1Department of Pharmacology, Assiut University, Egypt
2Department of Forensic Toxicology, Suez Canal University, Egypt
*Corresponding author: Abdel-Wahab BA, Department of Pharmacology, College of Medicine, Assiut University, Assiut, 22 University street, Assiut University, Assiut, Egypt
Received: August 25, 2014; Accepted: November 03, 2014; Published: November 06, 2014
Citation: Abdel-Wahab BA and Metwally ME. Protective Effect of Alpha Lipoic Acid against Lead-induced Hippocampal Neurotoxicity and Neuronal Oxidative Stress in Rats. Austin J Pharmacol Ther. 2014; 2 (11).1058. ISSN: 2373-6208.
Acute lead intoxication is usually accompanied by neurological symptoms, including seizures, which result from the toxic effects of lead on the nervous system. These effects may be attributed to lead-induced oxidative stress and neuronal injury. This study was designed to investigate the protective effect of alpha lipoic acid (α-LA) against seizures, and hippocampal oxidative stress, induced by acute lead acetate (PbA) intoxication, in rats.
Methods: The protective effect of α-LA (at doses of 50 and 100 mg/kg i.p.) against seizures induced by the ED50 of PbA was tested. Valproic acid (VPA) was used as reference drug. The changes of hippocampal glutamate, lipid peroxidation, reduced glutathione (GSH), and 8-hydroxy-2´-deoxyguanosine (8- OHdG), as well as glutathione peroxidase (GSH-PX) activity, were measured. Furthermore, the neuronal damage produced by PbA on hippocampal neurons was studied by using Fluoro-Jade B staining technique.
Results: α-LA showed a protective effect against PbA-induced seizures in rats. There was a corresponding decrease in hippocampal lipid peroxidation and 8-OHdG levels upon pretreatment with α-LA. Additionally, there was an increase in hippocampal GSH level and GSH-PX activity compared to the estimated levels in PbA-treated animals. The increase induced by PbAc in hippocampal glutamate level was not inhibited by α-LA. Moreover, the Fluoro-Jade B staining study showed that α-LA, at the tested doses, significantly decreased the number of hippocampal neurons that were injured by PbA.
Conclusion: α-LA has a neuroprotective effect against PbA-induced neurotoxicity and DNA damage. These results may represent the basis for the use of α-LA in the treatment of lead intoxication.
Keywords: Alpha lipoic acid; Lead acetate; Oxidative stress; DNA damage; 8-OHdG; Fluoro-Jade B
Lead is a pervasive and persistent environmental pollutant that can be detected in almost all phases of the environment and biological systems. Because of its low cost and physical properties, lead and its compounds have been widely used in a variety of products for different purposes [1]. However, lead is one of the most highly toxic metals found in the environment. Its toxicity mainly affects the central nervous system, where the symptoms of neurological disturbances are commonly associated with lead intoxication. In case of severe acute toxicities with lead, seizures usually develop and they may be followed by coma and/or death [2]. In the brain, toxic effects caused by lead extend to affect neurons, vascular endothelial cells, astroglia, and oligodendroglia. In addition, lead can induce excitotoxicity and disturbances in the storage and release of neurotransmitters and second messengers as well as lead to neuronal apoptosis [1].
Lead-induced neurotoxicity can be mediated by different cellular, intracellular, and molecular mechanisms. One of these mechanisms includes the induction of oxidative stress. Lead-induced oxidative stress can pose a hazard to biological systems by either increasing the production of Reactive Oxygen Species (ROS), such as superoxide and hydroxyl radicals, or by depletion of major antioxidant defenses of cells [3]. Lead-induced oxidative stress may be either direct or indirect through increasing the production of lipid peroxidation products where it can disturb lipid metabolism and cell membrane functions [3]. Rats exposed to lead toxicity showed an increase in the rate of lipid peroxidation and decrease in the antioxidant defense mechanisms in their brains [4].
Antioxidant enzymes and glutathione play an important role in the body’s defense against ROS. After exposure to lead toxicity, the level of oxidized Glutathione (GSSG) in different organs increased with a concomitant decrease in reduced glutathione (GSH) [5]. Glutathione is necessary for proper functioning of the antioxidant enzymes Glutathione Peroxidase (GSH-Px), Glutathione-S-Transferase (GST), and Glutathione Reductase (GR), all of which participate in the elimination of ROS. The activities of these enzymes are significantly modified by the influence of lead [6]. Moreover, by stimulating oxidative stress, lead can induce cellular apoptosis and oxidative DNA damage in aging brain cells [7].
