
Opinion
Austin J Pharmacol Ther. 2023; 11(3): 1180.
“In Rats, the Chemotherapeutic Drug Vincristine-Induced Neuropathic Nociception is Suppressed When Cannabinoid CB1 and CB2 Receptors are Activated”
Hari Prasad Sonwani*
Apollo College of Pharmacy, Anjora, Durg CG, India
*Corresponding author: Hari Prasad Sonwani Apollo College of Pharmacy, Anjora, Durg CG, India. Email: [email protected]
Received: November 10, 2023 Accepted: December22, 2023 Published: December 29, 2023
Abstract
Rats were used to test the potential of cannabis to reduce mechanical hypersensitivity, or mechanical allodynia, which was brought on by vincristine chemotherapy. After that, action sites were located. Method of experimentation: After ten daily injections of vincristine, mechanical hypersensitivity developed in comparison to those that received saline at the same periods. The effects on chemotherapy-induced neuropathy were assessed for the CB1/CB2 receptor agonist WIN55, 212-2, the receptor-inactive enantiomer WIN55, 212-3, the CB2-selective agonist (R,S)-AM1241, the opiate agonist morphine, and vehicle. To determine the locations of action, WIN55, 212-2 was injected either locally in the hind paw or intrathecally (i.t.). By employing competitive antagonists for either CB1 (SR141716) or CB2 receptors (SR144528), pharmacological selectivity was demonstrated. Vincristine-evoked mechanical allodynia was decreased when WIN55, 212-2, but not WIN55, 212-3, were administered systemically. A change in the dose-response curve to the left was noticed after WIN55, 212-2 in comparison to morphine therapy. Antibodies of CB1 (SR141716) and CB2 (SR144528) inhibited WIN55, 212-2’s anti-allodynic actions. Via a CB2 mechanism, (R,S)-AM1241 reduced c-induced mechanical hypersensitivity. Without causing catalepsy, both cannabinoid agonists reduced the mechanical hypersensitivity brought on by vincristine. Cannabis-induced neuropathy may be modulated by cannabinoids at spinal sites of action. When delivered intraperitoneally, WIN55, 212-2 but not WIN55,212-3 inhibited vincristine-evoked mechanical hypersensitivity at dosages that were inert after local hindpaw injection. Spinal co-administration of CB1 and CB2 antagonists inhibited WIN55,212-2’s anti-allodynic effects.By activating CB1 and CB2 receptors, cannabinoids inhibit the maintenance of vincristine-induced mechanical allodynia. The spinal cord is involved in the mediation of these anti-allodynic actions, at least partially.
Keywords: CB1/CB2 receptor; Hypersensitivity; Hypersensitivity; Chemotherapy
Introduction
A common side-effect of several different kinds of chemotherapy drugs is painful peripheral neuropathy. Examples of these groups of drugs include vinca alkaloids (like vincristine), compounds derived from taxanes (like paclitaxel), and compounds derived from platinum (like cisplatin). According to Sandler et al. (1969), Polomano and Bennett (2001a), Bacon et al. (2003), Cata et al. (2006)b, and other studies, the incidence and severity of chemotherapy-induced neuropathy are influenced by the type of cancer, the dose schedule, the choice of chemotherapeutic drug, and the existence of concurrent medical issues.It has been suggested that vincristine causes cytoskeletal structural changes and microtubule disorientation to produce anti-tumor effects [56,58]. In the periphery, where the effects of disrupted axonal transport would first become apparent, neurofilament buildup in cell bodies and proximal axons may cause paraesthesiae and dysesthesiae (Topp) in 2000, et al. It has also been noted that chemotherapy-induced neuropathy occurs when primary afferents are not morphologically damaged; these subsequent investigations show that microtubule disruption is not a prerequisite for chemotherapy-induced neuropathy [48]. Dysregulation of cellular calcium homoeostasis due to aberrant mitochondrial action may be the cause of chemotherapy-induced neuropathy (Flatters and Bennett, 2006; Siau and Bennett, 2006). According to Jackson et al. (1988), vincristine-induced neuropathy restricts the duration and dosage of anti-cancer treatments that may save lives. Patients are frequently recommended aspirin, ibuprofen, and celebrex to treat chemotherapy-induced neuropathy; nevertheless, their effectiveness is limited [32]. The discovery of potent substitute analgesics is a critical medical necessity since there are currently no approved therapies for chemotherapy-induced neuropathy. In animal models of traumatic nerve injury, cannabinoids—drugs that have the same target as cannabis's psychoactive component, D9-tetrahydrocannabinol—suppress neuropathic nociception through mechanisms specific to cannabinoid CB1 and CB2 receptors [6,14,17,24,31,51,64]. The central nervous system has the highest density of CB1 receptors. [68] on the CNS. The majority of CB2 receptors are expressed [7,39], although they are not the only ones (Van Sickle et al., 2005; Outside the central nervous system [5]. After spinal nerve ligation, CB2 is significantly upregulated in the rat spinal cord and dorsal root ganglion [5,65,67], indicating that CB2-mediated antihyperalgesic effects in neuropathic pain states may be mediated by additional neuroanatomical substrates. Paclitaxel-induced neuropathic nociception is suppressed by the mixed CB1/CB2 receptor agonist WIN55,212-2 via a CB1 mechanism [45]. Nevertheless, little is known about the mechanisms behind the emergence of excruciating peripheral neuropathies brought on by various chemotherapy drugs (for a review, see Cata et al., 2006b). Different symptoms of neuropathic pain complied with the International Association for the Study of Pain's recommendations for treating animals [69]. Following the relevant institutional procedures, bedding containing metabolized vincristine was handled as biohazardous waste and disposed of.
Typical Experimental Techniques
In order to stop behavioral sensitization to cutaneous stimulation from developing, the effects of the drugs were assessed using just one stimulus modality. On day zero, baseline reactions to mechanical or thermal stimulation of the hindpaw were determined. After undergoing behavioral testing, the rats were given intraperitoneal (i.p.) injections of vincristine sulphate (0.1 ml/kg/day i.p.) or saline (1 ml/kg/day i.p.) every day for a period of 12 days. The five daily injections were part of the treatment paradigm, which was followed by a two-day break during which no injections were given, and then five more daily injections as explained earlier on. [63]. The experimenter was blinded to the drug condition in every study. Every day, weights were recorded.
