
Special Article: Emergency Contraception
Austin J Obstet Gynecol. 2015;2(2):1037.
Emergency Contraception: Efficacy Difference between Levonorgestrel and Ulipristal Acetate According to Follicle Size at the Time of Unprotected Sexual Intercourse
Christian Jamin*
Department of Obstetrics and Gynecology, 169 Boulevard Haussmann, France
*Corresponding author: Christian Jamin, Service de Gynécologie-obstétrique, 169 Boulevard Haussmann, 75008 Paris, France
Received: March 23, 2015; Accepted: April 12, 2015; Published: April 22, 2015
Abstract
The most frequently used treatment worldwide for emergency contraception is the levonorgestrel (LNG) pill. However, its efficacy decreases if it is administered 3 days after unprotected sexual intercourse, whereas the ulipristal acetate (UPA) pill is effective up until 5 days afterwards. Pooled clinical data show that UPA is more effective than LNG when taken very shortly after intercourse (within 24 h) or, conversely, between 72 and 120 h after intercourse. UPA is also more effective than LNG in inhibiting follicular rupture when administered near the time of ovulation. We show here why overall UPA is more effective than LNG in reducing the rate of unwanted pregnancies by demonstrating the effect of each product according to follicle size at the time of unprotected sexual intercourse We also explain the difference between UPA and LNG in the maximum time to administration simply by the shift in ovulation and the fact that UPA has an effect on larger follicles than LNG (18 mm vs. 14 mm), without postulating a hypothetical endometrial effect. We also explain why UPA and LNG remain emergency contraceptives and should not be used for daily contraception.
Keywords: Emergency contraception; Ulipristal acetate; Levonorgestrel;Follicle diameter
Introduction
Emergency contraception (EC), or “morning after” or postcoital contraception, prevents pregnancy in the majority of cases when it is administered in the very first days after sexual intercourse [1]. It is indicated in an emergency following unprotected intercourse, whether consensual or otherwise (rape, forced intercourse), or following failure or incorrect use of a contraceptive method (condom rupture, for example) [1-3]. Currently available EC methods include the copper intrauterine device (IUD) and emergency contraceptive pills. Although these hormonal methods reduce the risk of pregnancy by up to 75% [4], the IUD is potentially the most effective method currently since the failure rate is well below 1% (0-0.2%), as against 0.2 to 5% with EC, depending on the time to administration after intercourse and the type of hormonal treatment [5-7]. In practice, however, this is not the case, as the need for insertion by a qualified professional and the risks of infection are restrictions to its use [8]. In this situation, the IUD is more a contragestive (preventing implantation) than a contraceptive (preventing conception). In fact, the IUD is still effective after the spermatozoa have passed through the female genital tract and for this reason it cannot act by rendering the spermatozoa infertile, as is the case with conventional contraception with IUD. Since the abandonment of combined estrogens and progestogens, there are currently two emergency hormonal contraceptives available. Levonorgestrel (LNG) is a progestogen derived from nortestosterone, while ulipristal acetate (UPA) is a progesterone receptor modulator. Their mechanism of action is the same and involves delaying ovulation by more than 6 days, the time necessary for the spermatozoa to lose their fertilizing potency. Among the different forms of EC, the standard treatment recommended by international health authorities is still the levonorgestrel (LNG) pill, taken in a single 1.5 mg dose within 3 days (72 h) of unprotected intercourse [2]. This pill is available without prescription in more than 60 countries [9] and in addition has been dispensed free of charge to minors in France since 2000 [10 -12]. However, its efficacy decreases with the time to administration after intercourse [13]. In fact, delaying its administration until the fifth day after intercourse increases the risk of pregnancy almost sixfold compared with administration on the first day and its efficacy then is no different from that of placebo [14]. In addition, some studies indicate a higher risk of contraceptive failure in overweight women [14], although others cast doubt on these results [15]. The other treatment is the UPA-based pill, which is effective with a longer time to administration after unprotected intercourse and is also recommended by the health authorities and by European and American scientific societies [1-17]. This second generation progesterone receptor modulator was marketed in Europe in 2009 and in the United States in 2010 and it has been approved for use in emergency contraception up to 5 days (120 h) after intercourse where there is