Alpha-lipoic acid (α-LA) is a natural product that was discovered in 1951 as a molecule that contributes in acyl group transfer processes in the cell. It has been identified as a natural antioxidant found in many types of food. Its antioxidant properties give it the ability to scavenge ROS, both in hydrophilic and in lipophilic media [8]. In addition, α-LA has the ability to regenerate endogenous antioxidants, including vitamins C and E, as well as intracellular GSH [9]. Moreover, α-LA can inhibit the synthesis and activity of inducible nitric oxide synthase (iNOS) [10] and scavenge reactive nitrogen species [11]. α-LA has been furthermore shown to exhibit a neuroprotective effect against neuronal apoptosis; this effect can be attributed to its ability to protect neurons against ROS-mediated neuronal and DNA damage through inhibition of ROS formation [12].
The aim of this study was to investigate the potential protective effect of α-LA against neurotoxicity, occurring during acute lead intoxication. The potential correlation between changes in hippocampal glutamate, lipid peroxidation, cellular antioxidant defense mechanisms, neuronal and DNA damage, and the protective effect of α-LA against lead-induced neurotoxicity was also studied.
Lead acetate (PbA), α-lipoic acid (water-soluble powder), valproic acid, and thiobarbituric acid, valproic acid (VPA) were purchased from Fluka Biochemika (Switzerland). Reduced glutathione (GSH), Ellman’s reagent [(5,5-dithiobis (2-nitrobenzoic acid), DTNB], Griss reagent [1% sulfanilamide in 5% phosphoric acid (sulfanilamide solution), and 0.1% N-1-naphthylenediamine dihydrochloride in bidistilled water (NED solution)] were purchased from INC Biomedicals Inc. (USA). The 8-hydroxy-2’-deoxy guanosine (8- OHdG) assay kit used herein was purchased from Nikken SEIL Co. (Japan). Fluoro-Jade B stain was purchased from Chemicon International (USA). All other chemicals were of analytical grade.
Male adult Sprague-Dawley rats, weighing 150–250 g, obtained from the Animal house of King Saud University (KSA) were used in all the experiments. Rats were housed in plastic cages with stainless steel mesh covers under a 12 h light/dark cycle at 25°C and were allowed free access to water and food (laboratory chow) ad libitum. All animal experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986, and associated guidelines, the EEC Directive of 1986 (86/609/EEC), or the NIH guide for the care and use of laboratory animals and with the approval of the institutional Animal Use Committee.
Rats were divided into ten groups with ten rats in each group. PbA was dissolved in distilled water, slightly alkalinized by sodium bicarbonate. The threshold for clonic convulsions was determined in control (vehicle-treated) rats by i.p. administration of PbA at doses ranging from 10 to 50 mg/kg. Following the injection of PbA, ras were placed separately in transparent Plexiglas cages (25×15×10 cm) and observed for 30 min for the occurrence of clonic seizures. Clonic seizure activity was defined as clonus of the whole body lasting for more than 3 s, with an accompanying loss of righting reflex. The number of animals convulsing out of the total number of rats tested was noted for each treatment condition. The convulsive action of PbA was evaluated as the CD50 (median convulsive dose, i.e., the dose of PbA that produced clonic seizures in 50% of the rats tested). Subsequently, a dose-response curve was determined from the percentage of rats convulsing according to the log-probit method of Litchfield and Wilcoxon [13]. Afterwards, the CD50 value of control animals was calculated from the equation of the dose-response curve for PbA.
