Review Article
Austin J Pharmacol Ther. 2013;1(1): 1002.
Review of Opioid Use in Palliative Care Patients with Refractory Dyspnea
Shyam Gelot1*, Enji Nakhla2, Howard Tuch3
1Department of Pharmacotherapeutics and Clinical Research, University of South Florida College of Pharmacy, USA
2Department of Pharmacy, Tampa General Hospital, USA
3Palliative Care Services, Tampa General Hospital, USA
*Corresponding author: : Shyam Gelot, Department of Pharmacotherapeutics and Clinical Research, University of South Florida College of Pharmacy, 12901 Bruce B. Downs Blvd., MDC 30, Tampa, FL 33612, USA
Received: December 13, 2013; Accepted: December 30, 2013; Published: December 31, 2013
Abstract
Palliative care is an interdisciplinary approach to improve the quality of life for patients and their families, at all stages of a serious illness. This approach is accomplished by managing symptoms of distressing conditions such as pain and dyspnea. Refractory dyspnea is a debilitating symptom of an advanced pulmonary and cardiovascular disease that is characterized by difficulty in breathing persisting at rest or with minimal exertion, despite optimal therapy of the underlying condition. Opioid use has been well documented in this setting; however, hesitancy remains when prescribing opioids until the terminal stages of the disease. Dyspnea can be the result of a variety of advanced underlying conditions such as cancer, COPD, and congestive heart failure (CHF), The palliative treatment models can be more efficacious in certain diseases. This article will review the current evidence of opioid use for the treatment of refractory dyspnea in the advanced conditions of COPD, cancer, and CHF, separately.
Keywords: Dyspnea; Opioids; Nebulized Morphine; Palliative Care.
Introduction
Palliative care is an interdisciplinary approach to improve the quality of life for patients and their families, at all stages of a serious illness. This approach is accomplished by managing symptoms of distressing conditions such as pain and dyspnea [1,2]. Refractory dyspnea is a debilitating symptom of an advanced pulmonary and cardiovascular disease that is characterized by difficulty breathing persisting at rest or with minimal exertion despite optimal therapy of the underlying condition. [3] The incidence of dyspnea increases in the last three months before death [4]. Dyspnea is often poorly controlled despite numerous potential interventions. One systematic review evidenced that breathlessness was experienced by more than 90% of patients with advanced COPD and more than 60% in patients suffering from advanced heart disease. [5]Additionally, according to Elkington and colleagues’ retrospective study, only half of the patients with the chronic obstructive pulmonary disease (COPD) with dyspnea, had partial relief of breathlessness [6].
The definition of dyspnea has recently been divided into breakthrough or episodic and continuous dyspnea [7,8].Episodic breathlessness is “characterized by a severe worsening of breathlessness intensity or unpleasantness beyond usual fluctuations in the patient’s perception” [9]. Episodic breathlessness can occur periodically for seconds to hours regardless if a patient has continuous dyspnea or not [9]. These terms have historically been used similarly to describe dsypnea in general; however, future understanding of these distinct patterns of dyspnea may lead to different clinical management.
Opioid use, especially morphine, has been well documented in refractory dyspnea; [10] however, prescribing opioids until the terminal stages of the disease remains a hesitancy [11]. This may be secondary to the lack of practice guidelines and consensus statements by professional societies, concern of opioids’ ability to hasten death as a result of the respiratory depressive effects, and the subjective nature of dyspnea[12,13]. Dyspnea can be the result of a variety of advanced underlying conditions such as cancer, COPD, cancer, and congestive heart failure (CHF). A study by Currow and colleagues investigated the progression of breathlessness in different advanced conditions (primary lung cancer, secondary lung cancer, heart failure, end–stage pulmonary disease, and no identifiable cardiorespiratory cause) as 5,862 patients neared death. Dyspnea was observed to be significantlygreater in those groups with non–cancer diagnoses at 53–60, 23–30, and 0–7 days before death (P<0.001). It was also noted in that dyspneancreased significantly (P<0.001) for patients with cancer at days 10 and 3 before death [14].
The palliative treatment models can be more efficacious in certain diseases given the breadth of literature. There are more studies involving opioid use in dyspneic patients with advanced COPD and cancer than with CHF. This article will review the current evidence of opioid use for the treatment of refractory dyspnea in the advanced conditions of COPD, cancer, and CHF, separately.