a risk of pregnancy [1-16]. Two independent randomized controlled studies in 1549 and 2221 women, respectively, each showed the noninferiority of UPA to LNG in emergency contraception between 0 and 72 hours after unprotected sexual intercourse or in the event of failure of the contraceptive method [12, 18]. A meta analysis of the pooled data from the two trials showed a significant reduction in the risk of pregnancy with UPA compared with LNG (p = 0.046) [12]. Moreover, unlike LNG whose effect decreases after a 72-hour interval before administration, the efficacy of UPA does not decline over time, so that UPA is more effective than LNG when taken between 72 and 120 h after intercourse [12]. In France, unlike the LNG pill, the UPA pill was issued on prescription only, making it potentially less accessible, but a recent decision by the European Medicines Agency asks Member States to authorize its issue without prescription. It is also free for minors. This decision is important since different data sources show that the use of EC has increased very substantially since the LNG pill has no longer been subject to medical prescription (1999) [17]. Nevertheless, access to UPA might also be facilitated if it could be prescribed in advance in order to be reimbursed by Social Security, which requires a prescription. According to an American study [19], UPA could have a better individual or public health cost benefit than LNG despite its higher price (including the cost of a medical consultation) if it were given as first-line EC. A number of arguments point to greater efficacy of this EC than that of LNG and to a more widespread use of this option in order to reduce the number of unwanted pregnancies and the resultant abortion rate. In this article, we compare the mechanisms of action of the two EC and demonstrate simply by the different effects of EC according to follicle size during the fertilizing intercourse why UPA overall is more effective than LNG in reducing the rate of unwanted pregnancies and over a longer timeframe. We explain the difference between the two products simply by the shift in the mechanism of ovulation without postulating a hypothetical endometrial effect. We also explain why UPA, like LNG, remains an emergency contraceptive and must not be used for daily contraception.
Probability of conception and onset of ovulation
A number of factors, such as the date of sexual intercourse relative to ovulation, the date of onset of ovulation or the actual number of fertile days during the woman’s menstrual cycle, play a role in the risk of pregnancy and, in fact, are likely to affect the efficacy of EC. Wilcox et al. were able to establish that the fertile period in women was composed of 6 consecutive days, ending on the day of ovulation [20]. It remains to be established at what point in the menstrual cycle this fertile window occurs, given that the day of ovulation varies with the cycle. Wilcox et al. in 2000 [21] showed that the date of this fertile window was highly unpredictable, even in women whose cycles are usually regular: it is between the 10th and 17th day of the cycle (the period suggested by the guidelines) in only 30% of women. These results [21] and those of a very recent study based on retrospectively calculated probabilities of conception in women who had become pregnant [22] demonstrate that in reality there are few days in the menstrual cycle when the woman is not theoretically at risk of becoming pregnant and the risk of being pregnant after a single act of intercourse only appears to be negligible in the first three days of the cycle [23], (Figure 1, red curve). In terms of the probability of having sexual intercourse during the period of the menstrual cycle, this appears to vary in a similar way to that of being in the fertile period during the cycle [24], (Figure 1, histogram). As it is not possible to predict the time of the fertile window and as the probability of having unprotected intercourse appears higher in the fertile period, it is impossible to predict the need to use EC after sexual intercourse. For this reason, EC must not just be prescribed to women who have had sexual intercourse at the time of the purportedly most fertile period of the cycle [23, 24]. In addition, to reduce the risk of conception after unprotected intercourse, EC must continue to be effective during the 6 days of the fertile period, which points to the preferential use of UPA over LNG in view of its longer maximum time to administration. EC must be administered regardless of the day of the cycle on which unprotected or inadequately protected intercourse took place, as advocated by the recommendations [1].
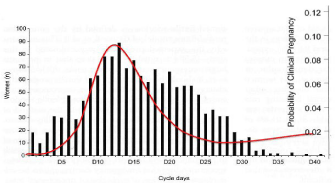
Figure 1: Probability of pregnancy after sexual intercourse relative to day of
menstrual cycle (red curve) versus number of women with unprotected sexual
intercourse relative to day of menstrual cycle (histogram).