The calculated CD50 of PbA was injected i.p. in another three groups of animals, either without or with prior (30 min) i.p. injection of α-LA at doses of 50 and 100 mg/kg body weight. Following an i.p. injection of CD50 of PbA there is a typical ten-minute sequence of observations. The first period is characterized as latency time and is followed by the first signs of clonic convulsions; these convulsions are associated with movements of the face, mouth, and the front limbs. After that and for two minutes the animal experiences clonic convulsions, followed by tonic clonic seizures; during that phase, extension of the front and back limbs is observed, directed first to the belly and then downwards, a movement that is usually followed by the death of the animal in no more than five minutes. The seizure latency and percentages of seizures and mortality in each group were taken as an index of seizure development. Two groups were treated with α-LA, at doses of 50 and 100 mg/kg body weight but did not receive PbA. The control group was treated analogously with distilled water. Valproic acid (VPA, 200 mg/kg, i.p) was used as reference anticonvulsant drugs for comparison. One hour after injection of PbA, the surviving animals in each group were sacrificed by decapitation. The brains of the sacrificed animals were obtained for biochemical measurements.
The brains were rinsed in ice-cold saline and the hippocampus was carefully separated, blotted, weighed, and homogenized in a phosphate buffer (pH 7.4). The homogenate was centrifuged at 3000 rpm for 15 min at 4°C, and the supernatant was collected for biological determinations.
Determination of lipid peroxidation: Lipid peroxidation was estimated by the measurement of malondialdehyde (MDA) levels in hippocampal tissues. The MDA level in the hippocampal homogenate was determined spectrophotometrically using thiobarbituric acid reactive substances (TBARS) as described previously [14].
Determination of intracellular GSH and glutamate levels: Determination of reduced GSH and glutamate levels in hippocampal homogenate was carried out as follows. A part of the hippocampal homogenate was added to an equal volume of Perchloric acid (1 Mol/l) and mixed by vortexing. The mixture was allowed to stand for 5 min at 25°C. After centrifugation at 10,000 ×g at 4°C for 5 min, the supernatant was collected. The GSH content of the neutralized supernatant was assayed using Ellman’s reagent according to the method of Griffith [15]. The glutamate content in the supernatant was measured spectrophotometrically according to the method of Lund [16]. A standard reference curve was prepared for each assay.
Determination of hippocampal nitrite level: Nitric oxide formation was measured in hippocampal homogenate by assaying nitrite spectrophotometrically using the Griss reagent [1% sulfanilamide in 5% phosphoric acid (sulfanilamide solution) and 0.1 % N-1-naphthylenediamine dihydrochloride in bidistilled water (NED solution)], as previously described [17]. A standard reference curve was prepared for assay.
Determination of GSH-PX activity: Glutathione peroxidase activity was measured spectrophotometrically as described [18]. A standard curve was plotted to calculate the concentration of glutathione peroxidase in the sample. The activity of GSH-PX was expressed as IU/mg protein.
Determination of 8-OHdG: 8-hydroxy-2’-deoxyguanosine (8-OHdG) is a biomarker of oxidative DNA damage. To measure the 8-OHdG level in hippocampal homogenate, a highly sensitive 8-OHdG assay kit (Nikken SEIL Co. Japan) was used, which is a competitive Enzyme-Linked Immunosorbent Assay (ELISA) kit utilizing a monoclonal antibody (clone N45.1) that is highly specific for DNA damage and does not cross-react with RNA oxidation products such as 8-hydroxy-guanine and 8-hydroxy-guanosine. The colored product was measured at 450 nm. All assay procedures were carried out as described in the manual provided by the supplier.
Determination of protein content: The protein content in hippocampal homogenate was measured according to the biuret method using bovine serum albumin as the standard [19].
For tissue analysis, 12 rats were used in four groups (n=3 per group). Animals were deeply anesthetized by thiopental and perfused with heparinized saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed, fixed for 1 h in 4 % buffered paraformaldehyde, and then submerged for 1 h into 20 % sucrose for cryoprotection. The entire hippocampus was sliced in the frontal plane into 40 μm thin sections with a cryostat and the free-floating sections were placed in 0.1 M phosphate buffer. Tissue sections were mounted onto gelatinized slides and allowed to dry at room temperature. Subsequently, sections were then stained with Fluoro-Jade B (FJB, Chemicon International, USA) according to the previously described method [20]. All the procedures were carried out according to the manufacturer’s instructions. FJB-positive, degenerating neurons were visualized by using an epifluorescence microscope (Nikon, USA) attached to a Nikon digital camera equipped with a filter system suitable for visualizing Fluorescein Isothiocyanate (FITC) (Nikon FITC filter cube B-2, which has an excitation filter at 450-490 nm and a barrier filter at 520 nm). The number of FJB-positive cells were counted with at least 100 cells from four randomly selected fields in each treatment.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) methodology was used to assess neuronal cell death in the CA1 (Cornu Ammonis) region of the hippocampus. Apoptosis occurring in vivo was assessed by TUNEL labeling [21]. An in situ cell death detection kit (Roche, Germany) was used to carry out TUNEL staining in sections according to the manufacturer’s instructions. Staining was visualized with diaminobenzidine. Each group contained 7 animals and from each animal 3 sections stained. The number of surviving neurons and TUNEL positive cells per millimeter linear length in the CA1 region [21] of the dorsal hippocampus was counted by an investigator who was blinded to the experimental conditions.