Evaluation of Mechanical Withdrawal Limits
A digital Electrovonfrey Anesthesiometer (IITC model Alemo 2290-4; Woodland Hills, CA, USA) with a stiff tip was used to measure mechanical withdrawal thresholds. Rats were arranged on a raised mesh platform beneath plastic cages that had been turned inside out. Prior to testing, the rats were given ten to fifteen minutes to acclimate to the chamber. The floor of the mesh platform was used to apply stimulation to the midplantar area of the hind paw. As paw withdrawal ended mechanical stimulation, there was no higher threshold limit established at which a trial may end. Each paw's mechanical withdrawal threshold was measured twice before and 24 hours after each vincristine or saline infusion. On day 11, the final injection of either saline or vincristine was given. Baseline mechanical withdrawal thresholds were measured on test day (day 12), which was around 24 hours after the last vincristine or saline injection. Additionally, the effects of pharmacological interventions were tested. When vincristine was administered to rats at pressures (g) that did not produce withdrawal symptoms prior to chemotherapeutic treatment, nocifensive responses were seen. Measuring mechanical paw withdrawal thresholds with the Electrovonfrey Anesthesiometer, vincristine-induced reductions were thus classified as mechanical allodynia. The animals treated with vincristine were given systemic injections of either vehicle (n ¼ 8) or WIN55, 212-2 (0.75, 1.5, or 2.5 mg kg—1 i.p.; n ¼ 8 per group) after their baseline mechanical withdrawal thresholds were assessed on day 12. Various groupings were given The available options are the CB2-selective agonist AM1241, the receptor-inactive enantiomer WIN55, 212-3 (2.5 mg kg—1 i.p.; n ¼ 8), or the opiate agonist morphine (2.5 or 8 mg kg—1 i.p.; n ¼ 8 and 4, respectively). The low-dose morphine was chosen since it was shown to both elicit antinociception [25] and decrease neuropathic pain behavior in a spinal nerve ligation model [27,31]. The dosage of AM1241 used was comparable to the amount that, after spinal nerve ligation, restored mechanical paw withdrawal thresholds [50]. Groups were given WIN55,212-2 (2.5 mg kg—1 i.p.) in conjunction with either SR141716 (2.5 mg kg—1 i.p.; n ¼ 8) or SR144528 (2.5 mg kg—1 i.p.) to ascertain the pharmacological specificity. (i.p.; n ¼ 8) and AM1241 (2.5 mg kg—1 i.p.) were jointly administered An antagonist given alone (n ¼ 8 per group), SR141716 (2.5 mg kg—1 i.p.; n ¼ 8) or SR144528 (2.5 mg kg—1 i.p.; n ¼ 8). In every study, mechanical withdrawal thresholds were assessed on day 12, about 24 hours after the final vincristine injection. The drug or vehicle withdrawal thresholds were measured prior to injection (baseline), as well as thirty and sixty minutes thereafter. 31 days after the last vincristine injection, rats treated with vincristine and given a vehicle were also assessed for the existence of mechanical allodynia as a potential indicator of a remission of the painful peripheral neuropathy caused by the drug.
Evaluation of Thermal Latencies during Paw Withdrawal
Paw withdrawal latencies to radiant heat were measured for each paw in duplicate using a commercially available device and the Hargreaves test [16]. The IITC model 33 plantar stimulation machine, located in Woodland Hills, California, USA. Rats were arranged on an elevated glass platform, under inverted plastic cages. Before testing, rats were given ten to fifteen minutes to become accustomed to the equipment. The floor of the glass platform allowed radiant heat to reach the midplantar area of the hind paw. To avoid damaging the tissue, stimulation was stopped when the paw was removed or after twenty seconds. The report presents the average of two sets of duplicate results, averaged across paws, for thermal paw withdrawal latencies. Thermal withdrawal latencies were measured prior to (day 0) and after (days 3, 6, 9, and 12) after either As previously mentioned, either saline (n ¼ 6) or vincristine (n ¼ 12). The same animals were then examined for the mechanical allodynia (on day 12) as determined by the previously mentioned techniques.
Catheter Insertion Intrathecal
Through an incision in the atlanto-occipital membrane, intrathecal catheters (PE10 tubing, Clay Adams, Parsippany, NJ, USA) were surgically implanted under pentobarbital/ketamine anesthesia into the spinal subarachnoid space [22,66]. The distal end of the catheters was heat-sealed after they were inserted to a depth of 8.5 cm and fastened to the skull. Any indicators of motor impairment caused by catheter implantation, such as difficulty walking on a wire cage cover or impaired righting reflex, were instantly fatal to the animals. Ten percent or so of the animals that had catheters implanted had motor impairment, and as a result, they were never tested again or given vincristine or saline treatment. Animals had permission to must heal for a minimum of five days after surgery before baseline paw withdrawal thresholds are established and vincristine or saline is started.
Location of the Incident
A preliminary study was conducted to ascertain whether intraperitoneal injection (i.t.) of the b-cyclodextrin vehicle (n ¼ 6) affected mechanical withdrawal thresholds in comparison to groups that had catheter implantation surgery but did not receive an i.t. injection (n ¼ 4). An additional vincristine-treated groups were given either WIN55,212-3 (10 mg i.t., n ¼ 6) or WIN55,212-2 (10 mg or 30 mg i.t.; n ¼ 6 each group). To ascertain In order to determine the pharmacological specificity of cannabis activities, two different groups were given WIN55,212-2 (30 mg intravenously) in combination with either SR141716 (30 mg intravenously; n ¼ 8) or SR144528 (30 mg intravenously; n ¼ 8)), WIN55,212-2 (30 mg intravenously) coadministered utilizing both SR141716 (30 mg intramuscular) and SR144528 (30 mg intramuscular) given simultaneously (n ¼ 6) or SR144528 (30 mg intramuscular; n ¼ 6) or SR141716 (30 mg intramuscular; n ¼ 5) given separately. Mechanical paw withdrawal thresholds were assessed every day in all of the investigations as previously said to confirm that the administration of vincristine caused mechanical allodynia in comparison to groups that were given saline (n ¼ 9) at the same periods. After testing, a post-mortem injection of Fast green dye and subsequent dissection were used to confirm the catheter's location.
No animals showed signs of tissue injury from the catheter implantation. Mechanical withdrawal thresholds were assessed in every study (on the day12) around twenty-four hours after the previous vincristine injection. The baseline paw withdrawal thresholds were tested in duplicate, as well as five, thirty, and sixty minutes after the injection. of a substance or car. In order to assess potential cannabinoid activity peripheral areas, WIN55, 212-2 or vehicle was applied topically to the paw. On the day of the test, each animal received a unilateral intraplantar (i.pl.) injection into the plantar surface of the hindpaw (day 12). Rats treated with vincristine were given either WIN55, 212-2 (30 or 150 mg; n ¼ 9 per group) or vehicle locally in the hindpaw (n ¼ 7). The subjects received injections into their right and left paws in equal amounts. All animals had their thresholds measured before (baseline) and 30 minutes after injection, in both the injected and non-injected paws.
Catalepsy Examination
On test day 12, catalepsy testing was carried out on rats that had previously been assessed for their reactivity to temperature stimuli using the bar test [38,46]. After the measurement of thermal paw withdrawal latencies, the rats were placed back into their home cages for a minimum of half an hour before the baseline catalepsy assessment was initiated.
The animals were positioned as previously mentioned (Martin et al., 1996) with their forepaws hanging over a stainless steel bar that was suspended 9 cm above a level platform. In animals treated with vincristine and given either a vehicle (n ¼ 6) or WIN55, 212-2 (2.5 mg kg—1 i.p.; n ¼ 6), catalepsy was reassessed. A different set of mice treated with vincristine (who did not have thermal test) were given AM1241, which is 2.5 mg kg-1 i.p.; n ¼ 6. For example, WIN55, 212-2 (2.5 or 10 mg kg—1 i.p.; n ¼ 6 per group) was given to two groups of otherwise naive rats. The time I stood there at the bar was measured for each group in triplicate at 30, 45, and 60 minutes after the medication injection.
