Review Article
Austin J Pharmacol Ther. 2014; 2 (2).1015
Autism and Oxidative Stress Interventions: Impact on Autistic Behavior
*Ana Maria Castejon and Jordan Ashley Spaw
Department of Pharmaceutical Sciences, College of Pharmacy Nova Southeastern University, USA
*Corresponding author: : Ana Maria Castejon, Department of Pharmaceutical Sciences, College of Pharmacy Nova Southeastern University, 3200 S University Drive Fort Lauderdale, FL, USA
Received: February 03, 2014; Accepted: February 10, 2014; Published: February 13, 2014
Abstract
Autism is an increasingly prevalent neurodevelopmental disorder in the United States, which relies on applied behavioral therapy as means of treatment. This disorder has been linked to increased levels of oxidative stress and lower anti–oxidant capacity. Metabolites in the interconnected transmethylation and transsulfuration pathways are significantly altered in autism, causing decreased glutathione synthesis. This review article was performed to support the role of oxidative stress in autism and its clinical symptoms. The use of the glutathione redox ratio as a biomarker for disease and treatment status was supported. Anti-oxidant supplementation, or ways to improve the altered metabolite levels in the interconnected pathways, has been associated with decreased autistic behaviors and severity. These interventions should be further studied in order to determine their effectiveness at improving metabolic imbalances in Autism Spectrum Disorder (ASD). Overall, oxidative stress related metabolites could have potential use as biomarkers and help determine treatments.
Introduction
Autism was first noted in 1943 as a neurodevelopmental disorder [1] and now affects 1 out every 88 children in the United States [2]. This disorder is clinically characterized by deficits in social interactions, hyper–focused repetitive behaviors, and impaired verbal and non–verbal expressive speech [2,3]. The etiology of autism stems from genetic, neurological and environmental factors [3], which are also supported by other neurological diseases. Environmental insults, like oxidative stress, are known to play a role in some neurological conditions such as Parkinson’s Disease [4], Alzheimer’s Disease [5], schizophrenia [6] and bipolar disorder [7]. Excessive oxidative stress and it’s clinical implications in autism is now of particular interest.
Autism Spectrum Disorder (ASD) encompasses the wide range of clinical symptoms seen in those individuals whom have been diagnosed with some form of autism. Behavioral assessments often note inattention, aggression, impulsivity, hyperactivity, excessive compulsions, affective instability, and occasional psychosis in autistic children [8]. Most children are clinically diagnosed with autism by age 3 using a myriad of standardized behavioral examinations [9]. The heterogeneity of this disorder has made it very difficult to diagnose and treat using pharmacological therapy. Atypical antipsychotics, selective serotonin reuptake inhibitors and psychostimulants have all shown some clinical benefits in this disorder but they are often associated with significant side effects, showing the need for better and safer treatments [10]. Alternative pharmacological therapies, like nutritional interventions and vitamin⁄mineral supplements, cancorrect abnormal transmethylation and transsulfuration pathways, increase anti–oxidant capacity and possibly improve autistic behavior in a safer way with less side effects and better tolerability.
Background
Metabolic abnormalities have been noted in autism and are related to the interconnected pathways of folate, methionine and glutathione metabolism [3,11]. Abnormal glutathione redox status stems from variations in these pathways; they are important for the regulation of normal redox homeostasis, cellular methylation potential and DNA synthesis [3,11]. Pathway imbalances most often lead to oxidative stress.