Pathophysiology of dyspnea
The pathophysiology of dyspnea is complex and requires more studies to fully elucidate the mechanism(s) of this condition. In the early stages of dyspnea pathophysiology, there is an apparent or perceived imbalance between the oxygen demand and the body’s abilityto supply or ventilate oxygen appropriately [13]. In the neurobiological model, airflow adequacy is monitored by somatic, proprioceptive and bronchopulmonary afferent neurons in the upper and lower respiratory tract, skin, and musculature to provide negative feedback to the central nervous system (CNS) via afferent signals. Absence or diminution in signals to the CNS results in a mismatch with the outgoing medullary respiratory motor efferent impulses to unconsciously control relaxation and contraction of respiratory muscles [3,13,15]. This mismatch between afferent and efferent impulses results in an urge and conscious effort to breathe. In later stages of dyspnea, lack of oxygenation induces anaerobic metabolism to produce lactic acid, which can decrease blood pH and is a stimulant for respiratory effort [13].
Neuroimaging studies with positron emission tomography and functional magnetic resonance imaging scans are being used to determine the location in the brain where dyspnea is perceived. It has been proposed that different pathways exist to process respiratory sensation. One pathway is the awareness of unpleasantness, which is mediated by the afferent neurons to right insula and amygdale [16]. Another pathway is the awareness of intensity. This is carried from the afferent neurons to the brainstem medulla, then to the ventroposterior thalamus and finally to the somatosensory cortex [16].
Clinically, dyspnea can be the result of increased oxygen demand due to airway obstruction (e.g. COPD, mass lesions, and pulmonary embolism), a weakness of respiratory muscles to maintain adequate respirations, and requirements from various conditions such as anemia in cancer patients and fever [17].
In addition to anatomical causes of dyspnea, emotional and spiritual stress along with lack of oxygenation evokes panic and anxiety [3,17]. Anxiety has been correlated with difficulty in breathing, thus leading to a vicious cycle of respiratory distress [3,18]. Kvaal and colleagues reported the incidence of anxiety to be higher in patients with COPD, than with cancer or heart disease [19].
Mechanism of opioids in dyspnea
Opioids are the mainstay in the treatment of dyspnea [20]. Exogenous opioids (e.g. morphine and hydromorphone) workby mimicking the body’s endogenous opioids (e.g. β–endorphins, enkephalins, and dynorphins). Opioids are thought to bind to three main receptors (mu, delta, and kappa) that are found both centrally and peripherally. The number of these receptors and subtype receptors vary amongindividuals depending on their genetic make–up [3]. Recent evidence has shed light on the role of endogenous opioids as a natural mechanism in relieving dyspnea. A trial was conducted, where 17 patients with COPD underwent exercise treadmill testing.21 Patientswere randomized to naloxone 10mg (an opioid antagonist) or saline administered intraveounsly. Patients self reported dyspnea scores. A three–fold increase in β–endorphins was observed from thestart of the test to after the 10–minute treadmill test in both groups. The mean ratings of breathlessness throughout the exercise and peak ratings of breathlessness were higher in the naloxone group, suggesting the role of endogenous opioids in decreasing dyspnea [21].
Both animal and human models have shown benefit for using opioids to treat dsypnea. Coleman and colleagues administered fentanyl andan endogenous opioid, dermorphin, to rats, which lead to decreased respirations [22].Upon the co–administration of naloxone, the decreased respiratory effects were noted to be blocked with fentanyl, as oppose to dermorphin. This occurred due to entanyl having a stronger affinity for mu1 than mu2 receptors, which is the receptor that naloxone mainly targets. Dermorphin has similar affinity for both mu1 and mu2 receptors.
A meta–analysis involving 18 double–blinded placebo controlled trials evaluated the use of opioids in patients with dyspnea [23]. In nine studies, the route of opioid administration was via nebulization, while in the other nine studies the opioid was administered either orally or parenterally (most studies involved morphine). A statistically significant benefit for the use of opioids on the sensation of breathlessness (p=0.008) was noted. The oral and parenteral routes were more effective than the nebulized route (p=0.02). A subgroup analysis failed to evidence a benefit of nebulized opioids; however, most of these trials had a small patient population [23].
Despite the known fact of opioids suppressing respiration in both animal and human models, the exact mechanism(s) of how opioids decrease dyspnea is not fully understood [3,13,24].