Examinations of Statistics
For repeated measures, Analysis of Variance (ANOVA) or planned comparison unpaired t-tests were used for data analysis when applicable. The Greenhouse-Geissner adjustment was implemented for every element that was repeated. Additionally, post-drug thresholds and pre-vincristine thresholds were compared using paired t-tests. (baseline) cutoff points. Using the following formula, the percent (%) reversal of mechanical allodynia was determined at the moment of maximal cannabis anti-allodynic efficacy: Using Fisher's protected least significant difference (PLSD) test, post hoc comparisons were executed. It was decided that Po0.05 was statistically significant.
Chemicals and Drugs
Tocris Cookson provided the vincristine sulphate (Ellisville, MO, USA). R(þ)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl] WIN55,212-23-de pyrrolo[1,2,3]One (1) 1,4-benzoxazin-ylWIN55, 212-3 (S(–)-[2,3-dihydro-5-methyl-3-[(4-morpholinyl)methyl]), -(1-naphthalenyl)methanone mesylate3-de pyrrolo[1,2,3]One (1) 4,4-benzoxazinylSigma Aldrich (St. Louis, MO, USA) provided the morphine sulfate, b-cyclodex-trin, and -(1-naphthalenyl)metha-none mesylate salt. (S, R)AM1241, ((R,S)-(2-iodo-5-nitro-phenyl)-[l-(l-methyl-piperidin-2-ylmethyl)-lH-indol-3-yl]-methanone) was produced. sized at one of the authors' laboratories (AM). Asymmetric pyrazole-3-carboxamide N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)- 4-methyl-1H-pyrazole and N-[(1S)- endo-1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl] SR144528-5-(4-chloro-3- methylphenyl) NIDA supplied the -1-(4-methylbenzyl)-pyrazole-3-carboxamide. A 0.9% saline solution was used to dissolve vincristine sulfate. Every other medication was dissolved in awith one exception, a systemic delivery vehicle consisting of 10% ethanol, 10% emulation, and 80% saline delivered at a volume of 1 ml/kg bodyweight. Because of solubility restrictions, antagonists were supplied in conjunction with AM1241 in certain trials, with a total injection volume of 1.5 ml/kg. For intraperitoneal and intrapleural administration, drugs were dissolved in 45% b-cyclodextrin according to the earlier description [22]. For intraperitoneal and intrapleural administration, the drug or vehicle was given in amounts of 10 and 50 ml, respectively.
Outcomes
Overall outcomes: Before either saline or vincristine was administered, there was no difference in body weight between the groups. In rats treated with saline, normal weight growth was noted throughout the injection period (F1,40 ¼ 41.515, Po0.0002; Figure 1a). In contrast, individuals receiving vincristine demonstrated a lack of gaining weightat all times after injection (F11,440, 23.32, Po0.0002, Po0.001 for every comparison; Figure 1a). Figure 1a displays variations in body weight for the groups depicted in Figure 1b during vincristine or saline treatment. In vincristine-treated mice receiving vehicle (i.p.), mechanical hypersensitivity had fully gone by 31 days after the last injection, and normal weight growth was noted (data not shown).The way that subjects responded to mechanical and thermal stimuli did not change in research using systemic or intrathecal injections. For each group on any given day, the right and left paws; as a result, withdrawal thresholds are shown as the average of the repeated measurements, averaged across paws. Results for the injected and non-injected paws are presented separately in trials using unilateral i.pl. Injections. Vincristine reduced paw withdrawal thresholds to mechanical stimulation (i.e., in each paw) in every study (Po0.0002 for all experiments; Figures 1b, 2, 5a, and 7). In a subset of groups, there were slight baseline variations in paw withdrawal thresholds prior to vincristine delivery (Po0.01 for each study; Figures 3a, c, and 6a). On test day, however, there was no difference in mechanical withdrawal thresholds prior to pharmacological interventions in any research between vincristine-treated groups. Three animals were not employed because they did not experience vincristine-induced hypersensitivity. In the pharmacological tests that followed. Evaluation of mechanical allodynia after WIN55, 212-2 is administered systemically WIN55, 212-2 caused a dose-dependent rise in mechanical withdrawal thresholds in rats given vincristine. The day 12 (preinjection) paw withdrawal thresholds established before to pharmacological treatments (F6, 56 6.628, Po0.0002) and in relation to the vehicle (F3, 28 ¼ 5.141, Po0.006, Figure 3a). The effects of the intermediate (1.5 mg kg—1 i.p.) and low (0.75 mg kg—1 i.p.) doses of WIN55, 212-2 were outlasted by the high dose (2.5 mg kg—1 i.p.) of the drug (Po0.02 for all comparisons). The high dose also generated the maximum suppression of mechanical hypersensitivity. Mechanical withdrawal thresholds were efficiently normalized in relation to previncristine levels by the high dose of WIN55, 212-2 (one-tailed t-test, P ¼ 0.059). Thirty minutes after the medication injection, WIN55, 212-2 caused a dose-dependent reversal of mechanical allodynia (F3,28 14.829, Po0.0002; Figure 3b). More than 50% of mechanical allodynia was reversed by the intermediate and low doses of WIN55, 212-2 (0.75 and 1.5 mg kg—1 i.p.) (Po.01 for all comparisons). The large amount 30 minutes after injection, the highest suppression of mechanical hypersensitivity was achieved with a dose of WIN55, 212-2 (2.5 mg kg—1 i.p.) (Po0.002 for all comparisons; Figure 3b).
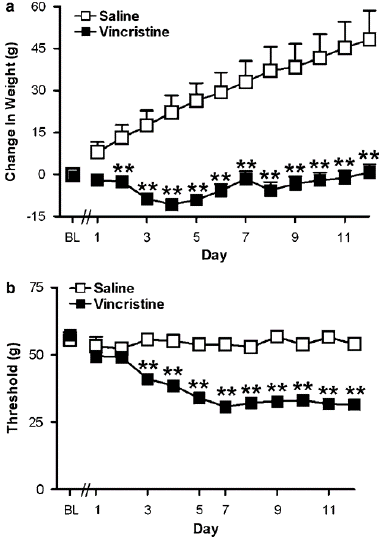
Figure 1: (a) Normal weight gain was absent in groups treated with the chemotherapeutic agent vincristine, relative to saline-treated controls. (b) Time course of vincristine-induced mechanical allodynia, as demonstrated by a lowering of the threshold for paw withdrawal to punctuate mechanical stimulation. Data are mean 7s.e.m. **Po0.001 different from control conditions (ANOVA and Fisher’s PLSD post hoc test).
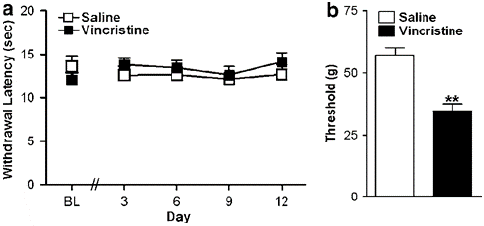
Figure 2: (a) Vincristine did not induce hypersensitivity to thermal stimulation relative to the control condition. (b) The same vincristine-treated animals showed robust mechanical allodynia (on day 12). Data are means7s.e.m. **Po0.001 different from control conditions (ANOVA). N ¼ 6–12 per group.