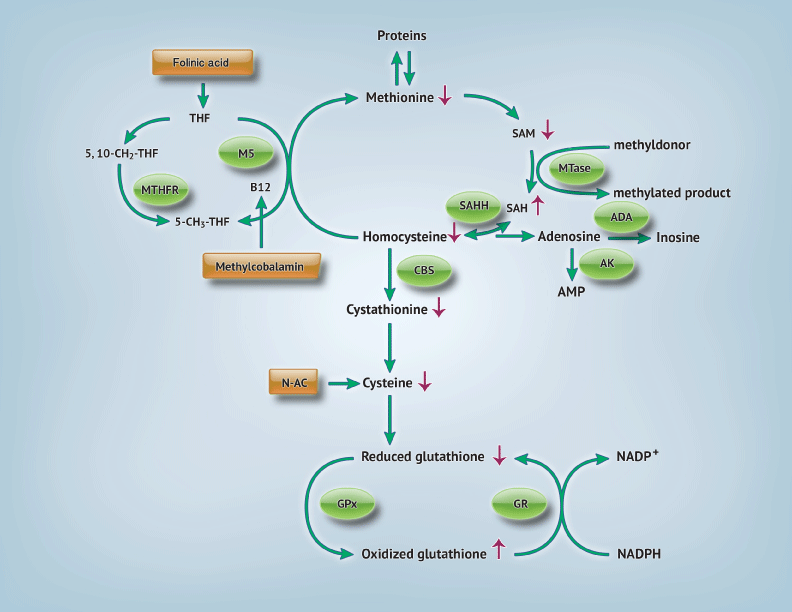
The transmethylation and transsulfuration pathways are shown in Figure 1. The methionine, or transmethylation pathway, describes the pathway where a methyl group is given to homocysteine by methylcobalamin via methionine synthase [11] Methylcobalamin obtains the methyl group needed for this methionine regeneration from methyltransferase activity and 5–methyltetrahydrofolic acid. Methionine can then produce increased amounts of S–adenosylmethionine (SAM), another methyl donor, and regenerate homocysteine. Homocysteine links the transmethylation and transsulfuration pathways; it can then either be used again in the transmethylation cycle or irreversibly removed by cystathione–B synthase (CBS) forming cysteine. The presence of cysteine is required for glutathione synthesis.
Figure 1: Transmethylation and transsulfuration pathways: Abnormalities found in autism and possible interventions. Arrows indicating (↑): levels are increased [3,11,13]. [Arrows indicating: (↓) levels are decreased [3,11,13]. Circles indicate participating enzymes. Rectangles show possible interventions. Abbreviations: 5,10–CH2–THF: 5,10– methylenetetrahydrofolate; 5–CH3–THF: 5–methyltetrahydrofolate; ADA: Adenosine Deaminase; AMP: Adenosine Monophosphate; AK: Adenylate Kinase; CBS: Cystathionine Beta Synthase; GPx= Glutathione Peroxidase; GR: Glutathione Reductase; N–Ac: N–Acetylcysteine; NADPH: Nicotaminde Adenine Dinucleotide Phosphate; MS: Methionine Synthase; MTase: Methyltransferase; MTHFR: Methylenetetrahydrofolate reductase; SAH: S–Adenosylhomocysteine; SAHH: S–Adenosylhomocysteine Hydrolase; SAM: S–Adenosylmethionine; THF: Tetrahydrofolate.
Children with autism exhibit lower levels of adenosine deaminase (ADA), which leads to increase levels of adenosine or homocysteine [3,11]. This accumulation inactivates S–adenosylhomocysteine hydrolase (SAHH) therefore increasing s–adenosylhomocysteine (SAH) and inactivating methytransferase. Methylation is hindered in children with autism. Protein synthesis is also decreased due to insufficient methionine levels [11], which has major downstream effects. Cysteine levels are reduced and are the ultimate cause of decreased glutathione production in this disorder [3,11].
Glutathione is a non–protein thiol, composed of cysteine, glycine and glutamate, which acts as an important endogenous antioxidant and detoxifier. It can be present in two forms, a reduced (GSH) or oxidized (GSSG) form, which help it neutralize dangerous free radicals present in the body. The glutathione redox ratio (GSH:GSSG) is an indicator of overall glutathione status and intracellular reducing environment [11]. Furthermore, it plays a role in normal immune function, redox–sensitive enzyme activity, detoxification and membrane redox signaling [11–13]. Glutathione is present in pools within mitochondria and freely in the cytosol. Reduction of mitochondrial glutathione levels has been associated with neuronal susceptibility to oxidative stress [14]. Glutathione deficiencies increase vulnerability to oxidative stress in children with autism [11]. Excessive ROS and depleted antioxidants⁄ antioxidant enzymes can create a negative cycle within mitochondria, which has been linked to mitochondrial dysfunction in autism [14–16]. Various ways to ameliorate the abnormalities in the transmethylation and transsulfuration pathways and their implications have been studied in heart disease, cancer, autoimmune disease and neurodegenerative conditions [17]. Increased antioxidant support is needed to maintain proper health in these conditions and could be a novel pharmacologic intervention in autism.