According to one theory, opioids work to alleviate dyspnea by decreasing the respiratory drive. Moreover, opioids can dampen the chemoreceptors ability in the brainstem to detect hypercapnia and hypoxia [24,25].With a decreased respiratory output, due to opioids, an assumption is made of a decrease in corollary discharge to thesensory cortex. It is thought that the brainstem sends discharges out to the motor neurons and a copy of this discharge signal is sent to the sensory cortex during automatic reflex breathing. In the case of voluntary breathing, the cortical motor center sends discharges to the motor neurons and a copy of this signal to the sensory cortex. This copy of the signal transmitted to higher brain centers is called the respiratory motor command corollary discharge and results in the conscious awareness of outgoing motor signals to the muscles controlling ventilation [25].This pathway has been identified in some animal models, however, remains under investigation in humans.
Opioids may decrease respirations; however, this is dependent on the rate at which drug levels rise. Steady state drug levels have little effect on the respiratory drive [13,26]. Decreasing respirations would theoretically produce worse blood gasses, leading to dyspnea exacerbation. Although, yet to be identified in humans, there is a region in the ventrolateral medulla that generates respiratory rhythm in several animal models called the pre–Bötzinger complex [26].It has been suggested that this region contributes toinspiration and the action of opioids can cause irregular, skipped breaths from subthreshold action potentials [27].
According to another growing theory, opioids can affect the central processing of dyspnea. Neuroimaging studies via functional MRI evidenced how the experience of air hunger was associated with higher brain centers such as the anterior insular, cingulated gyrus, and amygdala activation [28].Pattinson and colleagues reported the urge to breathing scores were decreased in subjects who received remifentanyl. As a result of this study, it was noted that the urge to breath was associated with activity in the bilateral insula andfrontal operculum [29].In a small study involving six subjects, the areas of activity after fentanyl administration, using a PET scan, was the caduate nuclei, the cingylate, orbitofrontal, and medial prefrontal cortices [30].Much research remains in this area; however, brain region activity after opioid administration occurs in similar locales as areas associated with dyspnea, suggests altering of central processing.
Peripheral mechanism is another theory explaining how opioids decrease dyspnea. It is believed that opioid receptors are located in the alveolar walls and in the trachea⁄ main bronchi [31]. Demonstrated in dogs and guinea–pigs, opioid agonists inhibit the release ofacetylcholine, which mediate the constriction of pulmonary smooth muscle and decrease mucus secretion [31]. Further studies need to be demonstrated in humans.
Evidence of opioid use for dyspnea in palliative copd patients
Chronic obstructive pulmonary disease is one of the leading causes of death in the U.S. with a death rate of 40.8 per 100,000 in 2010 [32]. COPD is characterized by unpredictable pulmonary exacerbations, leading to functional decline and high symptom burden [33]. Dyspnea is the most common symptom of COPD and significantly impairs the patients’ quality of life and emotional well –being [34]. Despite being optimized on medical management, patients still suffer from breathlessness [35]. Opioid use in treating dyspnea associated with COPD was first reported by Light and colleagues in 1989 using oral morphine solution (0.8mg/kg), which resulted in lower Borg scores and increased exercise capacity [36]. Since that time a growing body of evidence has emerged supporting its efficacy
The first adequately powered randomized study supporting the use of opioids for symptomatic relief of dyspnea was conducted byAbernethy and colleagues [35]. Their study involved 48 opioid naïve patients (42 with COPD), who were randomized to four days of 20mg of sustained release morphine daily, followed by four days of placebo or vice versa. The primary outcome of the study was sensation on a visual analogue scale (0 to 100 mm with 100 being the worst possible breathlessness) on the final day of the treatment period. Thirtyeight patients completed the study and participants reported better dyspnea scores in the morphine group versus placebo (6.6mm 95%CI 1.6 to 11.6mm, P=0.011 in morning and 9.5 mm, 95% CI 3 to 16.1mm, P=0.006 in the evening). Patients in the morphine group alsoreported better sleep (P=0.039). This study was not powered enough to detect differences of side effects, but two participants withdrew due to nausea, vomiting and sedation in the morphine group.