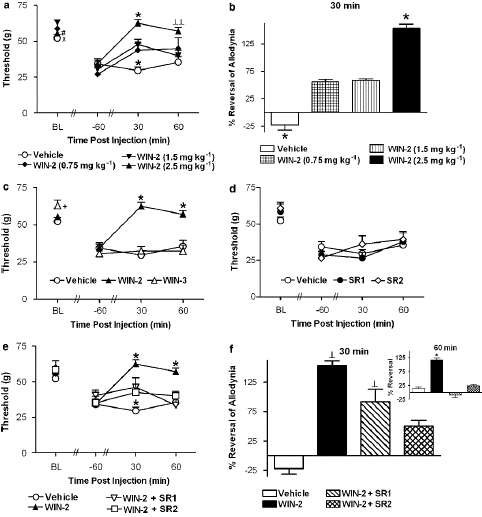
Figure 3: (a) The CB1/CB2 agonist WIN55,212-2 (WIN-2; 2.5, 1.5 and 0.75 mg kg—1 i.p.) induced a dose-dependent suppression of vincristine-induced mechanical allodynia, as demonstrated by an increase in the mechanical paw withdrawal threshold (on day 12). In all panels, BL denotes the baseline, day 0, paw withdrawal threshold assessed before vincristine or saline treatment. (b) WIN55,212-2 (2.5 mg kg—1 i.p.) produced a maximal reversal of mechanical allodynia at 30 min post-injection. (c) WIN55,212-2 (2.5 mg kg—1 i.p.) suppressed vincristine-evoked mechanical allodynia relative to the receptor-inactive enantiomer WIN55,212-3 (WIN-3; 2.5 mg kg—1 i.p.) or vehicle. (d) The CB1 antagonist SR141716 (SR1; 2.5 mg kg—1 i.p.) and the CB2 antagonist SR144528 (SR2; 2.5 mg kg—1 i.p.) did not alter vincristine-induced mechanical allodynia relative to vehicle. (e) Blockade of WIN55,212-2-induced anti-allodynia by SR141716 and SR144528.
According to Figure 3c, WIN55, 212-2 (2.5 mg kg—1 i.p.) suppressed mechanical hypersensitivity in comparison to treatment with vehicle or the receptor-inactive enantiomer WIN55, 212-3 (2.5 mg kg—1 i.p.); this increase in mechanical withdrawal thresholds was caused by WIN55, 212-2 and was receptor-mediated (F2,21 17.78, Po0.0002 for each comparison). Paw withdrawal thresholds were likewise raised in comparison to day 12 preinjection thresholds by the active enantiomer but not by the inactive one (F4, 42 ¼ 11.236, Po0.0005; Figure 3c). At no stage did the mechanical withdrawal thresholds of the animals treated with WIN55, 212-3 change from the vehicle.
Pharmacological Specificity
In vincristine-treated rats, administration of the CB1-selective antagonist SR141716 (2.5 mg kg—1 i.p.) or the CB2-selective antagonist SR144528 (2.5 mg kg—1 i.p.) did not alter paw withdrawal thresholds relative to vehicle (Figure 3d).However, both antagonists blocked the suppression of vincristine-evoked mechanical allodynia induced by WIN55,212-2 (F3,28 ¼ 5.79, Po0.004; Po0.05 for each comparison; Figure 3e) and this blockade was time-dependent(F6,56 ¼ 9.51, Po0.0002). Post hoc comparisons failed to reveal a differential blockade of the anti-allodynic effects of WIN55, 212-2 following treatment with either antagonist. Paw withdrawal thresholds were higher in groups receiving WIN55, 212-2 alone compared to either antagonist coadminis- tration group. Partial and complete blockade of the WIN55,212-2-induced attenuation of vincristine-induced mechanical hypersensitivity was observed at 30 and 60 min post-injection, respectively (Po0.05 for each comparison; Figure 3e).WIN55,212-2 (2.5 mg/kg i.p.) produced 4100% reversal of vincristine-evoked mechanical allodynia relative to vehicle treatment at 30 min post-injection (F3,28 4.009, Po0.02; Figure 3f). At this time point, SR144528 (Po0.005, planned comparison t-test), but not SR141716, reliably attenuated the anti-allodynic effects of WIN55, 212-2. Planned comparisons failed to reveal significant differences in reversal of vincristine- evoked mechanical allodynia observed following WIN55, 212-2 coadministration with either SR144528 or SR141716 (P40.26). By 60 min post-injection, both SR141716 and SR144528 produced a complete reversal of the WIN55, 212-2-induced suppression of mechanical allo- dynia (F3,28 ¼ 9.123, Po0.0003; Po.002 for all comparisons; Figure 3f, inset). Assessment of mechanical allodynia following systemic administration of AM1241 and morphine WIN55, 212-2 (2.5 mg kg—1 i.p.) and morphine (8 mg kg—1 i.p.) suppressed vincristine-evoked mechanical allodynia (F4,31 ¼ 9.513, Po0.0002; Figure 4a) relative to treatment with either vehicle, the CB2-selective agonist AM1241 or the lower dose (2.5 mg kg—1 i.p.) of morphine (Po0.01 for each comparison). The time course of anti-allodynic effects observed was differentially affected by the experimental treatments (F8, 62 ¼ 3.926, Po0.002). The suppression of vincristine-evoked mechanical allodynia induced by WIN55, 212-2 (2.5 mg kg—1 i.p.) was comparable to the high dose (8 mg kg—1 i.p.) of morphine. By contrast, paw with- drawal thresholds in groups receiving the lower dose of morphine (2.5 mg kg—1 i.p.) did not differ from vehicle at any time point. A leftward shift in the dose–response curve for post-drug paw withdrawal thresholds was also observed for WIN55, 212-2 relative to morphine (Figure 4b). AM1241 (2.5 mg kg—1 i.p.) also suppressed vincristine-evoked mecha- nical allodynia relative to vehicle and the low dose of morphine (2.5 mg kg—1 i.p.). This suppression was maximal at 30 min post-injection (Po0.05 for all comparisons; Figure 4a). The anti allodynic effect of WIN55,212-2 (2.5 mg kg—1 i.p.) was greater (Po0.05) and of longer duration than that induced by AM1241 (Figure 4a). The AM1241-induced suppression of vincristine-induced mecha- nical hypersensitivity was similar to that induced by the low and middle doses of WIN55,212-2 (0.75 and 1.5 mg kg—1 i.p., respectively); thresholds were elevated at 30 min post- injection and returned to vehicle levels by 60 min post-drug (Po0.04 for all comparisons; Figures 4b and c). The AM1241-induced suppression of mechanical allodynia was mediated by CB2 receptors (F2, 21 ¼ 8.58, Po0.002, Figure 4d). The anti-allodynic effects of AM1241 were blocked by the CB2 antagonist SR144528 (2.5 mg kg—1 i.p.; Po0.003) but not by the CB1 antagonist SR141716.
