
Special Article - Vitamin B Deficiency
Ann Nutr Disord & Ther. 2016; 3(1): 1032.
Purification and Modification of Nanoclay for Adsorption of Vitamin B6 as Nanocarriers
Akbari Alavijeh M, Sarvi MN* and Ramazani Afarani Z
Department of Mining Engineering, Isfahan University of Technology, Iran
*Corresponding author: Mehdi Nasiri Sarvi, Department of Mining Engineering, Isfahan University of Technology, Isfahan 84156-83111, Iran
Received: October 25, 2016; Accepted: November 18, 2016; Published: November 21, 2016
Abstract
Nanomaterials are widely used for preparation of nanocarriers especially in order to overcome problems associated with deficiency of vitamins. Among them nanoclay has been introduced as a versatile carrier. In this study nanoclay was prepared, modified and applied for adsorption of vitamin B6 as carrier. The nanoclay was prepared from bentonite as a naturally available ore and then modified using a dispersing agent (sodium Hexamethaphosphate (NaHMP)) for improvement of adsorption of vitamin B6 on it. The results showed that the adsorption of vitamin B6 on nanoclay was occurred in early stages of experiments and adsorption was stopped after 5 minutes indicating a high affinity of vitamin B6 to the nanoclay due to cation exchange mechanism. The adsorption kinetics was quit similar in different pH. However, the amount of vitamin B6 adsorbed at pH=6 was higher than pH=3.2. Amount of adsorption was increased when small amount of dispersant was used for modification of nanoclay however it reduced by increasing the amount of dispersant. The results indicated that the nanoclay can be tuned for improvement of adsorption of vitamin B6 in order to develop versatile nanocarriers to fight deficiency of such important species.
Keywords: Nanoclay; Montmorillonite; Vitamin B6; Biomolecule adsorption; Cationic exchange mechanism
Introduction
Vitamin B6 is one of the water soluble vitamins which occur naturally as pyridoxine, pyridoxal, and pyridoxamine, either free or combined with other substances such as phosphate [1]. It helps red blood cell regeneration and fat and carbohydrate need it as co-factor for neurotransmitters [2,3]. In addition, this vitamin is necessary for proper adsorption of vitamin B12. Maintaining healthy nerve and muscle cells, production of DNA and RNA are responsibility of pyridoxine. Deficiency of vitamin B6 causes Changes in mood, such as irritability, anxiety and depression, confusion, muscle pains, fatigue, irritability, difficulty concentrating and worsening symptoms of anemia. The food sources of vitamin B6 are meat, poultry, fish, egg, potato, starchy vegetables. Banana, nut, whole grains and fortified soy are other sources [3].
Considering, lack of several very important nutrients such as vitamins attempt have been done to prepare food and pharmaceutical supplements to overcome this problem [4-6]. In this regards, carriers of food and pharmaceutical supplements played an important role which among them, nanocarries attracted much attention to compensate deficiency of vitamins and proteins [7-9]. Between these carriers nanoclay is a promising nano-carrier for this purpose due to its superior characteristics such as high surface area, cation exchange capacity, non-toxicity, high biocompatibility, and low price [10].
Among clay minerals montmorillonite has been widely used for preparation of nanoclay as nano-carrier due to its phenomenon cation exchange capacity [11-13]. Montmorillonite is the major component of bentonite that is a natural clay mineral with layered structure. Due to its 2:1 layered structure with exchangeable cations, different molecules could be adsorbed in between the layers.[14]There are many drugs and biomaterials that have been loaded on montmorillonite that proteins (DNA [15], cytochrome c [16], lysozyme and bovine serum albumin [17]), antibiotics (metronidazole [18] and tetracycline [19]) and vitamins (B12 [10], B1 [12], B6 [20], E [11]).
As the vitamin B6 is protonated in the solution it is possible to adsorb it to the montmorillonite through an ionic exchange mechanism. In this regards the accessible sites for adsorption of vitamin to adsorb is not well investigated. In addition, one of the disadvantages of nanoclay in the adsorption of food supplements and drugs is that they tend to accumulate together and form larger cluster. Consequently, clay nanoparticles adhere together and reduce the accessible sites for adsorption. To the best of our knowledge there is not enough information about improvement of adsorption of vitamin B6 onto montmorillonite by increasing the accessible sites for adsorption. Hence, in this study we are planning to apply a dispersing agent for increasing the accessible adsorption sites for vitamin B6 and to improve the adsorption of vitamin B6 onto nanoclay. Adsorption kinetics and Isotherms were tested and calculated by modified montmorillonite with different amount of NaHMP in two different pH for this purpose.