The purpose of this systematic review was to provide an in–depth analysis of the transmethylation⁄ transsulfuration and glutathione metabolism pathways in autism as they relate to oxidative stress and clinical symptoms. Various therapeutic intervention strategies, like anti–oxidant supplementation, in treating this neurological disorder could have positive clinical benefits.
Analysis
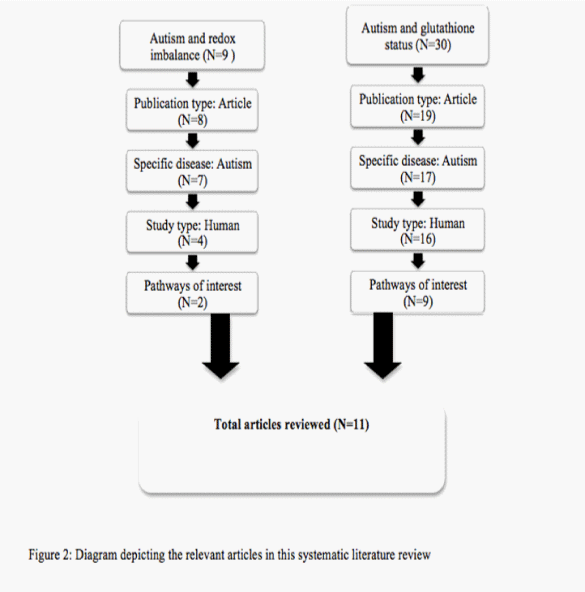
An in–depth literature search was conducted using EMBASE on December 2, 2013. All articles of interest were in English, focused solely on autism and glutathione and were based on human⁄ human cell lines. Review articles, related disease states and non–human based studies were excluded. Search terms ‘autism’ and ‘redox imbalance’ gave 9 articles; only 2 of were relevant to the pathways of interest. The search ‘autism and glutathione status’ gave 30 articles of which 9 included. A total of 11 articles were reviewed in total. The process in which these articles were selected is shown in Figure 2.
Figure 2: Diagram depiciting the relevant articles in this systematic literature review
Metabolic differences between those with autism and controls exist in transmethylation and transsulfuration pathways. Overall, seven studies focused on metabolic and redox imbalance related to glutathione metabolism. Two studies showed significant differences in glutathione and oxidative stress related enzymes in human blood and plasma. A study by Al–Gadani et al. [18] measured anti–oxidant metabolites and enzymes in 20 autistic children vs 20 matched controls in Saudi Arabia. Significant decreases in reduced glutathione (p=0.001) and GSH:GSSG (p=0.001) were found. There was also a significant increase in oxidized glutathione (p=0.001), glutathione peroxidase (p<0.05) and superoxide dismutase (p<0.0.5) levels in those with autism indicating increased oxidative stress [18]. These results were shown in a similar study by Al– Yafee et al. [19] which measured metabolic biomarkers within the transsulfuration pathway in 30 autistic individuals as compared to controls. The autisticgroup showed significantly lower levels of plasma total glutathione, GSH:GSSG ratio, and significantly higher amounts of oxidizedglutathione (GSSG) [19].
Two other metabolite studies related metabolite levels to autism severity. In a study by Adams et. al. [20], an association between the nutritional and metabolic status of 55 children with autism with that of 44 neuro–typical children was established. Autistic children exhibited significantly lower reduced glutathione (GSH; p<0.0001), higher oxidized glutathione (GSSG; p=0.001) and decreased GSH:GSSG (p<0.0001). Other metabolites in the interconnected pathways, free (p<0.00001) and total sulfate (p<0.0001), and S–adenosylmethionine (p<0.0001) were significantly altered in autistic individuals, implicating the importance of this pathway. Regression analysis showed a relationship between Pervasive Developmental Disorder Behavior Inventory (PDD–BI), Autism Treatment Evaluation Checklist (ATEC) and Severity of Autism (SAS) clinical tests and increased free sulfate (p<0.01), S–adenosylmethionine (p<0.05) and oxidized glutathione levels (p<0.05) furthermore linking glutathione levels to oxidative stress and disorder severity [17]. Another study by Essa et al. [21] related autism severity directly with glutathione peroxidase and indirectly with reduced glutathione and superoxidse dismutase levels. Significant differences in reduced glutathione (GSH;p<0.0001), superoxide dismutase (P<0.0001), catalase (p<0.0001) and glutathione peroxidase (p<0.0001) levels were once again shown in the autism group comprised of 19 Omani children in comparison to controls [21]. These studies indicate the role these pathways may play in the phenotype of autism.