Comparison of the mechanisms of action of UPA and LNG
The differences in the mechanism of action of UPA and LNG explain the greater efficacy of UPA than that of LNG. Among the proposed mechanisms, interference with ovulation appears the most likely in both cases. LNG, in particular, can either inhibit rupture of the follicle or arrest its development, but only if LNG is administered during the preovulatory phase before the start of the LH (Luteinizing Hormone) peak [25, 26]. Given 2-3 days before the LH peak, LNG inhibits and even delays and flattens the peak, whereas when administered on the day before or on the day of the peak itself it has no effect [25, 26]. According to studies of ovarian function following administration of LNG in the periovulatory period [27, 28], the efficacy of LNG is negatively correlated with follicle size at the time of administration of EC and can only prevent a follicle from rupturing if it is ≤ 14 mm.
Conversely, UPA can act on follicular maturation even if it is given just before ovulation. A randomized, crossover, placebocontrolled trial showed that UPA administered in the presence of a dominant follicle inhibited rupture of the follicle for at least 5 days in 100% of cases if it was administered before any increase in LH levels, in 78.6% at the start of the peak, and in 8.4% after the LH peak [29]. An intact follicle was found in almost 60% of cases on the fifth day after treatment, and analysis showed that follicular rupture occurred on average 6 days (from 4 to 10 days) after taking UPA vs 2 days for placebo, P = 0.028. A similar result was obtained in an analysis of the pooled data from 3 pharmacodynamic studies using the same methodology [27-29], which compared the capacity of UPA, LNG, and the combination of LNG + meloxicam to delay ovulation when these treatments were administered in an advanced follicular phase in the presence of a follicle ≥ 18 mm [28].In fact, follicular rupture was delayed for at least 5 days in 58.8% of cycles in the group treated with UPA versus 14.6%, 38.7% and 4% in the LNG, LNG + meloxicam, and placebo groups, respectively [30]. When treatment was administered before the peak or at the time of the LH surge, UPA proved the most effective with 100% and 78.6%, respectively, of dominant follicles not ruptured 5 days after treatment versus 25 and 14% for LNG and 0 and 10% for placebo (Figure 2). The median time to rupture was also significantly longer during the cycles with UPA treatment than with the other treatments (6 vs 2 days, p = 0.0015). Conversely, no treatment was effective after the ovulatory peak [30].
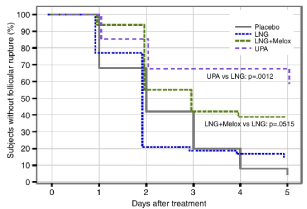
Figure 2: Percentage reduction of follicular rupture during the 5 days
following administration of different compounds in the late follicular phase:
LNG: levonorgestrel, UPA: ulipristal acetate, Melox: meloxicam.
UPA is therefore capable of delaying ovulation for at least 5 days in a significantly larger number of women than LNG when it is given in the late follicular period, i.e, at the time when the LH peak is imminent (4 to 5.5 times higher probability of non rupture of the follicle, p = 0.002), a period when LNG proves as ineffective as placebo in delaying or blocking ovulation [30]. This difference is crucial in view of the fact that this is the time when the probability of conception and sexual intercourse are highest and when the majority of women will seek EC, considering themselves to be at risk of pregnancy.
Why is UPA overall more effective than LNG?
In (Figure 3) we have drawn up a theoretical comparative chart of the possible time of use of UPA and LNG and their respective efficacy according to follicle diameter at the time of unprotected intercourse, taking into account the different data that we presented earlier, i.e,: 1) fertility is greatest in the 48 h preceding ovulation, at the time when the probability of sexual intercourse is highest, when the size of the follicle is between 14 and 18 mm; 2) it is not possible to predict reliably the date of ovulation, which is the last day of a six-day window of fertility whose position in the menstrual cycle varies; 3) a follicle grows on average by 2 mm a day; 4) LNG delays ovulation if the size of the follicle is ≤ 14 mm; 5) UPA delays ovulation if the size of the follicle is ≤ 18 mm.
