1Life Force Research, Ljungskile, Sweden
2Department of Complementary of Medicine, University of Westminster, UK
*Corresponding author: Michael McMullen, Life Force Research and Department of Complementary of Medicine, University of Westminster, Backegardsv, 4, 45930 Ljungskile, Sweden
Received: September 08, 2014; Accepted: November 17, 2014; Published: November 19, 2014
Citation: McMullen MK. Oops! Who Forgot to Measure Cardiac Contraction Force (dP/dt)?. Austin J Pharmacol Ther. 2014; 2 (11).1061. ISSN: 2373-6208.
Cardiovascular parameters are often used by researchers without sufficient understanding of the integrated nature of cardiac parameters, vascular tonus and baroreflex activity. Coupled with this is the use of research equipment that measures only a limited number of cardiac parameters such as heart rate or heart rate variability. This report emphasises the need to measure stroke volume, cardiac contraction force (dP/dt) and total peripheral resistance for the understanding of the impact of short-term cardiovascular interventions. Using previously published work, the integration of cardiovascular system is demonstrated using clinical data. This includes (1) how a sympathetic response can lead to decreased heart rate and (2) how an intervention can increase total peripheral resistance and lead to reductions of cardiac workload via the baroreflex. The effect of the baroreflex on heart rate variability is explained and the usefulness of heart rate variability measures, without dP/dt measures, is questioned.
Keywords: Cardiac contraction force; dP/dt; Heart rate; Heart rate variability; Stroke volume; Total peripheral resistance
ANS: Autonomic Nerve System; BP: Blood Pressure; bpm: Beats Per Minute; BRS: Baroreflex Sensitivity; CO: Cardiac Output; CVS: Cardiovascular System; DP: Diastolic Pressure; dP/dt: Cardiac Contraction Force, HR: Heart Rate; HRV: Heart Rate Variability; SP: Systolic Pressure; SV: Stroke Volume; TPR: total peripheral resistance
Cardiovascular parameters are used by researchers in many fields but often without sufficient understanding of the integrated nature of cardiac parameters, vascular tonus and baroreflex activity. Coupled with this is the use of research equipment that measures only a limited number of cardiac parameters. Unfortunately this poor quality research is often published and becomes the standard protocol because many journal reviewers are not sufficiently knowledgeable in the complicated physiology of blood circulation and autonomic control.
The measure dP/dt(max) is a widely used as an index of cardiac contractility [1] and is commonly abbreviated to dP/dt. It is available on beat-to-beat pulse wave monitors such Finopres, Portopres, Finometer and Nexfin as the maximum slope of the vascular pulse wave during the upstroke. Actually there are various ways of deriving dP/dt as well as other measures of cardiac contractibility. This article aims to convince the reader that measuring dP/dt, or some other measure of cardiac contractibility, is essential for studies assessing short term interventions on cardiovascular function. In particular, without a measure of cardiac contractibility, it is impossible to know whether decreases in heart rate reflect increased sympathetic Autonomic Nerve System (ANS) activity or increased parasympathetic ANS activity. Obviously this also has implications for interpreting changes of Heart Rate Variability (HRV) measures.
The Cardiovascular System (CVS) is a highly integrated system and under the control of the ANS. There are two ANS control systems which regulate the CVS. The first is the centrally driven ANS innervation while the second involves the baroreflexes and modulates the central ANS outflow. Sympathetic nerves innervate both the heart and the vasculature while parasympathetic nerves innervate only the heart. Additionally the heart has an intrinsic/local nerve system which beats independently of ANS control. For humans the intrinsic Heart Rate (HR) is circa 100 to 110 Beats Per Minute (bpm). The intrinsic HR is subject to tonic innervation from both the sympathetic and parasympathetic branches of the ANS. Sympathetic innervation acts to increase heart rate whereas parasympathetic innervation acts to decrease heart rate [1,2]. Humans have a resting heart rate commonly between 55 and 75 bpm and so exist primarily in neural “parasympathetic dominance”. The dual-tonic heart innervation allows for fast responses as both branches of the ANS are involved. Vagal responses occur within the beat-to-beat interval whereas sympathetic responses take three to four seconds to manifest at the cardiac level [3]. In contrast to this dual-innervation controlling HR, both cardiac contraction and vascular tone are solely under the control of the sympathetic branch of the ANS. Stroke Volume (SV), the amount of blood ejected from the heart to the systemic circulation on any beat is dependent on the amount of blood flowing from the lungs (sympathetically controlled), the contraction force of heart (sympathetically controlled) and the resistance or impedance of blood already present in the aorta (aortic impedance) (sympathetically controlled). Cardiac output (CO) refers to the amount of blood ejected from the heart into the systemic circulation over the period of a minute. Commonly the two branches of the ANS act in unison to effect CVS changes. For example on standing, initial increases of HR result from vagal withdrawal whereas later increases of HR are due to sympathetic activation [1].