Redox homeostasis and metabolic abnormalities were investigated within human cells. James et al. [22] analyzed intracellular redox homeostasis using lymphoblastoid cell lines from autistic children, in contrast to the previous studies which measured extracellular redox imbalance. This abnormality was conveyed with significant decreases in GSH:GSSG ratios (p<0.0001) in autistic lymphoblastoid whole cells and mitochondria as compared to controls [22]. Exposure to prooxidant reagents caused a greater decrease in glutathione redox ratios in lymphoblastoid cells from autistic children suggesting increased oxidative damage to cells in body[22].
Also, the role of glutathione in autism pathophysiology was explored in two post mortem autistic brain studies. Chachuan et al. [23] studied anti–oxidant capacities of the cerebellum, temporal, occipital, parietal and frontal cortices in autistic and typical brains. Significant differences in reduced glutathione (p<0.0001), oxidized glutathione (p>0.0001) and GSH:GSSH (p<0.0001) were only found in the cerebellum and temporal cortex [23]. Similar results for glutathione levels were shown by Rose et al. [24] in a later study. Increases in other oxidative damage biomarkers were shown in autistic samples compared to controls in these brain regions. Oxidative stress stemming from decreased redox homeostasis⁄ antioxidant capacity could cause brain– region specific functional damage, linking the location of imbalance to autistic phenotype [24].
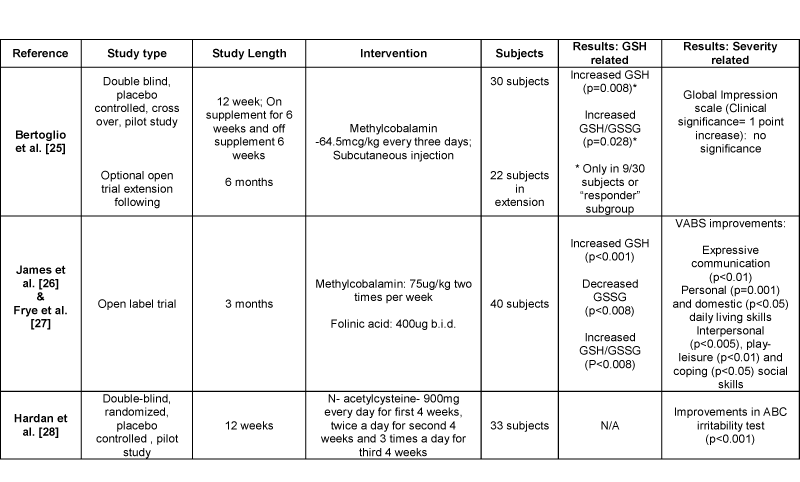
Anti–oxidant supplementation was suggested to improve abnormalities in interconnected pathways and redox homeostasis. Possible interventions in the transmethylation and transsulfuration pathways that could improve metabolite levels are shown in Figure 1. Four of the eleven studies reviewed analyzed methylcobalamin, folinic acid, and N–acetylcysteine interventions (Table 1). One study investigated methylcobalamin supplementation alone in children with autism. Bertoglio et al. [25] did not find a significant relationship between methylcobalamin supplementation, glutathione status and autistic behavior. Nine of the thirty autistic children showed improved glutathione status conveying the presence of a responder subgroup to this intervention [25]. In a set of another two studies, supplementation of methylcobalamin with folinic acid was investigated as an open label trial. The results were analyzed in two studies, suggesting glutathione status and behavior improvements. James et al. [26] noted reduced glutathione levels (p<0.001) and glutathione redox ratios (p<0.008) were significantly increased with this intervention. Also, the amount of oxidized glutathione was significantly decreased (p<0.008) [26]. In addition, Frye et al. [27] analyzed an association between improved glutathione redox ratios and autistic behaviors. Treatment with methylcobalamin and folinic acid was related to increased Vineland Adaptive Behavior Scale (VABS) scores in various categories (Table 1) [27]. Moreover, one study by Hardan et al. [28] investigated the use of N–acetylcysteine in autism. N– acetylcysteine intervention increased antioxidant capacity in these children and was related to significant improvements in an Aberrant Behavior Checklist (ABC) irritability test. The fact that these interventions improved some autistic behaviors supports the role of metabolic abnormalities, glutathione deficiency and oxidative stress in autism behaviors.