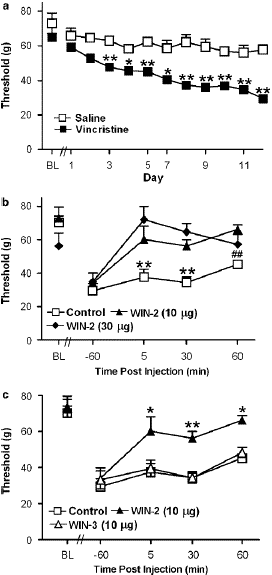
Figure 4: (a) Time course of development of vincristine-induced mechanical allodynia in rats implanted with i.t. catheters. (b) The CB1/CB2 agonist WIN55, 212-2 (WIN-2; 10 and 30 mg i.t.) suppressed vincristine-induced mechanical allodynia. (c) WIN55, 212-2 (10 mg i.t.) suppressed vincristine-evoked mechanical allodynia relative to the receptor-inactive enantiomer WIN55, 212-3 (WIN-3; 10 mg i.t.) or the control condition. Data are means7s.e.m. **Po0.01, *Po0.05 different from all groups, ##Po0.01 different from WIN55, 212-2 (10 mg i.t.) (ANOVA and Fisher’s PLSD post hoc test). N ¼ 6–9 per group.
Assessment of Spinal Site of Cannabinoid Action
Mechanical withdrawal thresholds did not differ between vincristine-treated groups receiving the b-cyclodextrin vehicle (i.t.) and controls that were surgically implanted with catheters but did not receive an injection (i.t.). Therefore, these groups were pooled into a single control group for subsequent statistical analysis of drug effects. In vincristine- treated rats, administration of the CB1/CB2 agonist WIN55, 212-2 (10 and 30 mg i.t.) increased mechanical withdrawal thresholds relative to either the control condition (F2, 19 ¼ 11.499, Po0.0006, Figure 5b) or to day 12 preinjection levels (F6, 57 ¼ 2.698, Po0.04; Figure 5b). Post hoc analyses failed to discriminate between the two doses of WIN55, 212-2 (10 and 30 mg i.t.) at any time point. The WIN55, 212-2-induced increase in mechanical with- drawal thresholds was receptor-mediated (F2, 19 7.152, Po0.005; Figure 5c). WIN55, 212-2 (10 mg i.t.) suppressed vincristine-evoked mechanical hypersensitivity relative to treatment with its receptor-inactive enantiomer WIN55,212-3 (10 mg, i.t) or the control condition (Po0.02 for each compa- rison). Mechanical withdrawal thresholds in WIN55,212-3- treated animals did not differ from control levels at any time point (Figure 5c).Spinal administration of either SR141716 (30 mg i.t.) or SR144528 (30 mg i.t.) did not alter paw withdrawal thresholds relative to the control condition (Figure 6a). However, coadministration (i.t.) of both SR141716 and SR144528 concurrently with WIN55, 212-2 blocked the cannabinoid induced suppression of vincristine-evoked mechanical allo-dynia (F4,33 ¼ 4.503, Po0.006, Po0.05 for each comparison; Figure 6b). By contrast, a trend toward partial blockade of WIN55, 212-2-induced anti-allodynia was observed following i.t. administration of the agonist with either the CB1 (Po0.13) or CB2 (Po0.08) antagonist alone, respectively. Planned comparisons confirmed that the CB2 antagonist induced a partial blockade of the anti-allodynic effects of WIN55,212-2 at 5 and 30 min post-injection (Po0.05 for each comparison). Intrathecal coadministration of both antagonists with WIN55, 212-2 blocked the cannabinoid- induced suppression of vincristine-evoked mechanical hypersensitivity at all time points (Po0.006 for each comparison; Figure 6b).
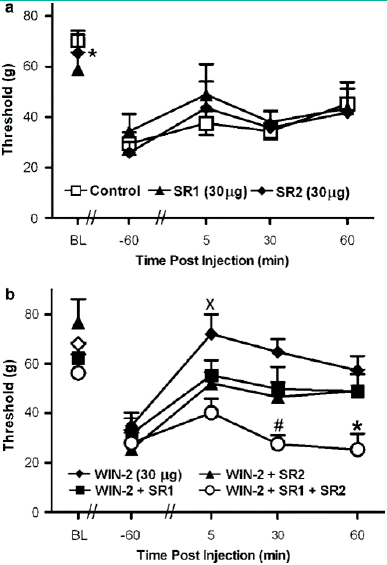
Figure 5: (a) The CB1 antagonist SR141716 (SR1; 30 mg i.t.) and the CB2 antagonist SR144528 (SR2; 30 mg i.t.) did not alter vincristine- induced mechanical allodynia relative to vehicle. (b) WIN55,212-2 (WIN-2; 30 mg i.t.) increased mechanical withdrawal thresholds relative to all other groups. Concurrent (i.t.) administration of SR141716 and SR144528 blocked the WIN55, 212-2-induced sup- pression of vincristine-evoked mechanical allodynia. Data are mean 7s.e.m. *Po0.05 different from all groups, #Po0.05 different from WIN55, 212-2 þ SR2 and WIN55, 212-2 (30 mg i.t.) XPo0.05 different from WIN55, 212-2 þ SR2 and WIN55,212-2 þ SR1 þ SR2 (ANOVA and Fisher’s PLSD post hoc test). N ¼ 58 per group.
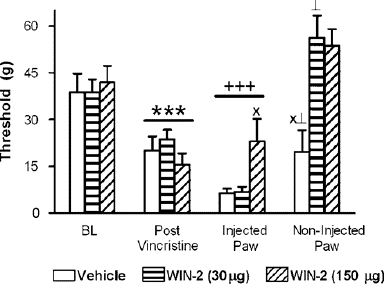
Figure 6: Local administration of the CB1/CB2 agonist WIN55, 212-2 (WIN-2; 30 mg or 150 mg i.pl.) failed to suppress vincristine-induced mechanical hypersensitivity in the injected paw. Hypersensitivity was observed at the site of local injection following vehicle or WIN55, 212-2 (30 mg i.pl.) administration relative to post-vincristine thresholds. Paw withdrawal thresholds in the non-injected paw were elevated relative to the injected paw in all groups. Data are means7s.e.m. ***Po0.05 different from baseline, post-i.pl.-injec- tion and non-injected paw thresholds þþþ Po0.05 different from baseline and non-injected paw thresholds, XPo0.05 different from all groups for the same comparison (ANOVA, and Fisher’s PLSD post hoc test), >Po0.05 different from corresponding group baseline previncristine threshold measures (t-test). N ¼ 7–9 per group.
Assessment of Peripheral Site of Cannabinoid Action
The i.pl. Injection lowered mechanical withdrawal thresh- olds relative to day 12 preinjection levels (F1, 22 7.47; Po0.02; Figure 7), consistent with the development of hypersensitivity at the site of injection. Enhanced hyper- sensitivity was differentially observed in the injected paw.