Materials and Methods
Chemicals
Pyridoxine hydrochloride (>98%, Sigma Aldrich) and Sodium hexametaphosphate (>96%, Sigma Aldrich), and Hydrochloric acid, HCl (37% w/w, Merck) was used as received. Deionized water (the water was purified to a resistivity of =18.2 MO.cm) was used in all experiments. The bentonite sample was provided by Salafchegan bentonite mine (Iran).
Preparation of nanoclay
In order to prepare nanoclay from bentonite montmorillonite particles smaller than 2.5μm were collected using centrifuge force. In addition, the dispersant agent was used in different amount in order to prepare nanoclay with dispersed particles of montmorillonite. Typically, 12 g of dry raw montmorillonite and defined amount of NaHMP (0.1, 0.5, 1.5 and 5 g) dispersed in 400mL of deionized water and the mixture was mixed for 16hours. Centrifuge force is used for physical purification and separating <2.5μm particles of montmorillonite [21]. Veiskarami et al. showed that according to type of centrifuge, nanoclay and falcon’s diameter montmorillonite particles smaller than 2.5μm was achieved by centrifuging at 1000 rpm for 260s [22]. The time of centrifugation was calculated based on stock’s law as followed [21]:
In this equation parameters are defined as follows: t: time of centrifuge (s), R: distance from the sediment surface to the axis of the centrifuge rotor (13.1cm), S: distance from the liquid to the axis of the centrifuge rotor (4.5cm), N: rotation speed, r: maximum radius of the desired particles and the liquid dispersion (0.00528gcm-1), ?: viscosity of the fluid (0.00748P for deionized water).
Hence in order to achieve <2.5μm particles of montmorillonite the mixture was centrifuged at 1000rpm for 260s and 25°C. The supernatant as a final product was collected and used for adsorption experiments.
Adsorption vitamin B6 on montmorillonite
Adsorption kinetics was carried out at initial vitamin B6 concentration of 0.8mg/mL in deionized water solution at 25°C and two different pH (3.2 and 6). 5mL of abovementioned solution in desired pH was added to 50mg of montmorillonite. In order to plot adsorption kinetics mixing times was set for different durations of 5,10,20,30,45, 60,90,120,180,270, and 360 minutes. Then the mixture was centrifuged at 5000rpm, 25°C for 5min and supernatant concentration was measured by UV-visible spectroscopy at the detection wavelength of 291nm. The amount of vitamin B6 adsorbed on nanoclay was calculated by the difference between initial and final concentration. For adsorption isotherm 5mL of water with different concentration of vitamin B6 (0.1,0.3,0.8,1.5,2.5 and 4.5 mg/mL) was added to 20mg of nanoclay and mixed for 6 hours (equilibrium time). After that the mixture was centrifuged and amount of vitamin remained in the solution was measured by UV-visible spectroscopy.
Characterization
Normal XRD analysis in the range of 2? from 3 to 80 degree for determination of impurities in nanoclay samples was done using Philips PW1800. Vitamin B6 concentration was measured using Unico UV-2100 spectrophotometer and calculated using the standard curve.
Results and Discussion
Table 1 shows the amount of NaHMP that is used to prepare each type of nanoclay samples. Figure 1 shows the results of XRD analysis of nanoclay samples along with raw montmorillonite. The results represent that physical purification increased purity of montmorillonite. According to the peak of montmorillonite in the range of 7 to 8 degree the peak became broader by increasing the concentrations of NaHMP as a dispersant and this can be a reason for decreasing particle size of montmorillonite. There are three peaks at 9,12 and 17-19 degree that related to muscovite, illite, and gypsum respectively. These peaks were removed when NaHMP was used and purity of montmorillonite. However, indication of cristobalite and calcite can be seen in the XRD patterns of all samples and physical purification and modification with NaHMP couldn’t remove this impurity completely while quartz was removed.
sample
Particle size (μ)
Amount of NaHMP used in the preparation (g)
2-Mt
< 2.5
-
2-Mt-0.1
< 2.5
0.1
2-Mt-0.5
< 2.5
0.5
2-Mt-1.5
< 2.5
1.5
2-Mt-5
< 2.5
5
Table 1: Synthesis parameters of different nanoclay samples.
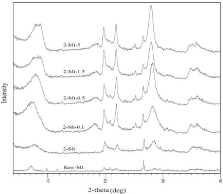
Figure 1: XRD patterns of raw montmorillonite and purified and modified
nanoclay.