Table 1: Results of supplementation studies and effects on autistic behaviors
Abbreviations: ABC: Aberrant Behavior Checklist; GSH: Reduced glutathione; GSSG: Oxidized glutathione; VABS: Vineland Adaptive Behavior Scale.
Interpretation
Autism is a neurodevelopmental disorder that is becoming increasingly prevalent. Oxidative stress has recently been linked to the etiology of this disorder along with genetic and environmental factors. Redox imbalance and metabolic abnormalities are present within the transmethylation and transsulfuration pathways in autism and ultimately lead to insufficient amounts of glutathione levels.
Oxidative stress stems from an imbalance between reactive oxygen species and anti–oxidant capacity, which results in macromolecule damage [29]. Reactive oxygen species can cause oxidative damage to proteins, lipids and DNA if they are not quenched. The organ most susceptible to oxidative damage is the brain, due to its composition and utilization of oxygen [30–31]. Neuronal insult has been suggested with chronic oxidative stress and mitochondrial dysfunction in children with autism. ATP depletion is the ultimate result of electron transport chain (ETC) I damage from pro–oxidant cellular conditions [16]. In addition to autism, oxidative damage has been noted in other neurological disorders and may play a role in neuronal function. Increased vulnerability to oxidative stress seen in autism is related to decreased glutathione levels and is brain–region specific. Both the cerebellum and temporal cortex of autistic children had greater differences in glutathione concentrations compared to controls. The cerebellum plays an important role in motor control and cognitive functions, like attention and language [32–33]. Moreover, the temporal cortex is involved in social perception, joint attention and expressive language [34]. Increased damage to these particular regions due to low glutathione redox status could explain some behavioral traits.
Glutathione is the most critical endogenous anti–oxidant and detoxifier, which provides an essential reducing environment necessary for a variety of cellular processes [29]. Reactive oxygen species, such as hydrogen peroxide, are reduced by the glutathione redox cycle. It is unknown whether glutathione deficiencies are the main disturbance in autism or if they are a ripple of a larger factor. Improvements in glutathione status, as shown in this review, have been successful with vitamin supplements/treatments that normalize the interconnected pathways, allowing glutathione synthesis to occur.
Metabolic studies presented in this review show supplementation may improve glutathione concentrations, redox status and benefit clinical symptoms. Methylcobalamin is the active co–enzyme form of vitamin B12 and is the only type present in the central nervous system for the transportation of methyl groups to proteins. Normal levels are needed for nerve cell activity, DNA replication, production of S–adenosylmethionine and glutathione metabolism [3]. This form of vitamin B12 is necessary to maintain neurological health and is often decreased in autism, leading to an impaired methylation capacity [3,11]. Larger amounts of methylcobalamin are needed to correct neurological defects and have shown to improve the severity of autism [20]. Methylcobalamin is not well orally absorbed and is usually given as an injection form for best results.
Methylcobalamin has the unique ability to directly activate the transmethylation and transsulfuration pathways. Children with autism have been shown to have lower levels of methyltransferase enzyme, therefore not creating enough methylcobalamin [20,30]. Supplementation provides a readily available form of methylcobalamin, which donates a methyl group to create methionine via methionine synthase. It can help ameliorate the effects of reduced levels of methionine synthase seen in those with autism [36]. Most children have shown response to this type of therapy along with improvements in executive function, speech and language, socialization and emotion [27]. The only side effect known is an increased activity level, which is far more tolerable than other treatments’ side effects [27,37].
