
Case Report
Austin Oncol. 2016; 1(2): 1006.
Acute Liver Failure from Anti-PD-1 Antibody Nivolumab in a Patient with Metastatic Lung Squamous Cell Carcinoma
Sarkissian Sarmen M.D. and Seery Tara M.D.
Department of Hematology/Oncology, University of California, Irvine, USA
*Corresponding author: Sarkissian Sarmen M.D, Department of Hematology/Oncology, University of California, Irvine, USA
Received: December 18, 2015; Accepted: March 21, 2016; Published: March 23, 2016
Abstract
Lung cancer remains one of the leading causes of cancer related mortality worldwide. The approval of the first immune checkpoint inhibitor, nivolumab, for squamous cell carcinoma of the lung has drastically increased the treatment options. Unfortunately, checkpoint inhibitor therapy is not innocuous, with hyperinflammation a known side effect. While hepatitis has been reported with this medication across several different cancer types, fulminant hepatic failure has not been reported. We present the case of a patient with metastatic squamous cell carcinoma of the lung that developed hepatitis and ultimately fulminant hepatic failure after 2 cycles of ivolumab therapy. Immunosuppressive therapy was initiated but was futile. This is the first reported case in the literature. We present this case to not only shed light on the inflammatory side effects that can develop with this family of biologics, but also help stress the importance of prompt recognition so that corticosteroid therapy can be initiated promptly.
Keywords: Nivolumab; Liver failure; Immunotherapy; Checkpoint inhibitor
Introduction
Lung cancer is the leading cause of cancer related mortality worldwide, with an estimated 221,200 new cases and 158,040 deaths in the United States for the year 2015 [1]. Primary prevention via reduced smoking prevalence has led to a drop in the annual death rate since the 1960’s [2]. 85% of lung cancer cases are non-small cell [3] with 21% of these being squamous cell carcinoma [4]. Prognosis is contingent on the stage of presentation, with a 2007 study showing a median 5 year survival of 2 percent and 17 percent for clinical and pathologic stage IV respectively [5].
The development of immune checkpoint inhibitors has changed the therapeutic landscape for squamous cell carcinoma. Nivolumab is an IgG humanized monoclonal antibody that targets the programmed cell death receptor on the surface of T lymphocytes. A pivotal phase III study in advanced squamous cell lung cancer patients from Spiegel et al. showed that second line nivolumab produced an increase in median OS from 6.0 to 9.2 months when compared head to head with docetaxel therapy [6].
The excess inflammation caused by immune checkpoint inhibitors is a well-known side effect. This includes dermatitis, colitis, cerebritis, pneumonitis, and importantly hepatitis [7]. Hepatotoxicity has been reported as less than 5% [8-10] of patients in trials with nivolumab. The time course is generally accepted to be around 6 weeks after the initiation of treatment the liver transaminase begin to elevate [11]. Recommended first line treatment includes high dose corticosteroids with at least 1-2 mg/kg daily. If there is no response to steroids then attempts with mycophenylate and infliximab can be pursued [12]. Anti-Thymocyte Globulin has also been tried in one case report [13].
While hepatitis is an uncommon but expected side effect, fulminant hepatic failure has never been reported for nivolumab in the literature. We present the case of a patient with advanced squamous cell carcinoma of the lung that developed severe hepatic impairment as a result of nivolumab therapy. He showed no response to high dose corticosteroids and was not a liver transplant candidate. He expired within 5 days of the diagnosis. We believe that his acute hepatic failure was a result of his immunotherapy. We present his case below.
Case Presentation
A 59 year old Caucasian male with squamous cell carcinoma of the lung, with metastases to the liver, was initially treated with 2 cycles of carboplatin and gemcitabine. He showed progression of disease on PET/CT and was initiated on treatment with nivolumab. The patient completed 2 infusions of nivolumab. Labs just prior to the second infusion showed normal liver function panels. The Patient presented to a community hospital emergency room, 21 days after his first infusion of nivolumab, with a 1 day history of cough, significant dyspnea on exertion and lower extremity edema. Patient denied sick contacts or traveling outside the United States.