Figure 2 shows the kinetics of adsorption of vitamin B6 on nanoclay at different pH at 25°C when the same amount of adsorbent was used. Figure 3a1 represents kinetics at pH=3.2. The amount of vitamin B6 adsorbed on nanoclay was 80, 100, 97, 95 and 85mg/g for 2-Mt, 2-Mt-0.1, 2-Mt-0.5, 2-Mt-1.5 and 2-Mt-5 respectively. The adsorption of vitamin B6 was very rapid for all samples and in the first 5 minutes maximum adsorption was occurred and after this time the adsorption was stopped. This could be due to high interaction forces between nanoclay and vitamin B6. Nanoclay has negative surface charge and vitamin B6 would be adsorbed to it through a cation exchange mechanism [7,23]. It is important that 2-Mt sample adsorbed lowest amount of vitamin B6 than other samples and when small amount of dispersant is used for preparation of nanoclay the amount of vitamin adsorbed increased. However, by increasing the amount of dispersant the amount of vitamin adsorbed decreased as well.
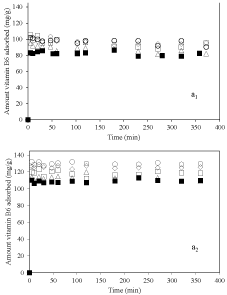
Figure 2: Kinetics of adsorption of vitamin B6 on nanoclay sample, ▪: 2-Mt;
○: 2-Mt-0.1; ◇: 2-Mt-0.5; ▢: 2-Mt-1.5; and △: 2-Mt-5; at pH=3.2 (a1) and pH=6
(a2).
As shown in Figure 2a2 the same trend was seen for kinetics of adsorption of vitamin B6 onto nanoclay samples at pH of 6 in which 2-Mt sample had lowest amount of adsorption and using NaHMP caused increasing in the adsorption of vitamin B6 onto nanoclay. Interestingly when similar initial concentration of vitamin B6 was used the maximum adsorption was higher at pH of 6 compare to that at pH of 3.2. In fact, the surface charge of nanoclay was more negative at higher pH 6 causing higher interaction of protonated vitamin B6.
In order to investigate the formation of adsorbed vitamin B6 onto nanoclay surfaces or interlayer spaces two models were fitted to the kinetics results: first the pseudo-second-order model and second the intraparticle diffusion. The first one is for better understanding of the mechanisms of adsorption which yields a rate constant, k, given by equation (1) [24-26]:
In this equation qt is the amount of vitamin B6 adsorbed at time t and qe is the adsorption capacity at equilibrium. This model was fitted to kinetic results and shows at figure 3 a1&a2 for pH 3.2 and 6, respectively. Table 2 summarized calculated parameters of the pseudo-second-order. The results displayed a good fit of pseudosecond- order model for adsorption of vitamin B6 on all types of purified nanoclay. The slope of the model is proportional to the inverse adsorption capacity at equilibrium. Therefore, the adsorption capacity at equilibrium for 2-Mt-0.1is more than 2-Mt and other samples (Figure 3 a1&a2) in both pHs as mentioned earlier.
Adsorbent
pH=3.2
pH=6
qe
(mg g-1)
k
(min g mg-1)
R2
qe
(mg g-1)
k
(mg g-1 min0.5)
R2
2-Mt
80
0.012
1
109
0.009
1
2-Mt-0.1
100
0.011
1
130
0.008
1
2-Mt-0.5
97
0.011
1
125
0.008
1
2-Mt-1.5
95
0.011
1
119
0.008
1
2-Mt-5
85
0.012
1
109
0.009
1
Table 2: Calculated parameters of the pseudo-second-order for adsorption of vitamin B6 onto nanoclay samples.
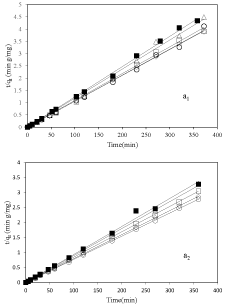
Figure 3: The pseudo-second-order model plots for adsorption of vitamin 6
on nanoclay sample, ▪: 2-Mt; ○: 2-Mt-0.1; ◇: 2-Mt-0.5; ▢: 2-Mt-1.5; and △:
2-Mt-5; at pH=3.2 (a1) and pH=6 (a2).