The primary function of the CVS is to provide oxygen and remove carbon dioxide. To achieve this goal blood pressure (BP) must be regulated. Peripheral feedback provided by the baroreceptors refines the central ANS innervation at the level of the medulla. BP is monitored in the aorta, carotid artery and other areas. Decreasing BP leads to increased HR while decreasing BP leads to increased HR [1]. The baroreflex acts on a beat-to beat basis and is so sensitive that it has been proposed as the mechanism by which HR is changed in response to BP changes induced by breathing i. e. respiratory sinus arrhythmia [4,5]. Baroreflex Sensitivity (BRS), the extent to which BP alters before HR changes, is also under ANS control. Higher levels of BRS, indicating greater BP changes are required to initiate a response, indicate greater parasympathetic activity whereas lower levels of BRS indicate greater sympathetic activity. This explains why Spontaneous BRS is commonly assessed using either spectral analysis or the sequence method [6]. BRS is posture dependent (Table 1) [7], greater lying than standing and this is one reason why HRV is greater lying than standing. BRS may be modified for hours by pharmaceutical interventions such as drinking water [8] or rapidly modified for short time periods by exposing oral taste receptors to drinks to which caffeine has been added [9].
The ANS responds to changes in both the internal and external environment producingvirtually an infinite number of CVS equilibriums. Simple body activity alters CVS parameters dramatically as is demonstrated in Table 1 [7], where CVS measures in the supine and upright postures are compared [7]. In this case the difference is due to the internal environment, posture, and the external environment, gravity! Consequently It is hardly surprising that the impact of pharmaceuticals on the CVS varies with posture, as ANS activity and vascular tonus vary with posture [7,10].
I advocate, that to assess the short-term (< 30 minutes) impact of pharmaceutical interventions on the CVS,it is necessary to have as a minimum measure of HR, DP/dt, SV, total peripheral resistance (TPR) and BP. CO follows as it the multiple of HR and SV (CO = HR x SV) while TPR is calculated from mean BP and CO (Mean BP = CO x TPR or TPR = Mean BP / CO). Note that BP is a function of TPR, rather than the reverse even though BP is used in calculating TPR. Intermittent measures of BP are of questionable value due to their inherent variability [11] and continuous BP is preferred [12]. Measures of central pressure may also be useful [13].
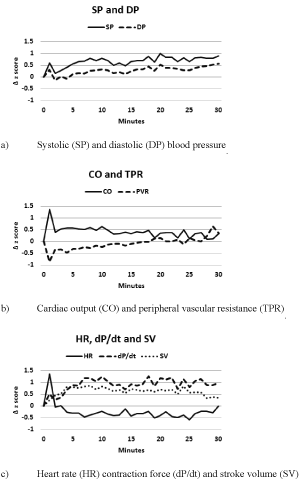
To demonstrate the importance of dP/dt for understanding haemodynamics we will examine the effect of ingesting 100 mL water on the CVS [14], see Figure 1. The vertical axes are z scores (mean = 0,standard deviation = 1) of changes from pre-ingestion measures. The use of z scores allows all parameters to be presented in the same units. The extent of changes can be visually gauged with reference to Cohen’s d effect sizes: 0. 2 small, 0. 5 medium/moderate and 0. 8 large [15].
The 30 minute post-ingestion period has a number of phases:
The post-ingestion period may last four hours or more after a large meal [19].
In assessing whether haemodynamic changes have occurred it is useful to start by examining whether the intervention elicited changes in BP, keeping in mind that the baroreflex constantly acts to minimise or nullify BP changes. The swallowing period should be excluded and the gastric and intestinal phases considered as separate physiological entities. If BP is altered then there has been a major impact on the CVS. In Figure 1a we observe, for both the gastric and intestinal phases, medium to large effects on SP and small to medium effects on DP.
Next query: are BP changes due to cardiac or vascular factors? In Figure 1b CO moderately increases in the gastric phase with small increases occurring in the intestinal phase. While for TPR small decreases occur in the gastric phase and no change in the intestinal phase. So the increased BP observed in Figure 1a is the result of increased cardiac activity.
Next query: were any cardiac parameters affected? In Figure 1c small to moderate decreases of HR occur in both phases, for dP/dt large increases occur in both phases, and for SV moderate to large increases occur in both phases. Thus the changes in BP can be attributed to dP/dt. If SV changes had been greater than dP/dt changes then some change of breathing would need to factored into the rationale. The physiology: stroke volume is influenced by the energy of cardiac contraction and aortic pressure; the energy of contraction can be raised by increased end-diastolic volume or increased dP/dt [1]. Note: stroke volume oscillations follow breathing [20] more accurately than heart rate oscillations (respiratory sinus arrhythmia) [21].
