
Special Article - Probiotics and Functional Foods
Austin J Nutr Metab. 2017; 4(1): 1046.
Transcriptomic Analysis of PBMCs from Allergic Patients after Probiotic Treatment
Bourdeau T¹*, Spertini F², Raymond F³, Audran R², Gosoniu L4, Mercenier A5, Nutten S1, Blanchard C1 and Elliott R6
¹Department of Allergy, Nutrition and Health Research, Nestle Research Center, Switzerland
²Division of Immunology and Allergy, Centre Hospitalier Universitaire Vaudois, Switzerland
³Department of Functional Genomics, Nestle Institute of Health Science, Switzerland
4Department of Biostatistics & Data Mgmt, Clinical Data Unit, Nestle Research Center, Switzerland
5Host-Microbe Interaction, Nutrition and Health Research, Nestle Research Center, Switzerland
6Institute of Food Research, Norwich Research Park, UK
*Corresponding author: Bourdeau T, Department of Immunology, Nutrition and Health Research, Nestle Research Center, Route du Jorat 57, PO Box 44, CH-1000 Lausanne 26, Switzerland
Received: February 01, 2017; Accepted: March 27, 2017; Published: April 25, 2017
Abstract
Background: Allergic rhinitis is one of the most prevalent manifestation of allergy, affecting over 15% of the population worldwide. Recent published clinical studies have shown that specific probiotics can improveallergic rhinitis clinical symptoms.
Findings: In this study, thirty one adult volunteers suffering from allergic rhinitis were enrolled in a crossover study evaluating the efficacy of the consumption of a product containing either L. paracasei-fermented milk or the placebo. Transcriptomic analysis was performed on unstimulated PBMC after each treatment period and analysis was adjusted for the crossover design. No differences were observed between PBMCs from probiotic treated allergen challenged allergic patients and PBMCs from placebo treated allergen challenged allergic patients.
Conclusion: This study shows that, in the blood compartment, PBMCs mRNA levels are too stable to mirror the changes of symptoms and alteration of cytokine expressions observed after a treatment with L. paracasei.
Trial Registration: NCT01150253. Registered 23 June 2010.
Keywords: Allergic rhinitis; Probiotic; Grass pollen; PBMC; Transcriptomic
Abbreviations
PBMC: Peripheral Blood Mononuclear Cell; RT-Q-PCR: Reverse Transcription-Quantitative-Polymerase Chain Reaction; IL: Interleukin; NPT: Nasal Provocation Test, B2M: Beta-2- Microglobulin
Introduction
Allergic rhinitis prevalence has dramatically increased over the last past decades reaching up to 15.7% of the populations [1]. Pharmacologic treatments for allergic rhinitis are available but with some side effects that affects the quality of life of the patients. Probiotics have been largely tested in the context of atopic dermatitis, allergic rhinitis and food allergy prevention or treatment [2-7] and have been shown to modulate the immune system dampening the Th2 response and potentiating T regulatory cell population and IL-10 production [8]. Ex vivo, Th2 skewed PBMCs stimulated with probiotics such as L. paracasei NCC2461 ST11 show an increase in TNF-α, IFN-ɣ and IL-10 productions and decrease in IL-5 production [9], or ex vivo in atopic patients [10,11]. Epigenetic mechanisms involving RNA stability have been described with L. paracasei NCC2461-stimulated PBMCs [12].Two previous clinical trials (CT) have shown that the probiotic L. paracasei NCC2461 can improve the nasal congestion or the nasal pruritus after a nasal provocation test [13,14]. Both trials strongly suggested that the consumption of L. Paracasei NCC2461 may benefit grass pollen induced allergic reaction. In the second trial [14], designed as a randomized double-blind placebo controlled crossover study, 31 adult volunteers, aged between 18 and 35 years old, suffering from allergic rhinitis were enrolled. The crossover study was based on two-times 4-week periods of product consumption either with L. paracasei-fermented milk or with the placebo, separated by a wash-out period of 6 weeks (Figure 1). This clinical trial demonstrated a significant reduction in nasal congestion in subjects receiving fermented L. paracasei milk compared to placebo treated subjects. Additionally, lower IL-5 expression level in grass pollen restimulated peripheral blood mononuclear cells (PBMCs) was found associated with the consumption of L. paracasei-fermented milk and with the decreased in nasal congestion. Similar decrease was observed for allergen specific immunoglobulin G4 (IgG4) level in the L. paracaseifermented milk treated group. Nasal pruritus was improved in the group treated with L. paracasei-fermented milk but without reaching significant difference.