The intraparticle diffusion model that shows the diffusion of vitamin B6 into layered structure of the nanoclay is presented by equation (2): [25-27]
xi, is a constant proportional to the mass transfer boundary layer thickness around the particle. This model was fitted to the kinetics results and is displayed in Figure 4. For two pH of adsorption (pH of 3.2 Figure 4a1 and pH of 6 Figure 4a2) two linear stages were fitted. The first one indicates adsorption of vitamin B6 onto the external surface of the nanoclay and the second one shows the diffusion of vitamin B6 in between nanoclay layers. In all samples the slope of second linear step was null showing no diffusion of vitamin B6 in between the nanoclay layers and all of it was adsorbed on external surface of nanoclay. The first linear stage for all samples had a high rate constant showing high amount of vitamin was adsorbed on the external surface of the montmorillonite in the first five minutes. This could be the reason of increment of maximum adsorption amount of vitamin B6 on nanoclay samples which were prepared using dispersant in which the particles are dispersed better providing higher external surfaces for vitamin B6 to adsorbed.
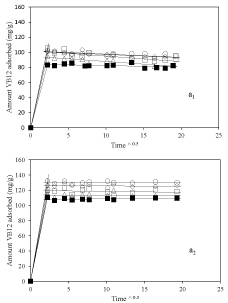
Figure 4: Intraparticle diffusion model resulting at adsorption of vitamin B6
onto nanoclay sample, ▪: 2-Mt; ○: 2-Mt-0.1; ◇: 2-Mt-0.5; ▢: 2-Mt-1.5; and △:
2-Mt-5; at pH=3.2 (a1) and pH=6 (a2).
Figure 5 a1&a2 shows the Equilibrium adsorption isotherm results at pH=3.2 and 6 respectively. In both cases, amount of vitamin B6 adsorbed was higher for nanoclay samples prepared using dispersant compare to nanoclay sample prepared without using dispersant. This proves the role of dispersion of nanoclay in improvement of adsorption of vitamin B6 which is mainly occurred on the external surface of the nanoclay. Interestingly, at pH 6 a huge amount of vitamin B6 adsorption amount for sample 2-Mt-0.1 around 450mg/g of nanoclay was achieved which makes it a good candidate as nanocarriers. In addition, adsorption amount was more at higher pH which could be because of more negative surface charge of nanoclay at higher pH and increasing interaction between vitamin B6 and nanoclay.
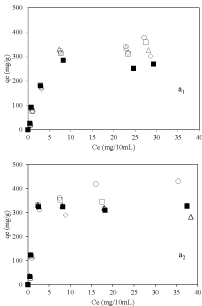
Figure 5: Equilibrium adsorption isotherms of vitamin B6 on nanoclay
sample, ▪: 2-Mt; ○: 2-Mt-0.1; ◇: 2-Mt-0.5; ▢: 2-Mt-1.5; and △: 2-Mt-5; at
pH=3.2 (a1) and pH=6 (a2).
Conclusion
Properties of adsorption vitamin B6 onto nanoclay were investigated. NaHMP had an important role in dispersing nanoparticles of nanoclay as well as on adsorption of vitamin B6. The results showed that the adsorption of vitamin B6 was occurred on the external surfaces of the nanoclay. Hence, applying a dispersant agent for preparation of nanoclay increased the amount of vitamin adsorbed. The adsorption vitamin on all montmorillonite samples was rapid and after five minutes adsorption reached to maximum state and was stopped. In addition, the adsorption mechanism was mainly due to an ionic exchange mechanism and consequently increasing the pH of adsorption increases the interaction forces between vitamin B6 and nanoclay. Results of this study provide important information about adsorption mechanism of vitamin B6 onto nanoclay which can be used for preparation of nanoclay derived nanocarriers.
Acknowledgment
The authors acknowledge access to infrastructure from the Mining Engineering Department of the Isfahan University of Technology.
References
- Toepfer EW, Polansky MM. Recent Developments in the Analysis for Vitamin B6 in Foods in Vitamins & Hormones. Harris IGWJALGFMRS, Kenneth VT, editors. In: Academic Press. 1964; 825-832.
- Cinta S, Morari C, Vogel E, Maniu D, Aluas M, Iliescu T, et al. Vibrational studies of B6 vitamin. Vibrational Spectroscopy. 1999; 19: 329-334.
- Allowances NRCCoD. Human Vitamin B6 Requirements: Proceedings of a Workshop: Letterman Army Institute of Research, Presidio of San Francisco, California, June 11-12, 1976. 1978: National Academy of Sciences.
- Ovesen L. Drugs and Vitamin Deficiency. Drugs. 1979; 18: 278-298.
- Samari F, Rezaei Z, Shamsipur M, Hemmateenejad B. A novel approach for rapid determination of vitamin B12 in pharmaceutical preparations using BSA-modified gold nanoclusters. Analytical Methods. 2012; 4: 4155-4160.