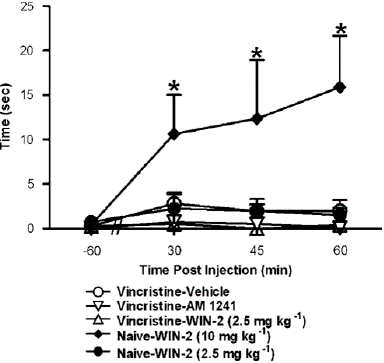
Figure 7: Anti-allodynic doses of AM1241 (2.5 mg kg—1 i.p.) and WIN55, 212-2 (2.5 mg kg—1 i.p.) failed to induce catalepsy in vincristine-treated rats. In otherwise naive rats, WIN55, 212-2 induced (10 mg kg—1 i.p.) catalepsy, as defined as an increase in time spent immobile in the bar test, at all post-injection time points. Data are means7s.e.m. *Po0.05 different from all groups, (ANOVA and Fisher’s PLSD post hoc test). N ¼ 6 per group.
Assessment of Spinal Site of Cannabinoid Action
Mechanical withdrawal thresholds did not differ between vincristine-treated groups receiving the b-cyclodextrin vehicle (i.t.) and controls that were surgically implanted with catheters but did not receive an injection (i.t.). Therefore, these groups were pooled into a single control group for subsequent statistical analysis of drug effects. In vincristine- treated rats, administration of the CB1/CB2 agonist WIN55, 212-2 (10 and 30 mg i.t.) increased mechanical with-
drawal thresholds relative to either the control condition (F2,19 ¼ 11.499, Po0.0006, Figure 5b) or to day 12 preinjection levels (F6,57 ¼ 2.698, Po0.04; Figure 5b). Post hoc levels that were lower than baseline in groups receiving the vehicle (i.pl.) and higher than baseline in groups receiving WIN55, 212-2 (30 mg i.pl.; Po0.03). In groups receiving WIN55, 212-2 (150 mg i.pl.), there was also a trend (Po0.08, t-test) towards higher paw withdrawal thresholds in the non-injected paw relative to baseline. In contrast, for all groups, the injected paw's paw withdrawal thresholds were lower than baseline (Po0.0002). 30 mg intraperitoneal dose of WIN55, 212-2 did not change mechanical withdrawal thresholds between the vehicle and the injected paw. In comparison to the vehicle or a lesser dose of WIN55, 212-2 (30 mg i.pl), WIN55,212-2 (150 mg i.pl) increased the mechanical withdrawal thresholds in the injected paw (F2,22 ¼ 4.083, Po0.05; Po0.03 for Figure 7; all comparisons) without inhibiting vincristine- caused the hypersensitivity of mechanics. Additionally, WIN55, 212-2 was unable to reduce the severity of vincristine-evoked mechanical allodynia at the injection site in comparison to day 12 thresholds, which were noted prior to the injection, at any dose.
Evaluation of Catalepsy
A dose of WIN55, 212-2 (10 mg kg—1 i.p.) known to impair motor activity was compared with systemic doses of AM1241, (2.5 mg kg—1 i.p.) and WIN55, 212-2 (2.5 mg kg—1 i.p.), which were shown to reduce vincristine-evoked mechanical allodynia (Figure 8). WIN55, 212-2-induced (10 mg kg—1 i.p.) catalepsy in the bar test (F4,25 4.34, Po0.01; Figure 8) in compared to preinjection levels (F12,75 ¼ 3.783, Po0.004) or all other conditions (Po0.05 for all comparisons). WIN55, 212-2 and AM1241, when given at dosages that inhibited in the bar test, vincristine-induced mechanical allodynia resulted in reduced motor activity (Figure 8).
Conversation
Vincristine selectively causes Behavior become more sensitive to mechanical stimulus than to thermal stimulation. The mechanical hypersensitivity caused by vincristine is lessened when the cannabinoid CB1 and CB2 receptor subtypes are activated. The animals maintained a pretty good state of health when using the vincristine injection paradigm used here, as seen by the lack of mortality seen with higher dose paradigms [1,2]. As in other studies, vincristine prevented normal weight growth in comparison to controls receiving saline treatment [63]. Only 5% of the animals showed signs of gastroenteritis. In the later phases of the experiment (that is, days 5–12), intraperitoneal bleeding—a common issue for chemotherapy patients [26,44,52,57]. No comparable symptoms and normal stool were observed by Weng et al. (2003) in the same paradigm of vincristine dosage. Variations may be due to the low frequency of symptom occurrence and the vast number of participants we investigated for our study. It is not possible to link the observed changes in mechanical withdrawal thresholds to the emergence of sensitization to repeated testing. When the animals were evaluated at the same time with saline treatment, mechanical allodynia did not occur, but it did in those treated with vincristine. By day three after vincristine, mechanical hypersensitivity had developed reaching its lowest level on day 7 and remained stable until day 12. Other studies similarly report that mechanical hypersensitivity is maximal by day 8 post-vincristine [42,63]. Vincristine-induced mechanical allodynia resolved completely by day 31 in our study, although lack of recovery has been reported with other dosing paradigms [42].
Hypersensitivity to thermal stimulation (or thermal hyperalgesia) was notably absent in vincristine-treated rats that nonetheless exhibited robust mechanical allodynia. By contrast, paclitaxel induces thermal hyperalgesia or thermal hypoalgesia (depending upon the dosing schedule), which may be absent in vincristine and cisplatin models of chemotherapy-induced neuropathy [1-3,8,32,42,63]. Thermal hyperalgesia has been observed in mice using a different vincristine dosing paradigm beginning at 4 weeks following initial vincristine treatment [28]. Nonetheless, vincristine may induce cold allodynia/hyperalgesia [3,32], consistent with clinical reports [9].
An upregulation of Neuropeptide Y (NPY) in medium and large diameter dorsal root ganglion cells has been postulated to underlie development of mechanical allodynia (in the absence of thermal hyperalgesia) following spinal nerve ligation [43]. More work is necessary to determine whether similar neurochemical changes accom- pany the development of vincristine-evoked mechanical allodynia in our study.
Subtype specificity of cannabinoid anti-allodynic actions WIN55, 212-2 (2.5 mg kg—1 i.p.) restored mechanical withdrawal thresholds to 4100% of previncristine levels. WIN55, 212-2 (1.5 mg kg—1 i.p.) reversed both mechanical and thermal hypersensitivity in a paclitaxel-induced neuro- pathy model [45] but did not reverse vincristine-induced mechanical hypersensitivity in our study. Doses of WIN55, 212-2 that eliminated vincristine- induced mechanical allodynia in our study did not induce motor deficits in the bar test. Thus, WIN55, 212-2-induced anti-allodynic effects are independent of any motor effects of cannabinoids. Similar or higher doses of WIN55, 212-2 (2.5—5 mg kg—1 i.p.) also attenuate mechanical allodynia in models of traumatic nerve injury [6,14,17,24,31,62] and diabetic neuropathy [59]. WIN55, 212-2 also attenuates deep tissue hyperalgesia in a murine model of cancer pain through a CB1 mechanism [30]. AM1241 (2.5 mg kg—1 i.p.) induced a CB2-mediated suppression of vincristine-induced mechanical allodynia without inducing antinociception. Metabolism of AM1241 may limit the duration of CB2-mediated anti-allodynia observed here. Nonetheless, CB2 agonists may represent preferred therapeutic agents relative to CB1 agonists due to their limited profile of CNS side-effects [15,35]. AM1241 is an effective anti-hyperalgesic agent in animal models of traumatic nerve injury [50] and inflammation [20,40,41,50]. Our studies suggest that CB2 is also a novel target for the treatment of chemotherapy- induced neuropathy. Activation of either CB1 or CB2 receptors suppressed the maintenance of vincristine-evoked mechanical allodynia. The anti-allodynic effects of WIN55, 212-2 were partially blocked by each antagonist alone at 30 min post-injection whereas complete blockade was observed at 60 min post- drug. Moreover, i.t. administration of both antagonists concurrently completely blocked the anti-allodynic effects of spinally administered WIN55, 212-2. Our data also raise the possibility that targeting multiple cannabinoid receptor subtypes simultaneously may act synergistically to suppress chemotherapy-induced neuropathy.