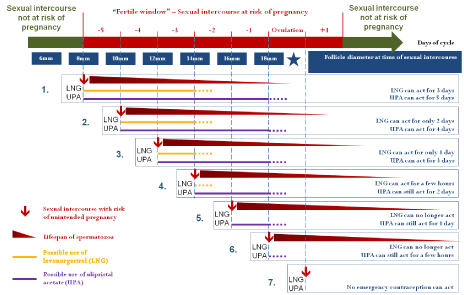
Figure 3: Time to possible use of emergency contraception with levonorgestrel (LNG) or ulipristal acetate (UPA) according to follicle diameter at the time of
unprotected sexual intercourse.
Thus, if a woman has sexual intercourse when the follicle is 6 mm in diameter, no contraception will be necessary as the risk of pregnancy will be nonexistent. In fact, given that the lifespan of spermatozoa is 5 days and that the follicle grows by 2 mm a day, all the spermatozoa will already be dead by the time of ovulation, 6 days after intercourse, when the size of the follicle will be ≥18 mm and the LH peak will have started.
Conversely, if a woman has intercourse when the size of the follicle is 8 mm (Figure 3, case 1), there is a risk of unintended pregnancy since the follicle will be 18 mm and ready for ovulation 5 days after unprotected intercourse, and some spermatozoa are still likely to be viable. In this case, EC is required to delay ovulation. If the woman opts to take LNG, she must do so in the 3 days following intercourse, since LNG is capable of inhibiting follicular rupture and thus delaying ovulation only if the size of the follicle is ≤ 14 mm (Figure 3, case 1). Beyond 3 days, the efficacy of LNG is markedly reduced and it can no longer inhibit rupture of the follicle. Administration of LNG on day 5 after intercourse when the follicle is 18 mm is totally ineffective, ovulation cannot be inhibited, and the still viable spermatozoa can fertilize the released ovule. Conversely, if the woman opts for UPA, she may take it up to 5 days after intercourse, i.e., at most when her follicle has reached a size of 18 mm. During this period when the follicle grows from 8 mm to 18 mm and up until the point when the LH peak is imminent, administration of UPA delays ovulation by at least 5 days [30], i.e., between 6 and 10 days at least after intercourse depending on the day of administration, after which the probability of finding still viable spermatozoa capable of fertilizing the released ovule is nonexistent.
Similarly, if unprotected intercourse occurs when the follicle is 12 mm in diameter (Figure 3, case 3), the woman will have no more than 24 h to take LNG, after which the follicle will have reached a size ≥ 14 mm and LNG will no longer be effective, whereas there will still be 3 days to take UPA before the follicle reaches a size > 18 mm, at which point UPA is no longer effective.
In the event that unprotected intercourse occurs when the follicle diameter is 14 mm (Figure 3, case 4), i.e., about 2 days before the probable start date of ovulation, LNG is no longer effective, whereas this is the time that fertility and the probability of intercourse are greatest and the risk of conception highest. During this period, UPA alone remains effective for a further 2 days until the start of the LH peak and the initiation of ovulation. This explains the greater overall efficacy of UPA compared with LNG, as it is only effective when fertility is greatest.
If intercourse occurs when the follicle has a diameter of 18 mm in the immediate preovulatory period, 24 h before ovulation (Figure 3, case 6), LNG is totally ineffective, whereas UPA is still capable of acting for a few hours. In fact, the results of Brache et al [30].showed that, with follicles of 18 mm diameter and over, UPA was still able to delay follicular rupture for at least 5 days in almost 60% of cases, which LNG was unable to do in the late preovulatory phase. Lastly, if sexual intercourse occurs at the time of the LH peak or after ovulation (Figure 3, case 7), neither of the two emergency contraceptives can work [30]. We have thus been able to explain simply the broader field of action of UPA than that of LNG (5 days versus 3 days) and its greater efficacy in preventing pregnancies due to its efficacy in the immediate preovulatory period by a difference in size of the follicle on which the two contraceptives are capable of acting, with UPA being able to act on larger and later follicles, whereas the efficacy of LNG is limited to follicles ≤ 14 mm in size.
Why must UPA not replace regular contraception?