Efficacy for the use of opioids in treatment of breathlessness, was established in the previous study and with the systematic reviewinvolving 18 clinical trials by Jennings and colleagues described earlier [23,35]. The minimum effective dose of sustained release morphine and long term data on safety and efficacy were unclear, however. A recent Phase II, open–label pharmacovigilence study by Currow and colleagues helped establish this information [37]. Eighty–three participants (45 with COPD) were initiated on 10mg of sustained release morphine daily. The dose was increased by 10mg daily each week to a maximum of 30mg for participants whofailed to respond with the 10mg daily dose. If patients, at the weeklyreview, had a 10% improvement in dsypnea over baseline, they were enrolled in a long–term Phase IV study at their current dose. Dyspnea intensity improvement was evaluated on a 100mm visual analogue scale aspreviously described. Fifty–two participants had a = 10% improvement in dyspnea over their baseline (average 35% improvement), making the response rate 62% and number needed to treat of 1.6 and number needed to harm 4.6. The appropriate dose to control dyspnea as 10mg daily for 70% of the participants. For the 52 patients who entered the phase IV study a benefit in dsypnea was still observed in a third of the patients on their current morphine dose. There were no incidents of respiratory depression, but constipation increased (P<0.001) despite use of laxatives in the study [37].
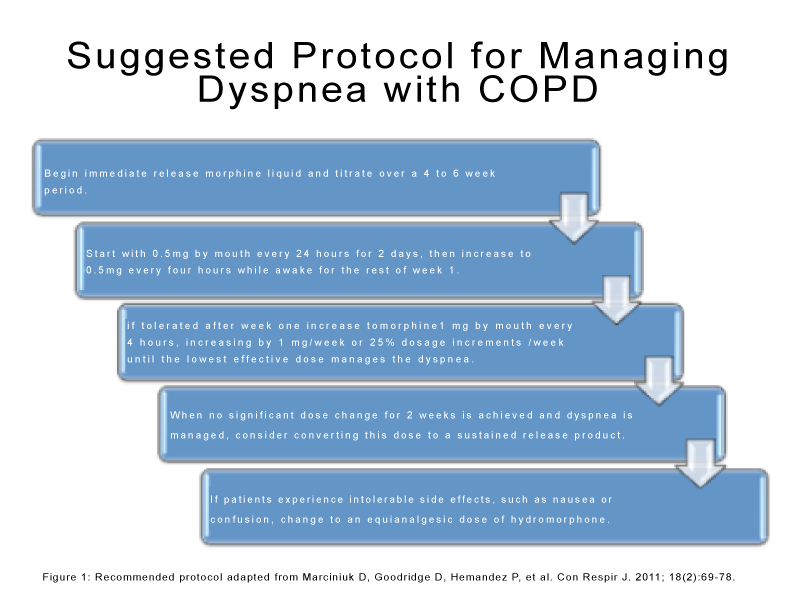
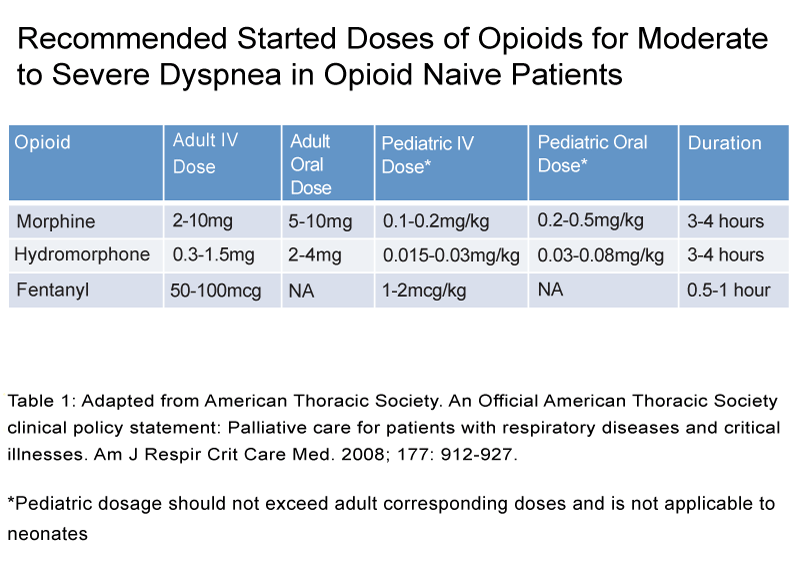
The American Thoracic Society, American College of Chest Physicians, and the Canadian Thoracic Society recommend the use of oral and parenteral opioids in the treatment of dyspnea. Their recommendations were based on the established trials for demonstrating efficacy,dose initiation and lack of major adverse effects, (See Figure 1 and Table 1). Nonetheless, the use of nebulized opioids were not recommended secondary to the lack of insufficient evidence [38–40].