Effects of Cannabinoids and Morphine on Vincristine-Induced Neuropathy
Opiates are commonly administered to cancer patients experiencing chemotherapy-induced neuropathy [9,32]. In our study, a leftward shift in the dose–response curve for mechanical withdrawal thresholds was observed for WIN55, 212-2 relative to mor- phine. WIN55, 212-2, at a dose of 2.5 mg kg—1, exhibited effects of approximately the same magnitude as morphine at a dose of 8 mg kg—1. Additional doses are required to enable calculations of the ED50 for each drug and verify differences in agonist potency. Our low dose of morphine (2.5 mg kg—1 i.p.) suppressed neuropathic nociception induced by spinal nerve ligation [27,31] and induced antinociception [25], but failed to suppress vincristine-induced allodynia in our study. The high dose of morphine (8 mg kg—1 i.p.) normalized paw withdrawal thresholds in our study but only partially (50%) reversed paclitaxel-evoked mechanical hypersensitivity [12]. Cannabinoids show enhanced antihyperalgesic efficacy relative to opiates in other neuro- pathic pain models [36,37]. Lower efficacy of morphine in reducing abnormal sensations related to myelinated as opposed to unmyelinated fibre activation [55] is consistent with the differential neuroanatomical distribution of m-opioid and cannabinoid receptors at spinal and primary afferent levels [6,19,22]. Thus, cannabinoids may be more potent and efficacious than opiates in suppressing diverse forms of neuropathic and deafferentation-induced pain.
Mechanisms and Site of Action
In our study, WIN55, 212-2 suppressed vincristine-induced mechanical allodynia when administered i.t. but not when administered locally into the paw. In fact, local injections of either vehicle or WIN55, 212-2 (30 mg i.pl.) in our study enhanced mechanical allodynia in the injected paw relative to preinjection levels. Changes in weight bearing due to sensitization at the site of i.pl. injection may contribute to the increases in paw withdrawal thresholds observed in all groups (including vehicle) in the non-injected paw. The In models of diabetic neuropathy [59] and traumatic nerve injury [14], the same local dose used here (30 mg i.pl.) reduced mechanical allodynia; however, in our study, it was unable to reduce vincristine-induced neuropathy or attenuate paclitaxel neuropathy [45]. The paw withdrawal thresholds in the non-injected paw were likewise raised above baseline (previncristine) levels by local injection of WIN55, 212-2 (30 mg i.pl. ), however this did not alleviate the hypersensitivity that was noted at the injection site. Paw withdrawal threshold variations in the non-injected paw may be related to cannabis leakage into the systemic circulation. WIN55, 212-2 with a larger local dose of 150 mg i.pl., which causes definite systemic effects [14] removed the hypersensitivity at the site of the injection of IPL. Nevertheless, this dosage did not normalize paw withdrawal thresholds to previncristine levels and did not decrease vincristine-evoked mechanical allodynia in comparison to preinjection levels.
Our findings directly demonstrate the involvement of spinal sites of action in the inhibition of chemotherapy-induced neuropathy mediated by CB1 and CB2 receptors. Remarkably, rats with traumatic nerve injury in their spinal cords have higher levels of CB2 receptor mRNA and protein [62,65,67]. A functional involvement for spinal CB2 receptors in neuropathic pain states is suggested by the direct spinal injection of a CB2 agonist, which also reduces mechanically evoked responses in wide dynamic range neurons in neuropathic rats but not in sham-operated rats [51].
Central sensitization is brought on by vincristine in Wide dynamic range neurons in the spinal cord, such as aberrant spontaneous activity, wind-up, and after-discharge reactions to mechanical stimulation applied above threshold [63]. The reported neuropathy brought on by chemotherapy may be mediated by these abnormal neurophysiological reactions. Cannabinoids inhibit spinal wide dynamic range neurons and C-fibre-mediated responses by means of CB1 [10,54] or CB2 [41] specific mechanisms. To understand the neurophysiological underpinnings of cannabinoid-mediated reduction of chemotherapy-induced neuropathy, more research is necessary [18].
Presynaptic facilitation, or enhanced primary afferent glutamate release, could potentially be involved in the aberrant behavioral phenotype and central sensitization brought on by chemotherapy. Reduced protein levels for the Excitatory Amino acid Synthase (EASN), Glial Glutamate Transporter-1 (GLT-1), and Glutamate-Aspartate Trans- porter (GLAST) are consistent with this theory. After paclitaxel treatment, carrier-1 (EAAC1) are seen [8]. Notably, however, glutamate and NMDA receptor antagonists do not restore hyperalgesia in models of chemotherapy-induced neuropathy [12,58], but they do in a nerve-injury model [37]. Therefore, different pathways could be involved in the development of neuropathic nociception brought on by chemotherapy and traumatic nerve injury, respectively.
An increase in intracellular Ca2 + [29] may be brought about by abnormal primary afferent input, presynaptic and/or descending [49,61] facilitation, and chemotherapy-induced dysregulation of calcium homoeostasis [53]. A T-type calcium antagonist called ethosuximide, along with other medications that lower intra- and extracellular Ca2 þ, also lower mechanical hypersensitivity brought on by vincristine [12,53]. Further research is necessary to ascertain whether the cannabis suppression of chemotherapy-induced neuropathy is connected to the cannabinoid suppression of central sensitization and Ca2 conductance [33,34].
References
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 2003a; 182: 12-20.
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. Description of a short-term Taxol-induced nociceptive neuropathy in rats. Brain Res. 2000; 887: 239-49.
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. A new animal model of vincristine-induced nociceptive peripheral neuropathy. Neurotoxicology. 2003b; 24: 797-805.
- Bacon M, James K, Zee B. A comparison of the incidence, duration, and degree of the neurologic toxicities of cisplatin- paclitaxel (PT) and cisplatin-cyclophosphamide (PC). Int J Gynecol Cancer. 2003; 13: 428-34.
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006; 23: 1530-8.
- Bridges D, Ahmad K, Rice AS. The synthetic cannabinoid WIN55, 212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001; 133: 586-94.
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur J Pharmacol. 2000; 396: 141-9.
- Cata JP, Weng HR, Chen JH, Dougherty PM. Altered discharges of spinal wide dynamic range neurons and down- regulation of glutamate transporter expression in rats with paclitaxel-induced hyperalgesia. Neuroscience. 2006a; 138: 329-38.
- Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol. 2006b; 72: 151-69.
- Drew LJ, Harris J, Millns PJ, Kendall DA, Chapman V. 2000.