The variability of the results was represented as the mean ± SEM. The significance of differences between the mean values was determined using one-way or two-way analysis of variance (ANOVA) as appropriate followed by Bonferroni’s post-hoc comparison between groups. P-values less than 0.05 were considered statistically significant.
The results presented in Table 1 indicate that injection of PbA at a dosage corresponding to the ED50 value (i.e., 250 mg/kg, i.p.) in rats produced clonic and tonic seizures with an onset at 25.1 min in 50% of tested animals and resulted in 20% mortality. Animals pretreated with α-LA at doses of 50 and 100 mg/kg, i.p., showed a significant increase in the seizure latency [F(2,27) = 6.780, p = 0.0041] . This increase in seizure latency was at a significance level of p < 0.05 at the lower dose and p < 0.01 at the higher dose of α-and 10%, respectively, relative to PbA group, with no mortality in the tested two doses of α-LA. All animals were free of seizures in the VPA group (p < 0.01).
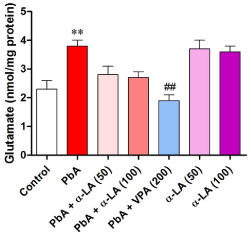
The measured values of hippocampal glutamate concentration showed significant changes in its level after the administration of PbA [F (2,54) = 0.7618, p < 0.001]. PbA caused a significant increase (p < 0.01) in the hippocampal glutamate level relative to the control level. α-LA at the two tested doses, 50 and 100 mg/kg, i.p., did not produce any significant change in the PbA-induced increase in the hippocampal glutamate level (Figure 1). In addition, a single α-LA treatment did not produce any significant change in the hippocampal glutamate level relative to the control level (Figure 1). Animals treated with VPA with PbA showed significant (p < 0.01) decrease in glutamate level compared with PbA group.
Results of the effect of PbAc and α-LA on the hippocampal lipid peroxidation, NO and GSH levels, and GSH-Px activity are shown in Table 2. Measurement of hippocampal lipid peroxidation product (MDA) showed a significant change in its level after treatment with PbA and α-LA [F (2,54) = 5.340, p < 0.0077]. PbA administration led to a significant increase (p < 0.01) in the hippocampal MDA level relative to the control group. Combined treatment with PbA and α-LA led to a significant decrease in the hippocampal MDA level (p < 0.05 at the α-LA dose of 50 mg/kg, i.p., and p < 0.01 at the dose of 100 mg/kg, i.p.) relative to its level in the PbA-treated group. Single α-LA treatment did not significantly change the hippocampal MDA level.
The hippocampal level of NO significantly changed by treatment with PbA and α-LA [F (2,54) = 4.638, p < 0.0138]. PbA significantly increased the hippocampal NO level compared with the control level. Combined treatment with PbA and α-LA significantly decreased the hippocampal NO level at the significance level of p < 0.05 at the dose 50 mg/kg, i.p., and p < 0.01 at the dose 100 mg/kg, i.p., of α-LA relative to its level in the group treated with PbA alone. Single α-LA treatment did not significantly change the hippocampal NO level.
Moreover, the hippocampal GSH level significantly changed by treatment with the test compounds [F (2,54) = 8.011, p < 0.001]. PbA significantly decreased the hippocampal GSH level compared with its level in the control group. Pretreatment with α-LA before administration of PbA significantly increased the hippocampal GSH level compared with its level in PbA-only treated animals. This effect was significant at the level of p < 0.05 at the dose of 50 mg/kg, i.p., and p < 0.01 at the dose of 100 mg/kg, i.p., of α-LA relative to its level in the PbA-treated group. Single α-LA treatment was accompanied by an increase in the hippocampal GSH level only at the dose of 100 mg/kg, i.p.