Figure 1: Recommended protocol adapted from Marciniuk D, Goodridge D, Hemandez P, et al. Con Respir J. 2011; 18(2):69-78
Table 1: Adapted from American Thoracic Society. An Official American Thoracic Society clinical policy statement: Palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008; 177: 912-927.
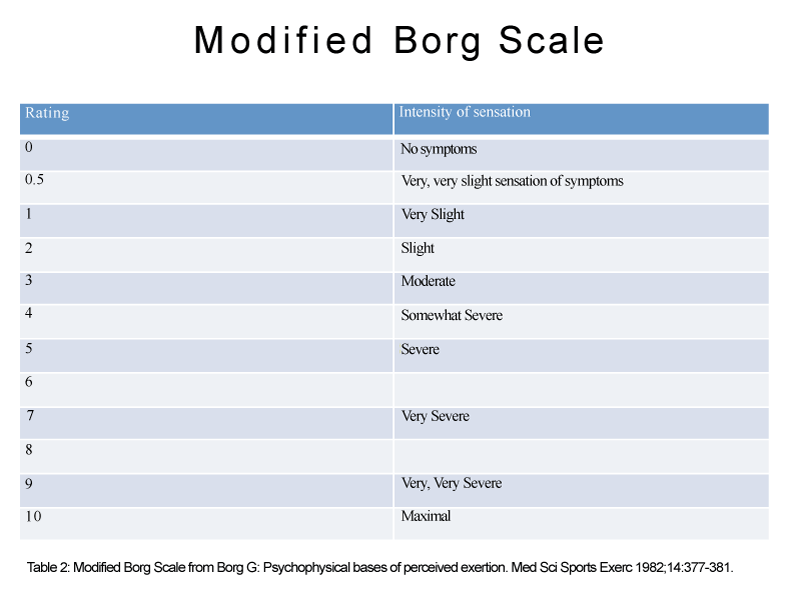
Per Jankelson and colleagues’ study, the use of nebulized morphine for dyspnea in COPD patients is not supported [41]. Nebulized morphine 20 and 40 mg per 5mL of were compared with normal saline in 16 patients with COPD. Post nebulization with each group, a 6–minute walk test was performed, then repeated one hour later to measure arterial oxygen saturation, modified Borg score (Table 2). There was no difference between either doses of morphine versus placebo with the aforementioned measures, nor was there an improvement in walking distances.
Table 2: Modified Borg Scale from Borg G: Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377-381
Similarly, another evaluation of 7 studies using nebulized opioids was not supportive [42]. Two studies only were able to evidencesome benefit. One study involving 11 patients showed improved exercise capacity; however, this effect was not reproducible on a larger scale. Another study in this systematic review involving 54 patients, showed benefit in terminal patients; however, this was a retrospective chart review. It remains to be proven whether the use of nebulized opioidshave a positive effect in a large randomized clinical trial.
Although patients may respond to opioids differently, predicting a response earlier would be optimal. Factors likely to increase the probability of opioid responsiveness in COPD patients are those that have dyspnea strongly correlated with fear and anxiety. Additionally, opioids have a better response if dyspnea intensity worsens over time, uncontrolled by pacing and behavioral interventions, and has an unpredictable onset [43].
Evidence of opioid use for dyspnea in palliative cancer patients
Dyspnea is a problem frequently encountered in terminally ill cancer patients, with 49% of patients affected and 20% reportingbreathlessness as moderate to severe [44]. Various estimates suggest that up to 70% of cancer patients suffered dyspnea during their last four weeks of life [45]. Most patients with cancer are being treated with an opioid for pain control, thus recommendations for specific dosing and titrations to control dyspnea are warranted in this population.
A study involving 15 dyspneic patients with cancer, found that subcutaneous doses of morphine 5mg helped with reducing dsypnea for 2 hours without respiratory rate changes [46]. Another study evaluating 33 terminally ill cancer patients reported that reduction ofdyspnea and tachypnea for four hours was sufficient in patients who received 25% of their 4–hourly scheduled opioid dose [47].