Often folinic acid is given in combination with methylcobalamin to those with autism. Folinic acid is a reduced folate derivative that is rapidly converted to 5– methyl– tetrahydrofolate, which is then transported into the brain where it aids in the conversion of sulfur–containing compounds to glutathione [30, 32] as shown in this review. Individuals with cerebral folate deficiencies, caused by alterations in the folate and interconnected pathways, show autistic like behaviors [38–39]. Supplementation with folinic acid increases plasma metabolites in the methionine pathway [24]. It has also been recently associated with improved cognitive, neurologic and motor functions in low functioning autistic children with cerebral folate deficiencies [39]. Improvements in autistic individuals have been related to increased antioxidant function [29] via corrections of the interconnected pathways.
Increasing glutathione concentration is the main treatment goal in relation to the methionine and transsulfuration pathways. Previously it was determined that oral glutathione supplements were not well absorbed therefore other alternatives were studied [40]. Since cysteine is the rate–limiting factor in glutathione synthesis, a precursor form of glutathione is now used and offers improved efficacy. N–acetylcysteine has beneficial effects in glutathione levels and autistic behaviors [11,28]. Autistic children taking this supplement presented a decrease in irritability and, repetitive and stereotypical behaviors [28]. The relationship in improving glutathione levels and clinical aspects of autism are once again suggested.
Overall, glutathione metabolism and redox homeostasis may play a role in the etiology of autism, and are biomarker candidates in this condition. The severity of this disorder can be attributed to different metabolic biomarkers, which are associated with both genetic and or environmental factors [23,32]. Treatments can improve glutathione redox status and based on the studies presented, could improve behavior outcomes in autistic patients. The various ways autism severity was determined in this review suggests the need for a uniform method to ensure comparability. Long–term studies using the interventions presented in this review are also needed to ensure their long–term safety and effectiveness. In the future, genetic analysis together with biomarker levels may determine the appropriate treatment for each patient.
Conclusion
Oxidative stress is associated with clinical symptoms and autistic behaviors. Ultimately, improving the glutathione redox ratio is the overall goal of new treatment strategies presented in this review. Alternative pharmacological therapies reviewed in this study ultimately improved anti–oxidant capacity and, in many cases ameliorated symptoms of autism with minimum side effects. Although these interventions correct metabolic abnormalities, they may never completely restore neuro–typical behavior. Treatments aimed to correct the abnormal transmethylation and transsulfuration pathways should be further studied in larger populations to determine their usefulness in alleviating behavioral symptoms in this condition.
References
- Ratajczak HV . Theoretical aspects of autism: biomarkers--a review. J Immunotoxicol. 2011; 8: 80-94.
- Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012; 61: 1-19.
- James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006; 141B: 947-956.
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deicits associated with the spectrum of autism. J Autism Dev Disord. 2000; 30: 205-223.
- Martin HL, Teismann P . Glutathione--a review on its role and signiicance in Parkinson’s disease. FASEB J. 2009; 23: 3263-3272.
- Bermejo P, Martín-Aragón S, Benedí J, Susín C, Felici E, et al. Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer’s disease from Mild Cognitive Impairment. Free Radic Res. 2008; 42: 162-170.
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007; 104: 16621-16626.
- Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008; 111: 135-144.
- Bertoglio K, Hendren RL . New developments in autism. Psychiatr Clin North Am. 2009; 32: 1-14.
- Kumar B, Prakash A, Sewal RK, Medhi B, Modi M, et al. Drug therapy in autism: a present and future perspective. Pharmacol Rep. 2012; 64: 1291- 1304.
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004; 80: 1611-1617.
- Ghanizadeh A, Akhondzadeh S, Hormozi M, Makarem A, Abotorabi-Zarchi M, et al. Glutathione-related factors and oxidative stress in autism, a review. Curr Med Chem. 2012; 19: 4000-4005.
- Geier DA, Kern JK, Garver CR, Adams JB, Audhya T, et al. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem Res. 2009; 34: 386-393.
- Wilkins HM, Kirchhof D, Manning E, Joseph JW, Linseman DA, et al. Mitochondrial glutathione transport is a key determinant of neuronal susceptibility to oxidative and nitrosative stress. J Biol Chem. 2013; 288: 5091-5101.
- Legido A, Jethva R, Goldenthal MJ . Mitochondrial dysfunction in autism. Semin Pediatr Neurol. 2013; 20: 163-175.