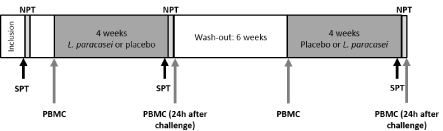
Figure 1: Schematic design of the crossover, placebo-controlled study.
Double blind and cross over study for comparison of two interventions (with
or without L. paracasei). SPT: Skin Prick Test; PBMC: Peripheral Blood
Mononuclear Cells; NTP: Nasal Provocation Test.
In order to test whether PBMCs could be used as a predictor of symptom response, we sought to identify whether the L. paracaseifermented milk treatment was able to modify the gene expression profile of unstimulated PBMCs compared to the placebo, and to correlate these gene expression levels with the cell counts in nasal fluids, immunoglobulin levels and cytokine expressions in grass pollen re-stimulated PBMCs.
Materials and Methods
RNA extraction and transcriptomics analysis
RNA was prepared from PBMC (107 cells) using RN Easy midi kits (Qiagen, Venlo, Netherlands), incorporating a DNase1 digestion step, according to the manufacturer’s instructions. Total RNA yield was determined using a NanoDrop® ND-1000 spectrophotometer (Thermo Scientific, Waltham, Massachusetts, United States) and purity assessed by the ratios of absorbance at 260:280 and 260:230 nm. RNA integrity was assessed using Agilent RNA6000nano LabChips® (Agilent Technology 2100 Bioanalyzer Version A.01.20 SI211, Santa Clara, California, USA). The RNA samples were aliquoted (10 μg) and stored at -80°C. RNA were prepared according to manufacturer’s instruction for the hybridization to Affymetrix human U133 Plus 2 arrays (Santa Clara, California, USA). The raw data for all the 48 arrays (.CEL files) were uploaded into GeneSpring 9.0.6 (Agilent Technologies, Santa Clara, California, USA) and analyzed. Statistical analyses (paired t-tests incorporating Benjamini and Hochberg multiple test correction) were then used to perform a number of specific group comparisons.
Real time PCR-PCT
Reverse transcription was performed using qScript reverse transcriptase (Invitrogen, Carlsbad, California, United States) and quantitative real-time polymerase chain reaction (RT-Q-PCR) was carried out using Platinum SYBR Green qPCR Supermix-UDG with ROX (Invitrogen) on an ABI Prism 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, California, United States). The PCR was performed with 40 cycles of the following conditions: denaturation for 15s at 95°C, annealing for 15s at 60°C and extension for 30s at 72°C. The primer sets used to perform real-time PCR are listed in Table 1. The mRNA sequences were used in the Primers3 software (https://simgene.com/Primer3) to select pairs of matching primers. Synthetic primers were obtained by Microsynth AG (Balgach, Switzerland). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the house-keeping gene and the results were calculated using a standard curve for each gene. Results are then expressed as a ratio compared to the house keeping gene.
Gene
Forward primer (3'-5')
Reverse primer (5'-3')
B2M
TTCATCCATCCGACATTGAA
CCTCCATGATGCTGCTTACA
IL5
GAGACCTTGGCACTGCTTTC
CAGTACCCCCTTGCACAGTT
IL6
AGGAGACTTGCCTGGTGAAA
CAGGGGTGGTTATTGCATCT
IL8
TAGCAAAATTGAGGCCAAGG
AAACCAAGGCACAGTGGAAC
INFg
TCCCATGGGTTGTGTGTTTA
AAGCACCAGGCATGAAATCT
TNFAIP6
TCACATTTCAGCCACTGCTC
AGACCGTGCTTCTCTGTGGT
TNFRSF17
GCAGTGCTCCCAAAATGAAT
GTCCCAAACAGGTCCAGAGA
PTGS2
TGAGCATCTACGGTTTGCTG
TGCTTGTCTGGAACAACTGC
GAPDH
GAGTCAACGGATTTGGTCGT
TTGATTTTGGAGGGATCTCG
Table 1: Human primers pairs used for real-time PCR.
Cytokines expression
Cytokines (IL-4, IL-5, IL-8, IL-10 and IFN-g) were measured on grass pollen re-stimulated PBMCs as previously described [14]. Detection limits of the multiplexed bead-based flow cytometry assay were 0.6, 0.2, 0.55, 1.19 and 0.87 pg/L, respectively.