Furthermore, the hippocampal GSH-PX activity significantly changed upon treatment with PbA and/or pretreatment with α-LA [F92.54) = 7.621, p < 0.0012]. Treatment with PbA significantly decreased the hippocampal GSH-PX activity (p < 0.001) compared with that in the control group. Pretreatment with α-LA before administration of PbA significantly increased the hippocampal GSH-PX activity compared with PbAc-only treated animals at the significance level of p < 0.001 with both tested doses of α-LA. Single α-LA treatment did not significantly change the hippocampal GSH-PX activity relative to the control.
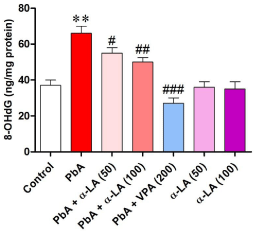
Measurement of hippocampal 8-OHdG showed significant changes in its level by treatment with the tested compounds [F (2, 54) = 5.835, p < 0.001]. Treatment of animals with α-LA alone did not significantly change the hippocampal level of 8-OHdG (Figure 2).
Treatment of animals with PbA significantly (p < 0.01) elevated the hippocampal level of 8-OHdG, reflecting the lead-induced DNA damage in hippocampal neurons. Animals pretreated with α-LA before PbA treatment showed a significant decrease in the level of 8-OHdG at the significance levels of p < 0.05 and p < 0.01 at the doses of 50 and 100 mg/k, i.p., respectively, relative to animals treated with PbA alone (Figure 3).
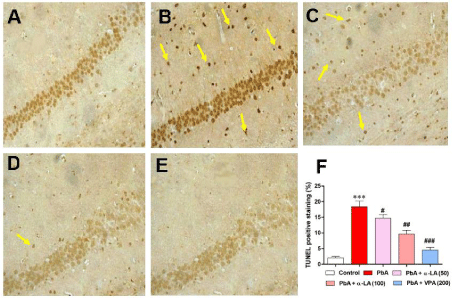
Representative images of the TUNEL staining in the hippocampus are shown in Figure 4. TUNEL-positive cells were stained a deep brown in the hippocampus. Cell counting of the neuronal apoptosis in the hippocampus of PbA-treated rats (18.18± 2.94%, P<0.001) were prominently more than in the vehicle control rats (3.82±1.78%). α-LA (50 and 100 mg/kg) and VPA treatments markedly attenuated neuronal apoptosis in PbA-treated rats (15.24± 1.47%, P < 0.05; 14.11±0.76%, P<0.01; and 6.48±0.64%, P < 0.001, respectively). These results indicated that α-LA attenuated PbA-induced apoptotic cell death in the hippocampus.
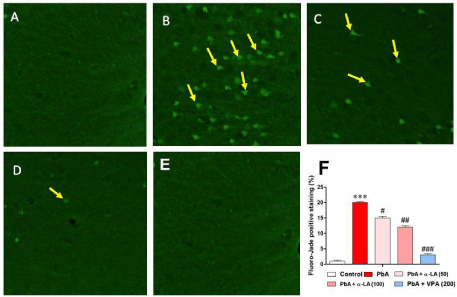
In control animals, no Fluoro-Jade B-positive neurons were observed in the studied area (frontal hippocampal region) (Figure 5A). In the PbA-treated group, a large number of hippocampal neurons were stained with Fluoro-Jade B, thus indicating neuronal damage (Figure 5B). Pretreatment with α-LA decreased the number of hippocampal neurons stained with Fluoro-Jade B at doses of 50 and 100 mg/kg (Figure 5C and 5D, respectively). The number of stained neurons with Fluoro-Jade B stain showed a significant decrease in neurons damaged by PbA [F (2,27) = 23.53, p < 0.001]. The number of stained neurons decreased significantly in the α-LA pretreated animals at doses of 50 mg/kg (p < 0.01) and 100 mg/kg (p < 0.001) relative to the PbA-treated animals (Figure 5E).