Labs on admission were notable fora hemoglobin 7.1g/dL and a leukocytosis 21.6 x 109 cells/L. CXRay showed a right upper lobe consolidation. Patient was initially treated with azithromycin and ceftriaxone for community acquired pneumonia. Home medications at that time included metformin, insulin, fluticasone/ salmeterol inhaler, losartan, lorazepam, zolpidem, hydrocodone/ acetaminophen, aspirin, ondansetron, and fenofibrate. Liver function panel on admission was notable for mild elevations as noted in the table (day 21). Patient’s symptoms continued to decline. On hospital day #4 a bronchoscopy was performed which was unremarkable. A transthoracic echocardiogram ruled out cardiomyopathy and a pericardial effusion. Ultrasound of the abdomen showed a nodular liver with moderate hepatomegaly and mild splenomegaly (patient has a history of alcohol abuse). Patient underwent an MRCP/ERCP which showed no evidence of gallstones or biliary ductal dilation.
Hospital Day
Day Count post Nivolumab
PT(s)
INR
AST (IU/L)
ALT (IU/L)
AlkP (IU/L)
TB (mg/dL)
DB (mg/dL)
NH3 (umol/L)
-21
0
NO Labs
NO Labs
19
15
68
0.8
NO Labs
NO Labs
-11
10
NO Labs
NO Labs
15
13
82
0.4
NO Labs
NO Labs
1
21
14.5
1.4
105
92
92
0.7
0
NO Labs
2
22
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
3
23
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
4
24
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
5
25
NO Labs
NO Labs
505
320
110
1.4
0
NO Labs
6
26
NO Labs
NO Labs
942
544
127
1.9
0.2
NO Labs
7
27
35.3
3.3
1,272
736
127
3.4
1.3
NO Labs
8
28
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
9
29
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
NO Labs
10
30
NO Labs
NO Labs
2,675
1.296
128
9
5.6
237
11
31
78.1
7.76
2,194
1,310
139
10.6
NO Labs
NO Labs
11
31
73.4
7.32
1,790
1,294
152
11.6
NO Labs
NO Labs
11
31
85.1
8.37
1,169
1,067
132
10.7
NO Labs
NO Labs
12
32
86.7
85.3
1,089
1,103
157
12.7
NO Labs
NO Labs
12
32
89.3
8.79
962
1,046
158
13.3
NO Labs
NO Labs
13
33
83.2
9.18
805
958
148
13.3
NO Labs
NO Labs
13
33
96.2
9.48
607
818
144
13.9
NO Labs
NO Labs
Table 1: AST: Aspartate Transaminase; ALT: Alanine Transaminase; PT: Prothombin Time; INR: International Normalized Ratio; AlkPhos: Alkaline Phosphatase; TB: Total Bilirubin; DB: Direct Bilirubin; NH3: Ammonia.
On hospital day 5 the patient’s liver function panel was rechecked and showed a dramatic increase in AST, ALT, bilirubin, and prothrombin time. Serum Acetaminophen level was also undetectable (<10). There was no evidence of recent alcohol, acetaminophen, or herbal supplement consumption. These parameters continued to increase daily. Hepatitis as a side effect of immunotherapy was not considered on the differential diagnosis.
The patient was started on methylprednisolone 40 mg IV Q8H on hospital day #8 and transferred to a university medical center for higher level of care. Unfortunately, the patient progressively became more disoriented the last 2 days prior to transfer. Labs on hospital day #9, the day of transfer, showed AST 2,675 IU/L, ALT 1,296 IU/L, total bilirubin 9.0 mg/dL and prothrombin time 78.1 seconds. He was hemodynamically stable during this time. Patient was continued on the same regimen of methylprednisolone with added N-Acetyl Cysteine, vitamin K, ursidiol, rifaximin, and lactulose. The patient was able to be aroused but not oriented. Computed tomography of the head showed no evidence of cerebral edema while the serum ammonia level was 237ug/dL. Viral hepatitis panel for hepatitis A, B, C were all negative. On Hospital Day 11 the patient passed from cardiopulmonary arrest. Autopsy was declined by family.