Statistical analysis
PCR cytokines expression levels and correlations were performed using R version 3.1.2 (https://cran.r-project.org/bin/windows/base/ old/3.1.2/) and graphs generated with GraphPad Prism 7 (GraphPad Software, Inc. La Jolla, California, USA).
Results
Transcriptomic analysis using Affymetrix human U133 Plus 2 arrays was performed and adjusted for the crossover design (Figure 1). Comparison of the ~47,000 transcripts expression profiles for samples collected immediately prior to both interventions identified no significant differences between the groups when a false discovery rate was applied (FDR<0.05) in the statistical analysis. Due to the challenge of detecting the often small changes in gene transcription resulting from nutritional interventions, such comparisons are often reported without corrections for multiple tests. However, even with the false discovery rate omitted from the analysis, the number of significant genes (p<0.05) was lower than the expected number of false positive tests for the number of comparisons performed before and after the intervention with placebo or L. paracasei. Similarly, no genes were identified as changing in expression as a result of the L. paracasei-fermented milk intervention compared to placebo when the false discovery rate was applied. However, in this case, when the false discovery rate was omitted, the number of genes for which the uncorrected p values was <0.05 was substantially higher (approximately 3-fold) than the expected number of false positive tests for the number of comparisons performed.
Because some cytokine transcripts are not always detected using microarray techniques, we used a candidate approach. To address this issue, a subset of candidate genes was selected based on the transcriptomic results and the known Th2 cytokines responding to probiotics intervention (IL-8; IFNγ). No significant differences were observed between the placebo group and the L. paracasei-fermented milk treated group for the different molecular markers analyzed by RT-Q-PCR in PBMCs (Figure 2) thus confirming the null findings obtained by transcriptomics. Interestingly, a negative correlation (Spearman’s r=-0.63, p=0.03) between interleukin-8 (IL8) mRNA expression and IL-8 secretion in the supernatant of grass pollen re-stimulated PBMC was observed in the L. paracasei-fermented milk treated group, while no statistical association was found for the placebo treatment (Spearman’s r=0.58, p=0.05, Figure 3A). A significant positive correlation was also observed with IFNG mRNA level in PBMC and interferon-γ (IFN-γ expression level in grass pollen re-stimulated PBMC in the placebo group (Figure 3B).
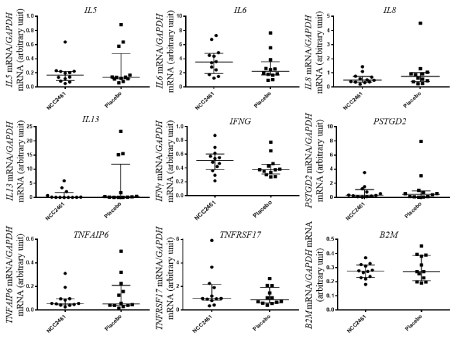
Figure 2: Relative mRNA expression of candidate genes. Effect of L. paracasei NCC2461 and placebo treatment of inflammatory cytokine gene expression in
PBMC. IL5, IL6, IL8, IL13, IFNG, TNFAIP6 and TNFRSRF17 mRNA expression levels were measured by RT-Q-PCR. Results are expressed as ratio relative to
housekeeping gene GAPDH.
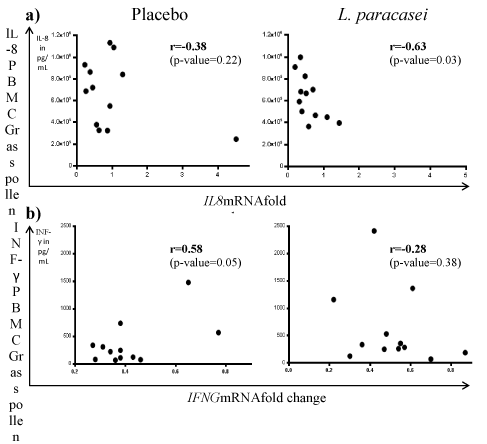
Figure 3: Correlation between mRNA and protein expression level in PBMCs. Comparison of cytokine expression in restimulated PBMC with grass pollen and
mRNA expression level by quantitative PCR in unstimulated PBMCs. Correlation ofIL-8 (a) and IFN-γ (b) for both the placebo and L. paracasei treated group are
shown.
