-554.
- Bourne M. Food texture and viscosity: concept and measurement. Elsevier. 2002.
- Lee CM, Resurreccion AVA. Resurreccion, Predicting sensory attribute intensities and consumer acceptance of stored roasted peanuts using instrumental measurements. Journal of food quality. 2006; 29: 319-338.