Discussion
Drug induced liver injury remains a diagnosis of exclusion and Hy’s law has traditional been used to assess whether a particular medication can be described as the culprit of hepatotoxicity. In this patient’s case we saw drastic increases in the transaminases as well as the bilirubin and prothrombin time within 3 weeks of initiating treatment with nivolumab. Common acute liver failure insults such as alcohol, acetaminophen and viral hepatitis were ruled out. Progressive disease was also an important consideration, but given the volume of disease four weeks prior (Figure 1), and given the accelerated evolution of liver function tests, metastatic disease burden was not believed to be the cause.
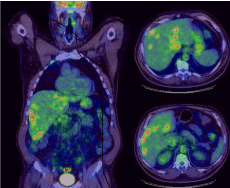
Figure 1: This PET scan representing tumor burden. Overall tumor burden
occupying less than 25% of total liver parenchyma volume. Strongest SUV
for liver lesions was 6.55.
This medication is well known to cause acute hepatitis and several reports on treating this side effect have been published. Ideally, a liver biopsy would have definitively ruled in the diagnosis. An iron panel including ferritin should ideally have been checked to rule out hemophagocytic syndrome secondary to disease progression. Whether these additional studies would have changed the outcome, is uncertain. A delaying in the initiation of corticosteroid therapy likely contributed to liver failure. While we cannot definitively conclude that a prompter treatment with methylprednisolone would alter the course, we feel that it could have. It is still, however, an extremely rare event.
The development of immune checkpoint inhibition has ushered in a new era of therapeutics for melanoma, lung cancer, renal cancer, and potentially several other malignancies. They unfortunately have a unique side effect profile, and practitioners must be cognizant of this adverse effect profile. Because the absolute number of patient-years in relatively small, very rare side effects take time to surface. A recent review from Spain et al. showed that grade 1-2 hepatitis secondary to nivolumab in NSCLC was prevalent in 1-3% of patients while grade 3-4 was seen in less than 1%. These were also noted to be silent, usually seen on incidental laboratory work [14].
Nivolumab has only been FDA approved for the last year and thus, experience with the medication is still in its infancy. One recommendation we can make is providing safety information cards for patients to carry. In the event of acute illness, these can be presented to hospital practitioners, who may not be experienced with these novel agents, advising them of the potential side effects. With this case we report the very first event of a fatal hepatotoxicity likely as a result of delayed immunosuppressive therapy from immune checkpoint inhibitor induced hepatitis.
References
- American Cancer Society, Cancer Facts & Figures. 2015.
- Alberg AJ, Samet JM. Epidemiology of lung cancer. 2003; 123: 21-49.
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. 2008; 83: 584-594.
- SEER Cancer Database.
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. 2007; 2: 706-714.
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. 2015; 373: 123-135.
- Howell M, Lee R, Bowyer S, Fusi A, Lorigan P. Optimal management of immune-related toxicities associated with checkpoint inhibitors in lung cancer. 2015; 88: 117-123.
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. 2015; 372: 320- 330.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal Clinical Oncology. 2014; 32: 1020-1030.
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. 2013; 369: 134-144.
- Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015.
- Cheng R, Cooper A, Kench J, Watson G, Bye W, McNeil C, et al. Ipilimumabinduced toxicities and the gastroenterologist. J Gastroenterol Hepatol. 2015; 30: 657-666.
- Chmiel KD, Suan D, Liddle C. Resolution of severe ipilimumab-induced hepatitis after antithymocyte globulin therapy. Journal of Clinical Oncology. 2011; 29.
- Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016; 44: 51-60.