- Palmieri L, Persico AM . Mitochondrial dysfunction in autism spectrum disorders: cause or effect? Biochim Biophys Acta. 2010; 1797: 1130-1137.
- Uttara B, Singh AV, Zamboni P, Mahajan RT . Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009; 7: 65-74.
- Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L . Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem. 2009; 42: 1032-1040.
- Al-Yafee YA, Al-Ayadhi LY, Haq SH, El-Ansary AK . Novel metabolic biomarkers related to sulfur-dependent detoxiication pathways in autistic patients of Saudi Arabia. BMC Neurol. 2011; 11: 139.
- Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab (Lond). 2011; 8: 34.
- Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxiication. Clin Chim Acta. 2003; 333: 19-39.
- James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009; 23: 2374-2383.
- Chauhan A, Audhya T, Chauhan V . Brain region-speciic glutathione redox imbalance in autism. Neurochem Res. 2012; 37: 1681-1689.
- Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, et al. Evidence of oxidative damage and inlammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. 2012; 2: e134.
- Bertoglio K, Jill James S, Deprey L, Brule N, Hendren RL, et al. Pilot study of the effect of methyl B12 treatment on behavioral and biomarker measures in children with autism. J Altern Complement Med. 2010; 16: 555-560.
- James SJ, Melnyk S, Fuchs G, Reid T, Jernigan S, et al. Eficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am J Clin Nutr. 2009; 89: 425-430.
- Frye RE, Melnyk S, Fuchs G, Reid T, Jernigan S, et al. Effectiveness of methylcobalamin and folinic Acid treatment on adaptive behavior in children with autistic disorder is related to glutathione redox status. Autism Res Treat. 2013; 2013: 609705.
- Hardan AY, Fung LK, Libove RA, Obukhanych TV, Nair S, et al. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol Psychiatry. 2012; 71: 956-961.
- Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, et al. Oxidative stress- related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med. 2012; 52: 2128-2141.
- Essa M, Braidy N, Waly M, et al. Impaired antioxidant status and reduced energy metabolism in autistic children. Research in Autism Spectrum Disorders. 2013;7(5):557-565.
- Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2012; 17: 290-314.
- Gendry Meresse I, Zilbovicius M, Boddaert N, Robel L, Philippe A, et al. Autism severity and temporal lobe functional abnormalities. Ann Neurol. 2005; 58: 466-469.
- Makris N, Schlerf JE, Hodge SM, Haselgrove C, Albaugh MD, et al. MRI- based surface-assisted parcellation of human cerebellar cortex: an anatomically speciied method with estimate of reliability. Neuroimage. 2005; 25: 1146-1160.
- Hodge SM, Makris N, Kennedy DN, Caviness VS Jr, Howard J, et al. Cerebellum, language, and cognition in autism and speciic language impairment. J Autism Dev Disord. 2010; 40: 300-316.
- Ali A, Waly MI, Al-Farsi YM, Essa MM, Al-Sharbati MM, et al. Hyperhomocysteinemia among Omani autistic children: a case-control study. Acta Biochim Pol. 2011; 58: 547-551.
- Muratore CR, Hodgson NW, Trivedi MS, Abdolmaleky HM, Persico AM, et al. Age-dependent decrease and alternative splicing of methionine synthase mRNA in human cerebral cortex and an accelerated decrease in autism. PLoS One. 2013; 8: e56927.
- Main PA, Angley MT, Thomas P, O’Doherty CE, Fenech M, et al. Folate and methionine metabolism in autism: a systematic review. Am J Clin Nutr. 2010; 91: 1598-1620.
- Djukic A . Folate-responsive neurologic diseases. Pediatr Neurol. 2007; 37: 387-397.
- Ramaekers VT, Blau N, Sequeira JM, Nassogne MC, Quadros EV, et al. Folate receptor autoimmunity and cerebral folate deiciency in low-functioning autism with neurological deicits. Neuropediatrics. 2007; 38: 276-281.
- Kern JK, Geier DA, Adams JB, Garver CR, Audhya T, et al. A clinical trial of glutathione supplementation in autism spectrum disorders. Med Sci Monit. 2011; 17: CR677-682.