In the present study, we investigated whether α-LA has a protective effect against neurotoxicity occurs during acute lead intoxication. Our results show that in rats, (a) α-LA has a protective effect against neurotoxicity and neuronal DNA damage, induced by acute PbA toxicity. (b) These effects can be related to the antioxidant effects of α-LA, mediated by reactivation of the antioxidant enzymes or by inhibition of free radical production induced by PbA.
Our first finding that α-LA can protect against the development of convulsions in different seizure models, an effect that is strongly correlated to its antioxidant properties. α-LA was found to be protective against oxidative stress induced by lead toxicity [10]. In addition, α-LA, at a dose of 100 mg/kg, i.p., attenuated seizure activity in a model of traumatic brain injury [22]. In addition, a strong protective effect of α-LA as an antioxidant was observed against oxidative stress in hippocampus during Pilocarpine-induced seizures [23]. Additional evidence comes from recent studies showing that α-LA can protect against potassium cyanide-induced seizures and mortality through its antioxidant properties, an effect that is related to inhibition of ROS production and maintenance of antioxidant defense mechanisms [24].
The results obtained in this study indicated that PbA at its CD50 can induce seizures and mortality in rats. These results are supported by and consistent with previous studies. Acute exposure to lead toxicity increased the incidence of picrotoxin-induced convulsions in rats [25]. In addition, Arrieta and co-workers [26] indicated that lead toxicity decreases the threshold of pentylenetetrazole-induced seizures in rats. The mechanism of PbA -induced seizures is not clearly understood. Our results showed that an increase in the hippocampal glutamate level accompanied the PbA-induced seizures. This finding is in agreement with previous studies that showed a disturbance of the glutamate/GABAergic transmission accompanies PbA-induced toxicity. This disturbance may be related to the ability of PbA to inhibit glutamate decarboxylase (GAD) resulting in a decrease in the level of GABA and an increase in the level of glutamate in cerebral neurons leading to excitotoxicity and seizures [27].
The results of this study showed an increase in the level of hippocampal NO upon exposure to PbA. This effect can be attributed to the ability to lead, to stimulate inducible nitric oxide synthase (iNOS), thus resulting in an increase in the rate of NO production [28]. In addition, lead enhances lipid peroxidation processes, a fact that was reflected in our results in the form of an increase in hippocampal MDA level after exposure to PbA. Lead exerts some of its toxic effects by inducing oxidative damage and peroxidation of the lipids in the cell membranes, thus compromising cellular functions. Furthermore, a decrease in antioxidant defense mechanisms in the brain of lead-exposed animals was observed [29]. The results of our study not only demonstrate that the hippocampal concentration of reduced Glutathione (GSH) but also the activity of GSH-PX were markedly reduced by animal exposure to PbA.
Lead not only induces neurotoxicity and increases oxidative stress but also induces neuronal DNA damage. This was reflected in our results that showed an elevation in the hippocampal level of 8-OHdG in hippocampi of animals treated with PbA. The neurotoxic effect of lead was further studied by microscopic examination of hippocampal slices stained by Fluoro-Jade B. This dye is an anionic fluorescein derivative useful for the histological staining of neurons undergoing degeneration. However, Fluoro-Jade B has an even greater specific affinity for degenerating neurons. Our microscopic study of hippocampal slices using Fluoro-Jade B dye showed staining of a large number of hippocampal neurons with the fluorescent dye in animals treated with PbAc, thereby indicating neuronal damage. These results were confirmed by Tunnel staining of hippocampal slices that showed icreased percentage of positively stained cells by Tunnel in hippocampi of PbA-exposed animals.
α-LA, at the tested doses protected animals from PbA-induced seizures and mortality. This effect was not accompanied by a significant change in the PbA-induced increase in hippocampal glutamate level, a finding that weakens the possibility of the involvement of glutamate in the protective effect of α-LA against PbAc-induced seizures. These results are in agreement with a previously published results regarding effects of α-LA on potassium cyanide-induced seizures and mortality [24].