- Activation of spinal cannabinoid 1 receptors inhibits C-fibre driven hyperexcitable neuronal responses and increases [35S]GTPgammaS binding in the dorsal horn of the spinal cord of noninflamed and inflamed rats. Eur J Neurosci. 2000; 12: 2079-86.
- Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel and vincristine-induced painful peripheral neuropathy. Pain. 2004; 109: 150-61.
- Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006; 122: 245-57.
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, et al. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001; 92: 91-100.
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. HU-308: a specific agonist for CB2, a peripheral cannabinoid receptor. Proc Natl Acad Sci USA. 1999; 96: 14228-33.
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988; 32: 77-88.
- Herzberg U, Eliav E, Bennett GJ, Kopin IJ 1997. The analgesic effects of R( )-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett. 1997; 221: 157-160.
- Hohmann AG. A cannabinoid pharmacotherapy for chemo- therapy-evoked painful peripheral neuropathy. Pain. 2005; 118: 3-5.
- Hohmann AG, Briley EM, Herkenham M. Pre and postsynap tic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 1999; 822: 17-25.
- Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A. 2004.
- Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J Pharmacol Exp Ther. 2004; 308: 446-53.
- Hohmann AG, Herkenham M. Regulation of cannabinoid and Mu Opioid receptor binding sites following neonatal capsaicin treatment. Neurosci Lett. 1998a; 252: 13-6.
- Hohmann AG, Tsou K, Walker JM. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN55,212-2. Neurosci Lett. 1998b; 257: 119-22.
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA. 2003; 100: 10529-33.
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, et al. CB2 cannabinoid receptor mediation of antinociception. Pain. 2006; 122: 36-42.
- Jackson DV, Wells HB, Atkins JN, Zekan PJ, White DR, Richards II F, et al. Amelioration of vincristine neurotoxicity by glutamic acid. Am J Med. 1988; 84: 1016-22.
- Joshi SK, Hernandez G, Mikusa JP, Zhu CZ, Zhong C, Salyers A, et al. Comparison of antinociceptive actions of standard analgesics in attenuating capsaicin and nerve-injury-induced mechanical hypersensitivity. Neuroscience. 2006; 143: 587-96.
- Kamei J, Tamura N, Saitoh A. Possible involvement of the spinal nitric oxide/cGMP pathway in vincristine-induced painful neuropathy in mice. Pain. 2005; 117: 112-20.
- Kawamata M, Omote K. Involvement of increased excitatory amino acids and intracellular Ca2 þ concentration in the spinal dorsal horn in an animal model of neuropathic pain. Pain. 1996; 68: 85-96.
- Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, et al. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003; 103: 175-86.
- LaBuda CJ, Little PJ. Pharmacological evaluation of the selective spinal nerve ligation model of neuropathic pain in the rat. J Neurosci Methods. 2005; 144: 175-81.
- Lynch III JJ, Wade CL, Zhong CM, Mikusa JP, Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain. 2004; 110: 56-63.
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA. 1992; 89: 3825-9.
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in att20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995; 15: 6552-61.
- Malan Jr PT, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinoci- ception. Pain. 2001; 93: 239-45.
- Mao J, Price DD, Lu J, Keniston L, Mayer DJ. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci Lett. 2000; 280: 13-6.
- Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995; 61: 353-64.
- Martin WJ, Hohmann AG, Walker JM. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: correlation between electrophysiological and antinociceptive effects. J Neurosci. 1996; 16: 6601-11.
- Munro S, Thomas KL, Abu-Shaar M. Molecular characteriza- tion of a peripheral receptor for cannabinoids. Nature. 1993; 365: 61-5.
- Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003; 119: 747-57.
- Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004; 92: 3562-74.
- Nozaki-Taguchi N, Chaplan SR, Higuera ES, Ajakwe RC, Yaksh TL. Vincristine-induced allodynia in the rat. Pain. 2001; 93: 69-76.
- Ossipov MH, Zhang ET, Carvajal C, Gardell L, Quirion R, Dumont Y, et al. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002; 22: 9858-67.
- Ozçay F, Kayiran SM, Ozbek N. Successful medical manage- ment of neutropenic enterocolitis (typhlitis) in a child with acute lymphoblastic leukemia. Turk J Pediatr. 2003; 45: 248-50.
- Pascual D, Goicoechea C, Suardíaz M, Martín MI. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nocicep- tion induced by paclitaxel in rats. Pain. 2005; 118: 23-34.
- Pertwee RG, Ross TM. Drugs which stimulate or facilitate central cholinergic transmission interact synergistically with delta-9-tetrahydrocannabinol to produce marked catalepsy in mice. Neuropharmacology. 1991; 30: 67-71.
- Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. 2001a; 2: 8-14.
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemo- therapeutic drug, paclitaxel. Pain. 2001b; 94: 293-304.
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan Jr TP, Ossipov MH, et al. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001; 21: 5281-8.
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis, A et al. Inhibition of inflammatory hyper- algesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003; 99: 955-60.
- Sagar DR, Kelly S, Millns PJ, O’Shaughnessey CT, Kendall DA, Chapman V. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur J Neurosci. 2005; 22: 371-9.
- Sandler SG, Tobin W, Henderson ES. Vincristine-induced neuropathy. A clinical study of fifty leukemic patients. Neurology. 1969; 19: 367-74.
- Siau C, Bennett GJ. Dysregulation of cellular calcium home- ostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg. 2006; 102: 1485-90.
- Strangman NM, Walker JM. Cannabinoid WIN 55,212-2 inhibits the activity-dependent facilitation of spinal nociceptive responses. J Neurophysiol. 1999; 82: 472-7.
- Taddese A, Nah SY, McCleskey EW. Selective opioid inhibition of small nociceptive neurons. Science. 1995; 270: 1366-9.
- Tanner KD, Levine JD, Topp KS. Microtubule disorientation and axonal swelling in unmyelinated sensory axons during vincristine-induced painful neuropathy in rat. J Comp Neurol. 1998; 395: 481-92.
- Tolstoi LG. Drug-induced gastrointestinal disorders. Medscape Pharmacother. 2002; 4: 437034.
- Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol. 2000; 424: 563-76.
- Ulugol A, Karadag HC, Ipci Y, Tamer M, Dokmeci I. The effect of win 55,212-2, a cannabinoid agonist, on tactile allodynia in diabetic rats. Neurosci Lett. 2004; 371: 167-70.
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characteriza- tion of brainstem cannabinoid CB2 receptors. Science. 2005; 310: 329-32.
- Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, et al. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006; 140: 1311-20.
- Walczak JS, Pichette V, Leblond F, Desbiens K, Beaulieu P. Behavioral, pharmacological and molecular characterization of the saphenous nerve partial ligation: a new model of neuropathic pain. Neuroscience. 2005; 132: 1093-102.
- Weng HR, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine- induced hyperalgesia and allodynia. Pain. 2003; 103: 131-8.
- Whiteside GT, Lee GP, Valenzano KJ. The role of the cannabinoid CB2 receptor in pain transmission and therapeutic potential of small molecule CB2 receptor agonists. Curr Med Chem. 2007; 14: 917-36.
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005; 135: 235-45.
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17: 1031-6.
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003; 17: 2750-4.
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999; 96: 5780-5.
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983; 16: 109-10.