Cognitive impairment occurs secondary to increased sedation when administering more systemic opioids, specifically in cancer patients whom require higher doses of opioids. Nebulized opioidsmay have a role in this target population due to systemic bioavailabilty of inhaled morphine being 5±3 %, which is less than when orally administered (24±13 %) [48]. In a cross over study, anattempt was made to compare subcutaneous morphine versus nebulized morphine in 11 patients [49]. Patient’s dsypnea was measured on a 0–10 intensity scale (with 10 being the worst possible shortness of breath). Dyspnea decreased from a median of 5 to 3 after subcutaneous morphine (P=0.025) and from 4 to 2 after nebulized morphine(P=0.007); however, this trial was not powered enough to detect a true difference among the treatment groups. Nonetheless, another study involving 35 cancer patients assessed patients’ perceptions of dsypnea, oxygen saturation and respiratory rates post using nebulized fentanyl 25mcg in 2mL of normal saline [50]. Eighty one percent of patients reported improvement in breathing. Oxygen saturation improved from baseline (94.6%) at 5 minutes (96.8%) and at one hour (96.7%) (P=0.0069) after fentanyl administration. Respiratory rates also improved after the single nebulization treatment from baseline of 28.4 breaths⁄minute to 25.9 after 5 minutes and 24.1 after 1 hour. This was a single dose study; therefore, it reported no adverse effects with the treatment.
Most evidence studying refractory dyspnea in cancer patients involve morphine. A recent systematic review included 13 trials involving the use of various formulations of fentanyl in dyspnea [51]. Eleven studies were case reports and two studies were randomizedcontrolled trials. Of the 88 total patients in the systematic review, 69 had lung cancer. All studies except for one randomized controlled trial were able to demonstrate improvement in dyspnea.
New formulations for breakthrough chronic cancer pain are emerging, which could also have implications in episodic breathlessness. Several case reports have demonstrated the benefit of oral transmucosal fentanyl citrate in rapid relief of dsypnea without signs of respiratory depression [52,53]. More research is needed involving these agents to potentially add another route of delivery if the patient is opioid tolerant.
Evidence of opioid use for dyspnea in palliative chf patients
Congestive heart failure is one of the leading causes of death in the U.S. with 5.1 million people, half of which will die within 5years of diagnosis [54]. Patients can experience symptoms of pain and fatigue; however, dyspnea is the most common symptom that is estimated to occur in 88% of patients. Currently, few trials exist that exclusively evaluate patients with heart failure, given thatbreathlessness is the most common symptom [55].
Two trials are single dose trials that are specific to CHF patients. One of these trials involved dihydrocodeine, which showedimprovement in exercise tolerance, breathlessness, and reduced exercise ventilation [56]. The second trial involved diamorphine,which showed improved aerobic exercise capacity [57].
A double blind, randomized placebo cross–over pilot trial involving 10 patients with New York Heart Association Class III and IV heartfailure, compared oral morphine 5mg four times daily for four days with placebo [58]. Sixty percent of patients reported improvementin breathlessness among the morphine group. Breathlessness was assessed using a 100mm visual analog scale. The reported median breathlessness score was reduced by 23mm (P=0.022) by day 2 in the morphine group and no changes occurred in the placebo group
The most recent study exclusively in heart failure patients, conducted by Oxberry and colleagues, assessed for breathlessnesswhen comparing oral morphine 5mg four times a day, oral oxycodone.5mg four times a day, and placebo [55]. Participants rated their change inbreathlessness on an 11 point numerical rating scale. Thirty–five patients completed all three study arms without showing any statistical difference. Large randomized controlled trials with opioids are still warranted in the setting of heart failure, despitethese findings.
Conclusion
Resolving refractory dyspnea completely in both palliative and terminal stages of COPD, Cancer, and CHF may not be possible ineverypatient. Meanwhile, the use of opioids may provide relief of dyspnea when other pharmacological and non–pharmacologicalmanagement have been fully implemented. The American College of Chest Physicians, the American Thoracic Society, and the Canadian Thoracic Society recommend an individualized approach to the choice of opioid and initiation/titration of doses fororal and parenteral routes. Nebulized opioids are not recommended, nonetheless, further investigations are needed in largerandomized controlled trials.