In addition, α-LA pretreatment decreased the lipid peroxidation induced by PbA, as reflected in the form of a decrease in the hippocampal MDA level. The increase in hippocampal NO levels induced by PbA were significantly reduced in groups treated with α-LA in a dose-dependent manner, the effect that may be attributed to inhibition of iNOS by α-LA. α-LA was found to have inhibitory effect on brian iNOS induced by different insults [30,31]. Moreover, both hippocampal GSH level and GSH-PX activity increased in α-LA-treated animals relative to PbA-treated animals, the fact that reflects the ability of α-LA to restore the PbA-depressed antioxidant defense mechanisms. Both α-LA and its reduced form, dihydrolipoic acid, have free radical scavenging properties [32]. The dithiol group in α-LA is utilized in the biosynthesis of endogenous antioxidants, such as vitamin E, vitamin C, and glutathione [8,10]. In addition, α-LA can increase intracellular GSH levels by increasing cysteine uptake, which is a rate-limiting step in GSH biosynthesis [11]. Thus, α-LA can antagonize the depletion of intracellular GSH. Moreover, it was found that α-LA can neutralize free radicals, in both the fatty and aqueous regions of the cells, in contrast to vitamin C (water soluble) and vitamin E (fat soluble), and that it functions as an antioxidant in both its reduced and oxidized forms. Furthermore, α-LA can readily cross the blood-brain barrier, a fact that helps it to reach the CNS in sufficient concentrations to exert its antioxidant effects [33].
In addition, α-LA can antagonize the decrease in the activity of GSH-dependent antioxidant enzymes including GSH-PX [32]. This finding was also seen in our results in the form of an increase in hippocampal GSH-PX activity depressed by PbAc toxicity. Therefore, the decrease in the level of lipid peroxidation products and the increase in cellular GSH level and GSH-PX activity in different brain tissues, including the hippocampus, in response to α-LA treatment in our study is indicative of increased free radical scavenging and enhanced detoxification of hydrogen peroxide and lipid hydroperoxides.
Results obtained from Tunnel and Fluoro-Jade B staining of hippocampal slices showed a marked decrease in the percent of positively stained neurons in groups treated with α-LA before PbA relative to the PbA-only treated group, thus indicating a neuroprotective effect of α-LA against lead-induced neuronal damage. Moreover, α-LA significantly reduced the hippocampal 8-OHdG level that was elevated by PbA, a finding that reflects its ability to protect against PbA-induced DNA damage, which can be related to the ability of α-LA to antagonize oxidative stress and production of damaging peroxy and nitroso radicals that cause DNA damage [33,34]. One limitation of our study is that our experiments have thus far been conducted only on rats. These findings augment the assumption that the observed neuroprotective activity of α-LA in our study against PbAc-induced neurotoxicity and DNA damage can be related to the antioxidant activity of α-LA.
Based on the results of the present study, we can conclude that α-LA has a protective effect against PbA-induced seizures and neuronal DNA damage, induced by acute PbA toxicity. This effect can be related to the antioxidant effects of α-LA, mediated by reactivation of the antioxidant enzymes or by inhibition of peroxy and nitroso radical production induced by lead toxicity. The results of this study suggest that α-LA plays an important role in abating the hazards of lead toxicity.
The protective effect of alpha lipoic acid, at doses of 50 and 100 mg/ kg, i.p., on seizures induced by predetermined CD50 of lead acetate (250 mg/kg, i.p.) in rats.
Treatment (mg/kg) |
n |
Seizure latency (Sec) |
Seizure incidence (%) |
Mortality (%) |
Control |
10 |
00.0 ± 0.0 |
0 |
0 |
PbA (250) |
10 |
25.1 ± 2.6 |
50 |
20 |
PbA + α-LA (50) |
10 |
42.2 ± 4.6a |
30 |
0 |
PbA + α-LA (100) |
10 |
46.5 ± 5.4b |
10 |
0 |
PbA + VPA (200) |
10 |
00.0 ± 0.0 |
0 |
0 |
α-LA: Alpha Lipoic Acid, PbA: Lead Acetate; VPA: Valproic Acid
Results represent mean ± SEM.
ap < 0.05 vs. PbA; bp < 0.01 vs. PbA.
The effect of alpha lipoic acid (at 50 and 100 mg/kg, i.p.) on lead acetate-induced changes in hippocampal malondialdehyde, nitrite, and intracellular reduced glutathione levels and glutathione peroxidase activity in rats.