- Watanabe F, Yabuta Y, Tanioka Y, Bito T. Biologically Active Vitamin B12 Compounds in Foods for Preventing Deficiency among Vegetarians and Elderly Subjects. Journal of Agricultural and Food Chemistry. 2013; 61: 6769-6775.
- Yu WH, Li N, Tong DS, Zhou CH, Lin CX, Xu CY. Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review. Applied Clay Science. 2013. 80-81: 443-452.
- Aguzzi C, Cerezo P, Viseras C, Caramella C. Use of clays as drug delivery systems: Possibilities and limitations. Applied Clay Science. 2007; 36: 22-36.
- Ruiz-Hitzky E, Aranda P, Darder M, Rytwo G. Hybrid materials based on clays for environmental and biomedical applications. Journal of Materials Chemistry. 2010; 20: 9306-9321.
- Akbari AM, Sarvi MN, Afarani ZR. Properties of adsorption of vitamin B12 on nanoclay as a versatile carrier. Food Chemistry. 2017; 219: 207-214.
- Feng SS, Mei L, Anitha P, Gan CW, Zhou W. Poly (lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel. Biomaterials. 2009; 30: 3297-3306.
- Joshi GV, Patel HA, Kevadiya BD, Bajaj HC. Montmorillonite intercalated with vitamin B-1 as drug carrier. Applied Clay Science. 2009; 45: 248-253.
- Kevadiya BD, Joshi GV, Patel HA, Ingole PG, Mody HM, Bajaj HC. Montmorillonite-Alginate Nanocomposites as a Drug Delivery System: Intercalation and In Vitro Release of Vitamin B-1 and Vitamin B-6. Journal of Biomaterials Applications. 2010; 25: 161-177.
- Park JH, Shin HJ, Kim MH, Kim Js, Kang N, Lee JY, Kim KT, et al. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. Journal of Pharmaceutical Investigation. 2016; 46: 363-375.
- Mignon P, Sodupe M. Theoretical study of the adsorption of DNA bases on the acidic external surface of montmorillonite. Physical Chemistry Chemical Physics. 2012; 14: 945-954.
- Hristova SH, Zhivkov AM. Adsorption of cytochrome c on montmorillonite nanoplates: Protein concentration dependence. Journal of Colloid and Interface Science. 2015; 446: 252-262.
- Lepoitevin M, Jaber M, Guegan R, Janot JM, Dejardin P, Henn F, et al. BSA and lysozyme adsorption on homoionic montmorillonite: Influence of the interlayer cation. Applied Clay Science. 2014; 95: 396-402.
- Calabrese I, Gennara C, Cinzia S, Mariano L, Marcello M, Luciana S, et al. Montmorillonite nanodevices for the colon metronidazole delivery. International Journal of Pharmaceutics. 2013; 457: 224-236.
- Porubcan LS, Serna CJ, White JL, Hem SL. Mechanism of Adsorption of Clindamycin and Tetracycline by Montmorillonite. Journal of Pharmaceutical Sciences. 1978; 67: 1081-1087.
- Joshi GV, Patel HA, Bajaj HC, Jasra RV. Intercalation and controlled release of vitamin B6 from montmorillonite–vitamin B6 hybrid. Colloid and Polymer Science. 2009; 287: 1071-1076.
- Thuc CNH, Grillet AC, Reinert L, Ohashi F, Thuc H, Duclaux L. Separation and purification of montmorillonite and polyethylene oxide modified montmorillonite from Vietnamese bentonites. Applied Clay Science. 2010; 49: 229-238.
- Veiskarami M, Sarvi MN, Mokhtari AR. Influence of the purity of montmorillonite on its surface modification with an alkyl-ammonium salt. Applied Clay Science. 2016; 120: 111-120.
- Chang PH, Jiang WT, Li Z, Kuo CY, Jean JS, Chen WR, et al. Mechanism of amitriptyline adsorption on Ca-montmorillonite (SAz-2). Journal of Hazardous Materials. 2014; 277: 44-52.
- Ho YS, McKay G. Pseudo-second order model for sorption processes. Process Biochemistry. 1999; 34: 451-465.
- Sarvi MN, Andrea O, Michelle G, Budianto B, Chuen G. Development of functionalized mesoporous silica for adsorption and separation of dairy proteins. Chemical Engineering Journal. 2014; 235: 244-251.
- Wu FC, Tseng RL, Juang RS. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chemical Engineering Journal. 2009; 153: 1-8.
- Nguyen TPB, Lee JW, Shim WG, Moon H. Synthesis of functionalized SBA-15 with ordered large pore size and its adsorption properties of BSA. Microporous and Mesoporous Materials. 2008; 110: 560-569.