References
- Goldstein N, Morrison R, eds. Evidence-base Practice of Palliative Medicine. Philadelphia, PA: Elsevier. 2013.
- Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008; 177: 912-927.
- Mahler DA. Opioids for refractory dyspnea. Expert Rev Respir Med. 2013; 7: 123-134.
- Elmqvist MA, Jordhøy MS, Bjordal K, Kaasa S, Jannert M. Health-related quality of life during the last three months of life in patients with advanced cancer. Support Care Cancer. 2009; 17: 191-198.
- Bausewein C, Farquhar M, Booth S, Gysels M, Higginson IJ. Measurement of breathlessness in advanced disease: a systematic review. Respir Med. 2007; 101: 399-410.`
- Elkington H, White P, Addington-Hall J, Higgs R, Edmonds P. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med. 2005; 19: 485-491.
- Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009; 12: 29-36.
- Simon ST, Higginson IJ, Benalia H, Gysels M, Murtagh FE. Episodic and continuous breathlessness: a new categorization of breathlessness. J Pain Symptom Manage. 2013; 45: 1019-1029.
- Simon ST, Weingärtner V, Higginson IJ, Voltz R, Bausewein C. Definition, Categorization, and Terminology of Episodic Breathlessness: Consensus by an International Delphi Survey. J Pain Symptom Manage. 2013
- Wiseman R, Rowett D, Allcroft P, Abernethy A, Currow DC. Chronic refractory dyspnoea--evidence based management. Aust Fam Physician. 2013; 42: 137-140.
- Horton R, Rocker G. What is the role of opioids in treatment of refractory dyspnea in advanced chronic obstructive pulmonary disease. In: Goldstein N, Morrison R, eds. Evidence-base Practice of Palliative Medicine. Philadelphia, PA: Elsevier. 2013.
- Currow DC, Ward AM, Abernethy AP. Advances in the pharmacological management of breathlessness. Curr Opin Support Palliat Care. 2009; 3: 103-106.
- Hallenbeck J. Pathophysiologies of dyspnea explained: why might opioids relieve dyspnea and not hasten death? J Palliat Med. 2012; 15: 848-853.
- Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010; 39: 680-690.
- O'Donnell DE, Banzett RB, Carrieri-Kohlman V, Casaburi R, Davenport PW. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc. 2007; 4: 145-168.
- von Leupoldt A, Dahme B. Cortical substrates for the perception of dyspnea. Chest. 2005; 128: 345-354.
- Abernethy A, Kamal A, Maguire J, Currow D. What interventions are effective for managing dyspnea in cancer. In: Goldstein N, Morrison R, eds. Evidence-base Practice of Palliative Medicine. Philadelphia, PA: Elsevier; 2013.
- Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000; 19: 357-362.
- Kvaal K, Macijauskiene J, Engedal K, Laake K. High prevalence of anxiety symptoms in hospitalized geriatric patients. Int J Geriatr Psychiatry. 2001; 16: 690-693.
- Kamal AH, Maguire JM, Wheeler JL, Currow DC, Abernethy AP. Dyspnea review for the palliative care professional: treatment goals and therapeutic options. J Palliat Med. 2012; 15: 106-114.
- Mahler DA, Murray JA, Waterman LA, Ward J, Kraemer WJ. Endogenous opioids modify dyspnoea during treadmill exercise in patients with COPD. Eur Respir J. 2009; 33: 771-777.
- Colman AS, Miller JH. Modulation of breathing by mu1 and mu2 opioid receptor stimulation in neonatal and adult rats. Respir Physiol. 2001; 127: 157-172.
- Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002; 57: 939-944.
- Yamanaka T, Sadikot RT. Opioid effect on lungs. Respirology. 2013; 18: 255-262.
- [No authors listed] Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999; 159: 321-340.
- Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008 100: 747-758.
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003; 37: 821-826.
- Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002; 88: 1500-1511.
- Pattinson KT, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR. Opioids depress cortical centers responsible for the volitional control of respiration. J Neurosci. 2009; 29: 8177-8186.
- Firestone LL, Gyulai F, Mintun M, Adler LJ, Urso K. Human brain activity response to fentanyl imaged by positron emission tomography. Anesth Analg. 1996; 82: 1247-1251.
- Zebraski SE, Kochenash SM, Raffa RB. Lung opioid receptors: pharmacology and possible target for nebulized morphine in dyspnea. Life Sci. 2000; 66: 2221-2231.