Correlations between interleukin-13 (IL13), interleukin-6 (IL6) and tumor necrosis factor alpha induced protein 6 (TNFAIP6) expression and neutrophil, leucocyte and eosinophil cell counts in nasal fluids, IgE in serum, and cytokine expressions in grass pollen restimulated PMBCs were all non-significant (data not shown).
Discussion
Transcriptomics analyses have been widely used in human tissues including some studies with PBMCs [15,16]. Persson H, et al. have recently demonstrated differential expression levels in therapy sensitive asthma patients versus treatment refractory asthma patients using peripheral blood leucocytes transcriptomic [17]. However blood PBMC is a mix of different cells that seems to be very well controlled for its overall gene expression [18]. Interestingly, amore recent study has shown that even when cells were isolated, proteomics emphasized only mild differences between allergic and non-allergic individuals [19]. It is thus not surprising retrospectively, that no differences were observed between PBMCs from probiotic treated allergen challenged allergic patients and PBMCs from placebo treated allergen challenged allergic patients. The intervention with L. paracasei-fermented milk may have influenced gene expression profiles but the effect was subtle resulting in a difficulty to separate real effects on the level of individual genes from random effects due to biological noise. It is possible that only a small subset of cells did respond to the probiotic treatment but the response may be diluted in the whole PBMC leading to an undetectable signal when looking at transcriptomic analysis of nonstimulated PBMCs. All together, these results suggest that the gene expression levels were highly stable in all individuals no matter the timing in the protocol or treatment at the time of the sampling. The negative correlation of IL8 mRNA and IL-8 secretion level in the supernatant of grass pollen re-stimulated PBMCs suggests that PMBC mRNA level for IL8 may inversely predict the responses in re-stimulated PBMCs in L. paracasei-fermented milk treated group. The discrepancy between the observed IL-8 results could be explained by the nature of the probiotics used. Depending on the physiological state of a given probiotic, specific mRNA may be specifically stabilized hence creating variations in effects after a probiotic treatment [12].
One should keep in mind that the results of the clinical trial have shown significant but mild decrease of clinical symptoms possibly explaining the lack of differences in our transcriptomic analysis. Another limitation of the study is the absence of results before the nasal provocation test (NPT) as the clinical trial was designed to assess clinical and molecular parameters (including gene expression in PBMCs) before and after probiotic treatment. As such the lack of PBMCs analysis before the NPT prevent analysis of the probiotic treatment efficacy on gene expression in PBMCs before challenge. Additionally the PMBCs were not stimulated ex vivo with the allergens, while this could have led to results better correlated to the cytokine expressions quantified after restimulation. Whalen K, et al. have shown that restimulated PBMC transcriptomic could separate healthy from allergic patients [20]. However the goal here was to assess baseline unstimulated gene expression changes that could be responsible for the differences observed ex vivo on cytokines secretion levels following ex vivo re-stimulation with the grass pollen extract.
Taken altogether, these results suggest that in the context of this probiotic intervention, the blood compartment and more specifically PBMC mRNA level is too stable to reflect the subtle changes of symptoms and alteration of cytokine expression triggered by a treatment with L. paracasei. The effect of a probiotic treatment on the systemic compartment may be too tenuous to reflect the clinical symptoms alterations.
Acknowledgement
We would like to thank the patients that participated to this study and Robert Mansourian for technical assistance with the transcriptomic analysis.
Declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Centre Hospitalier Universitaire Vaudois Ethics Committee. NCT01150253.
Consent for publication
All patients consented to participate to the study and gave their written approval and the study was performed in accordance with the principle of the Declaration of Helsinki.
Funding
The study was supported by Nestle Research Center.
Authors’ contributions
All authors approved the final manuscript. SN, RE, RA and AM participated in the study design or the conduct of the clinical trial. FS was principal investigator for the study. RE, CB and FR analyzed the transcriptomics profiles. TB analyzed the gene expression levels relevant to this publication and drafted the text. LG performed the correlation and the statistical analysis. CB led the writing of this manuscript.
References
- Genuneit J, Seibold AM, Apfelbacher CJ, Konstantinou GN, Koplin JJ, La Grutta S, et al. Overview of systematic reviews in allergy epidemiology. Allergy. 2017.
- Eigenmann PA. Evidence of preventive effect of probiotics and prebiotics for infantile eczema. Curr Opin Allergy Clin Immunol. 2013; 13: 426-431.
- Kalliomäki M, Antoine JM, Herz U, Rijkers GT, Wells JM, Mercenier A. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of allergic diseases by probiotics. J Nutr. 2010; 140: 713S-721S.