We saw earlier that in cases 6 and 7 when the woman had unprotected intercourse on the day of ovulation or the following day (Figure 3, cases 6-7), UPA was still effective for a few hours if contraception was taken immediately at the start of the LH peak, but that it became ineffective if it was taken at the time of the peak or afterwards. We also saw that LNG was as ineffective as placebo, regardless of when it was taken, if the follicle size exceeded 18 mm [30]. Since there still remains this small window just after the LH peak has started when UPA can no longer prevent ovulation, this is why it cannot be considered for use in place of standard contraception. In fact, in about 40% of women in the late follicular phase (follicle ≥ 18 mm), follicular rupture could not be prevented in the 5 days following treatment, and even if UPA was taken at the start of the LH peak, the failure rate was still about 20% [29]. For this reason, although it can be used for a longer period after intercourse than LNG and it is effective during the greater part of the period of maximum likelihood of conception, UPA is not 100% effective, particularly in the late follicular phase. Assuming sexual intercourse occurs once every 3 days, statistically the woman will have intercourse on the day of ovulation, i.e., when EC is not effective, once every three months. For this reason, this contraception must remain emergency contraception, to be taken as soon as possible after intercourse in order to optimize the chances of success. In fact, each day that passes after intercourse increases the risk of EC being used after the follicular size has exceeded its threshold of efficacy (14 mm for LNG and 18 mm for UPA). To this end, UPA should be prescribed in advance in order to be reimbursed by Social Security without waiting for a consultation or even purchased in advance “in case”, given in particular that sexual intercourse is more common on Saturday evening, and that the dispensing of EC in pharmacies occurs more often on Sundays (23%) [17], a day off when access to prescribers is more difficult. It has been shown that prescription in advance multiplies the administration of EC threefold [31]. In conclusion, the fact that UPA can act on larger diameter follicles than LNG enables it to be effective over a longer period after unprotected intercourse. As a result of this broader field of action in respect of follicular size which, in the period when the LH peak is imminent, covers the period of maximum fertility and the period when there is a greater number of sexual relations, UPA can prevent a greater number of pregnancies than LNG. This is why UPA should be chosen as first-line treatment in EC because of the lack of criteria of choice and because of its greater statistical efficacy. However, it needs to be remembered that UPA remains an EC and cannot replace daily contraception. To optimize its efficacy, it is essential to reduce the time to administration of contraception after unprotected or inadequately protected intercourse as far as possible, which is an argument in favor of prescribing EC in advance in order to improve its coverage by health insurance companies.
Acknowledgement
We are grateful to Marielle ROMET (Santé Active Edition, France) for her medical writing services and Drs. Glasier, Fine, Wilcox and Brache for allowing their figures to be reproduced.
References
- International Consortium for Emergency Contraception (ICEC). Emergency contraceptive pills. Medical and service delivery guidelines. 2013; 1-15.
- WHO. Emergency contraception. Fact sheet n. 2012; 244.
- Jamin C, André G, Audebert A, Christin-Maître S, Elia D, Harvey T et al. Pélissier, pour le Groupe de réflexion « oublis de pilule" Oublis de la contraception hormonale : réflexions sur leur prise en charge en pratique quotidienne. Gynécol Obstét Fertil. 2011; 39: 644-655.
- Trussell J, Rodriguez G, Ellertson C. New estimates of the effectiveness of the yuzpe regimen of emergency contraception. Contraception. 1998; 57: 363-369.
- Cheng L, Che Y, Gulmezoglu AM. Interventions for emergency contraception. The Cochrane database of systematic reviews 2012; 8: CD001324.
- Richardson AR, Maltz FN. Ulipristal acetate: review of the efficacy and safety of a newly approved agent for emergency contraception. Clin Ther 2012; 34: 24-36.
- Van Look PF, von Hertzen H. Emergency contraception. Br Med Bull. 1993; 49: 158-170.
- Turok DK, Gurtcheff SE, Handley E, Simonsen SE, Sok C, North R, et al. A survey of women obtaining emergency contraception: Are they interested in using the copper IUD? Contraception 2011; 83: 441-446.
- ICEC. The international consortium on emergency contraception, 2014.
- HAS. Note de cadrage contraception d'urgence. 2011.