- https://www.cdc.gov/copd/data.htm.
- Young J, Donahue M, Farquhar M, Simpson C, Rocker G. Using opioids to treat dyspnea in advanced COPD: attitudes and experiences of family physicians and respiratory therapists. Can Fam Physician. 2012; 58: e401-407.
- Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000; 55: 1000-1006.
- Abernathy A, Currow D, Frith P, Fazekas B, McHugh A, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnea. Br Med J. 2003; 327: 523-528.
- Light RW, Muro JR, Sato RI, Stansbury DW, Fischer CE. Effects of oral morphine on breathlessness and exercise tolerance in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989; 139: 126-133.
- Currow DC, McDonald C, Oaten S, Kenny B, Allcroft P. Once-daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. J Pain Symptom Manage. 2011; 42: 388-399.
- Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri-Kohlman V. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010; 137: 674-691.
- Marciniuk DD, Goodridge D, Hernandez P, Rocker G, Balter M, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011; 18: 69-78.
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012; 185: 435-452.
- Jankelson D, Hosseini K, Mather LE, Seale JP, Young IH. Lack of effect of high doses of inhaled morphine on exercise endurance in chronic obstructive pulmonary disease. Eur Respir J. 1997; 10: 2270-2274.
- Foral PA, Malesker MA, Huerta G, Hilleman DE. Nebulized opioids use in COPD. Chest. 2004; 125: 691-694.
- Horton R, Rocker G, Currow D. The dyspnea target: can we zero in on opioid responsiveness in advanced chronic obstructive pulmonary disease? Curr Opin Support Palliat Care. 2010; 4: 92-96.
- Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage. 2001; 21: 95-102.
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986; 89: 234-236.
- Bruera E, Macmillan K, Pither J, MacDonald RN. Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage. 1990; 5: 341-344.
- Allard P, Lamontagne C, Bernard P, Tremblay C. How effective are supplementary doses of opioids for dyspnea in terminally ill cancer patients? A randomized continuous sequential clinical trial. J Pain Symptom Manage. 1999; 17: 256-265.
- Masood AR, Thomas SH. Systemic absorption of nebulized morphine compared with oral morphine in healthy subjects. Br J Clin Pharmacol. 1996; 41: 250-252.
- Bruera E, Sala R, Spruyt O, Palmer JL, Zhang T. Nebulized versus subcutaneous morphine for patients with cancer dyspnea: a preliminary study. J Pain Symptom Manage. 2005; 29: 613-618.
- Coyne PJ, Viswanathan R, Smith TJ. Nebulized fentanyl citrate improves patients' perception of breathing, respiratory rate, and oxygen saturation in dyspnea. J Pain Symptom Manage. 2002; 23: 157-160.
- Simon ST, Köskeroglu P, Gaertner J, Voltz R. Fentanyl for the relief of refractory breathlessness: a systematic review. J Pain Symptom Manage. 2013; 46: 874-886.
- Benitez-Rosario MA, Martin AS, Feria M. Oral transmucosal fentanyl citrate in the management of dyspnea crises in cancer patients. J Pain Symptom Manage. 2005; 30: 395-397.
- Gauna AA, Kang SK, Triano ML, Swatko ER, Vanston VJ. Oral transmucosal fentanyl citrate for dyspnea in terminally ill patients: an observational case series. J Palliat Med. 2008; 11: 643-648.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association . Circulation. 2013;127:e6–e245.
- Oxberry SG, Torgerson DJ, Bland JM, Clark AL, Cleland JG. Short-term opioids for breathlessness in stable chronic heart failure: a randomized controlled trial. Eur J Heart Fail. 2011; 13: 1006-1012.
- Chua TP, Harrington D, Ponikowski P, Webb-Peploe K, Poole-Wilson PA. Effects of dihydrocodeine on chemosensitivity and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 1997; 29: 147-152.
- Williams SG, Wright DJ, Marshall P, Reese A, Tzeng BH. Safety and potential benefits of low dose diamorphine during exercise in patients with chronic heart failure. Heart. 2003; 89: 1085-1086.
- Johnson MJ, McDonagh TA, Harkness A, McKay SE, Dargie HJ. Morphine for the relief of breathlessness in patients with chronic heart failure--a pilot study. Eur J Heart Fail. 2002; 4: 753-756.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982; 14: 377-381.