- Kim SO, Ah YM, Yu YM, Choi KH, Shin WG, Lee JY. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014; 113: 217-226.
- Cuello-Garcia CA, Brożek JL, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Terracciano L, et al. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015; 136: 952-961.
- Fiocchi A, Pawankar R, Cuello-Garcia C, Ahn K, Al-Hammadi S, Agarwal A, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ J. 2015; 8: 4.
- Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for the treatment of eczema: a systematic review. Clin Exp Allergy. 2009; 39: 1117-1127.
- Klaenhammer TR, Kleerebezem M, Kopp MV, Rescigno M. The impact of probiotics and prebiotics on the immune system. Nat Rev Immunol. 2012; 12: 728-734.
- Holvoet S, Zuercher AW, Julien-Javaux F, Perrot M, Mercenier A. Characterization of candidate anti-allergic probiotic strains in a model of th2- skewed human peripheral blood mononuclear cells. Int Arch Allergy Immunol. 2013; 161:142-154.
- Prescott SL, Dunstan JA, Hale J, Breckler L, Lehmann H, Weston S, et al. Clinical effects of probiotics are associated with increased interferon-gamma responses in very young children with atopic dermatitis. Clin Exp Allergy. 2005; 35: 1557-1564.
- Snel J, Vissers YM, Smit BA, Jongen JM, van der Meulen ET, Zwijsen R, et al. Strain-specific immunomodulatory effects of Lactobacillus plantarum strains on birch-pollen-allergic subjects out of season. Clin Exp Allergy. 2011; 41: 232-242.
- Demont A, Hacini-Rachinel F, Doucet-Ladevèze R, Ngom-Bru C, Mercenier A, Prioult G, et al. Live and heat-treated probiotics differently modulate IL10 mRNA stabilization and micro RNA expression. J Allergy Clin Immunol. 2016; 137: 1264-1267.
- Perrin Y, Nutten S, Audran R, Berger B, Bibiloni R, Wassenberg J, et al. Comparison of two oral probiotic preparations in a randomized crossover trial highlights a potentially beneficial effect of Lactobacillus paracasei NCC2461 in patients with allergic rhinitis. Clin Transl Allergy. 2014.
- Wassenberg J, Nutten S, Audran R, Barbier N, Aubert V, Moulin J, et al. Effect of Lactobacillus paracasei ST11 on a nasal provocation test with grass pollen in allergic rhinitis. Clin Exp Allergy. 2011; 41: 565-573.
- De Mello VD, Kolehmanien M, Schwab U, Pulkkinen L, Uusitupa M. Gene expression of peripheral blood mononuclear cells as a tool in dietary intervention studies: What do we know so far? Mol Nutr Food Res. 2012; 56: 1160-1172.
- Myhrstad MC, Ottestad I, Günther CC, Ryeng E, Holden M, Nilsson A, et al. The PBMC transcriptome profile after intake of oxidized versus high-quality fish oil: an explorative study in healthy subjects. Genes Nutr. 2016.
- Persson H, Kwon AT, Ramilowski JA, Silberberg G, Söderhäll C, Orsmark- Pietras C, et al. Transcriptome analysis of controlled and therapy-resistant childhood asthma reveals distinct gene expression profiles. J Allergy Clin Immunol. 2015; 136: 638-648.
- Pniewska E, Sokolowska M, Kuprys-Lipinska I, Kacprzak D, Kuna P, Pawliczak R. Exacerbating Factors Induce Different Gene Expression Profiles in Peripheral Blood Mononuclear Cells from Asthmatics, Patients with Chronic Obstructive Pulmonary Disease and Healthy Subjects. Int Arch Allergy Immunol. 2014; 165: 229-243.
- Blüggel M, Spertini F, Lutter P, Wassenberg J, Audran R, Corthésy B, et al. Toward protein biomarkers for allergy: CD4+ T cell proteomics in allergic and nonallergic subjects sampled in and out of pollen season. J Proteome Res. 2011; 10: 1558-1570.
- Whalen KA, Legault H, Hang C, Hill A, Kasaian M, Donaldson D, et al. In vitro allergen challenge of peripheral blood induces differential gene expression in mononuclear cells of asthmatic patients: inhibition of cytosolic phospholipase A2alpha overcomes the asthma-associated response. Clin Exp Allergy. 2008; 38: 1590-1605.