Treatment (mg/kg) |
MDA (µM/g protein) |
Nitrite (µM/g protein) |
GSH (µM/g protein) |
GSH-PX (IU/g protein) |
Control |
318.48 ± 16.27 |
5.32 ± 1.52 |
2.92 ± 0.13 |
32.65 ± 3.62 |
α-LA (50) |
308.38 ± 19.24 |
5.29 ± 1.43 |
2.96 ± 0.14 |
34.83 ± 2.63 |
α-LA (100) |
292.58 ± 14.37 |
4.22 ± 1.63 |
3.42 ± 0.15a |
38.77 ± 2.13 |
PbA (250) |
435.77 ± 22.31b |
9.33 ± 0.85a |
2.33 ± 0.09a |
17.58 ± 2.23b |
PbA (250) + α- LA (50) |
356.37 ± 25.32c |
4.65 ± 0.24c |
3.76 ± 0.14c |
28.65 ± 2.56d |
PbA (250) + α- LA (100) |
333.28 ± 21.33d |
3.64 ± 0.19d |
2.88 ± 0.13d |
30.14 ± 2.55d |
PbA + VPA (200) |
322.25 ± 17.26 d |
3.25 ± 0.18d |
2.53 ± 0.12 d |
30.09 ± 2.34 d |
α-LA: Alpha Lipoic Acid, PbA: Lead Acetate; MDA: Malondialdehyde; GSH: Reduced Glutathione; GSH-Px: Glutathione Peroxidase. Results represent means ± SEM (n = 10).
ap < 0.05 vs. control; bp < 0.01 vs. control; cp < 0.05 vs. PbA; dp <0.01 vs. PbA.
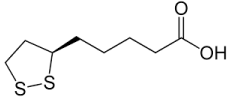
Chemical structure of alpha-lipic acid.
Effect of alpha lipoic acid at doses of 50 and 100 mg/kg alone and in combination with CD50 of lead acetate on hippocampal level of glutamate in rats.
α-LA: Alpha Lipoic Acid; PbA: Lead Acetate; 8-OHdG: 8-Hydroxy-2’-Deoxyguanosine.
Results represent means ± SEM (n = 10). **p <0.05 vs. control, ##p < 0.01 vs. PbA.
Effect of alpha lipoic acid at doses of 50 and 100 mg/kg alone and in combination with CD50 of lead acetate on hippocampal level of 8-hydroxy- 2’-deoxyguanosine in rats.
α-LA: Alpha Lipoic Acid; PbA: Lead Acetate; 8-OHdG: 8-Hydroxy-2’-Deoxyguanosine.
Results represent means ± SEM (n = 10). **p < 0.05 vs. control, #p < 0.05 vs. PbA, ##p <0.01 vs. PbA, ,###p < 0.001 vs. PbA.
TUNEL staining of paraffin-embedded hippocampal slices of (A) the control group, (B) lead acetate-treated rats, and (C) rats treated with lead acetate 30 min after receiving treatment with 50 mg/kg α-LA, and (D) rats treated with lead acetate 30 min after receiving treatment with 100 mg/kg α-LA. (E) Difference in TUNEL positive cells between lead acetate-only treated rats and rats treated with lead acetate 30 min after treatment with 50 or 100 mg/kg α-LA.
α-LA: Alpha Lipoic Acid; PbA: Lead Acetate.
Results represent means ± SEM (n = 3). ***p < 0.001 vs. control; #p < 0.05 vs. PbA, ##p < 0.01 vs. PbA, ###p < 0.001 vs. PbA.
Fluoro-Jade B staining of paraffin-embedded hippocampal slices of (A) the control group, (B) lead acetate-treated rats, and (C) rats treated with lead acetate 30 min after receiving treatment with 50 mg/kg α-LA, and (D) rats treated with lead acetate 30 min after receiving treatment with 100 mg/kg α-LA. (E) Difference in Fluoro-Jade B positive cells between PbA only treated rats and rats treated with PbA 30 min after treatment with 50 or 100 mg/kg α-LA.
α-LA: Alpha Lipoic Acid; PbA: Lead Acetate.
Results represent means ± SEM (n = 3). ***p < 0.001 vs. control #p < 0.05 vs. PbA, ##p < 0.01 vs. PbA ###p < 0.001 vs. PbA.