- Delotte J, Molinard C, Trastour C, Boucoiran I, Bongain A. Délivrance contraception d’urgence aux mineures dans les pharmacies françaises Gynécol Obstét Fertil. 2008; 36: 63-66.
- Glasier AF, Cameron ST, Fine PM, Logan SJ, Casale W, Van Horn J, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375: 555-562.
- Piaggio G, Kapp N, von Hertzen H. Effect on pregnancy rates of the delay in the administration of levonorgestrel for emergency contraception: A combined analysis of four WHO trials. Contraception. 2011; 84: 35-39.
- Glasier A, Cameron ST, Blithe D, Scherrer B, Mathe H, Levy D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011; 84: 363-367.
- EMA. Levonorgestrel and ulipristal remain suitable emergency contraceptives for all women, regardless of bodyweight. European Medicines Agency.2014.
- Royal College of Obstetricians and Gynaecologists Clinical effectiveness U: Emergency contraception. Clinical guidance. England, Faculty of Sexual and Reproductive Healthcare. 2011.
- Haute Autorité de Santé (HAS). Recommandations en santé publique. Contraception d'urgence : prescription et délivrance à l'avance, Avril 2013. Saint-Denis La Plaine, HAS. 2013.
- Creinin MD, Schlaff W, Archer DF, Wan L, Frezieres R, Thomas M, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108:1089-1097.
- Bayer LL, Edelman AB, Caughey AB, Rodriguez MI. The price of emergency contraception in the United States: what is the cost-effectiveness of ulipristal acetate versus single-dose levonorgestrel? Contraception. 2013; 87: 385-390.
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation — effects on the probability of conception, survival of the pregnancy, and sex of the baby. New Engl J Med. 1995; 333:1517-1521.
- Wilcox AJ, Dunson D, Baird DD. The timing of the "fertile window" in the menstrual cycle: day specific estimates from a prospective study. BMJ. 2000; 321:1259-1262.
- Stirnemann JJ, Samson A, Bernard JP, Thalabard JC. Day-specific probabilities of conception in fertile cycles resulting in spontaneous pregnancies. Hum Reprod. 2013; 28:1110-1116.
- Wilcox AJ, Dunson DB, Weinberg CR, Trussell J, Baird DD. Likelihood of conception with a single act of intercourse: providing benchmark rates for assessment of post-coital contraceptives. Contraception. 2001; 63: 211-215.
- Fine P, Mathe H, Ginde S, Cullins V, Morfesis J, Gainer E. Ulipristal acetate taken 48-120 hours after intercourse for emergency contraception. Obstet Gynecol 2010;115: 257-263.
- Gemzell-Danielsson K, Berger C, P GLL. Emergency contraception -- mechanisms of action. Contraception 2013; 87: 300-308.
- Marions L, Cekan SZ, Bygdeman M, Gemzell-Danielsson K. Effect of emergency contraception with levonorgestrel or mifepristone on ovarian function. Contraception.2004; 69: 373-377.
- Croxatto HB, Brache V, Pavez M, Cochon L, Forcelledo ML, Alvarez F, et al. Pituitary-ovarian function following the standard levonorgestrel emergency contraceptive dose or a single 0.75-mg dose given on the days preceding ovulation. Contraception 2004; 70: 442-450.
- Massai MR, Forcelledo ML, Brache V, Tejada AS, Salvatierra AM, Reyes MV, et al. Does meloxicam increase the incidence of anovulation induced by single administration of levonorgestrel in emergency contraception? A pilot study. Hum Reprod. 2007;22: 434-439.
- Brache V, Cochon L, Jesam C, Maldonado R, Salvatierra AM, Levy DP, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010; 25: 2256-2263.
- Brache V, Cochon L, Deniaud M, Croxatto HB. Ulipristal acetate prevents ovulation more effectively than levonorgestrel: analysis of pooled data from three randomized trials of emergency contraception regimens. Contraception. 2013; 88: 611-618.
- Polis CB, Grimes DA, Schaffer K, Blanchard K, Glasier A, Harper C. Advance provision of emergency contraception for pregnancy prevention (Review). Cochrane Library. 2010.