The statistical analysis of the differences between the pre-ingestion measures and the post-ingestion measures for the interval 5 to 15 minutes were: SP increase (p = 0. 002), DP increase (p = 0. 025), CO and TPR unchanged,HR decrease (p = 0. 033), dP/dt increase (p = 0. 002) and SV increase (p = 0. 060). In conclusion we can state that the intake of 100 mL water elicited a sympathetic response as would be expected from the literature [18]. But, you may ask, what about HR, how can the response be sympathetic if HR is decreased? The simplest explanation (Occam’s razor) is that the increase in BP enhanced baroreflex activity which caused in reduced HR. Consequently, and significantly, when assessing the impact of an intervention on the ANS, HR may be an unreliable indicator of autonomic changes in short-term studies. This example demonstrates that changes in all CVS parameters need to be examined to gain an understanding of the pharmaceutical effects of an intervention as simple as 100 mL water.
The impact of drinking water on the CVS during the gastric phase has received negligible attention from researchers, despite its importance for a wide range of fields including gastroenterology, nutrition and CVS function. For example, the postprandial reductions of SP in the elderly, which may be as large as 25 mm Hg, are largely confined to the initial 30 minutes post-ingestion period [22]. Prior to the study outlined above, the consensus view based on intermittent blood pressure readings every 10 or 15 minutes, has that even the ingestion of relatively large amounts of water (500 mL) by healthy adults elicits no pressor response [23,24]. Clearly this is view point is not consistent with the physiology of postprandial haemodynamics in the gastric phase: with gastric extension, the blood flow rapidly increases in the celiac artery there must be circulatory compensation or hypotension will occur [25]. This circulatory compensation involves an increase in cardiac output. Recently a report suggested that cardiac workload decreased following the ingestion of 500 mL water because HR decreased [8]. This study has been criticised because contraction force (dP/dt) was not measured and increases in stroke volume, indicative of increased cardiac workload, were ignored [26].
In pharmacological studies of the CVS any impact on the heart or the vasculature will result in a change factors determining BP, but any changes in BP will be opposed by the baroreflex. For example in the second part of the study outlined above, extracts of bitter tastants (1500 mg Gentiana lutea and 1500 mg Artemisia absinthium) were used to flavour the water [14]. These tastants altered the ANS response to the ingestion of water. Both tastants elicited increases in TPR (p = 0. 002 and p = 0. 003 respectively) which would be expected to increase BP. However, BP remained stable, as dP/dt, SV and CO all decreased with HR unchanged. The reduction in cardiac activity can be attributed to the influence of the baroreflex reacting to the increased TPR. The results indicate that bitter tastants can reduce cardiac workload and may be therapeutically useful in cases were the cardiac response to eating/drinking is inadequate. Such a usage of bitter tastants has been reported to relieve gastroparesis in Parkinson patients in the intestinal phase [27]. It is important to remember that interventions including water [8] and caffeine [9,28] may alter BRS.
Heart Rate Variability (HRV) is an analysis based on differences of the inter-beat interval and beat-to-beat recordings are required to produce HRV measures. HRV measures are referred to as second order measurements and this type of measure is very helpful in assessing variability in biological systems. Generally high HRV values are associated with a healthy CVS and low values with poor prognosis. The HRV Taskforce stated: ”any medications act directly or indirectly on the autonomic nervous system, and HRV can be used to explore the influence of various agents on sympathetic and parasympathetic activity” [29]. However, it is questionable whether short-term HRV (≤ 5 minute recording) are suitable for assessing acute pharmacological interventions unless they take into account changes of dP/dt. For example, the ingestion of water which can be expected to produce a sympathetic ANS response [18] involving increased BP, dP/dt and SV as well as decreased HR [14]. In contrast, it has been reported that the ingestion of water increases high frequency HRV values indicating an increased vagal tone [8]. This increase of vagal tone suggests a parasympathetic response to water ingestion rather than a sympathetic response. In this case the result HRV analysis does not reflect the actual situation because it gives information about a compensatory response and not the primary response.
The CVS is an integrated system and piecemeal measurements may lead to a faulty analysis of pharmacological impacts. Changes in a single parameter such as HR or HRV are of limited significance if measures of dP/dt, SV and TPR are unavailable.
Cardiovascular measures (means ± standard deviation) for 12 healthy adults in the supine and upright postures.
CVS parameter |
Supine |
Upright |
HR |
67.3 ± 8.7 |
85.2 ±11.3 |
dP/dt |
942 ± 257 |
1220 ± 348 |
iSV |
50.4 ± 7.4 |
33.6 ± 9.4 |
iCO |
3.4 ± 0.6 |
2.8 ± 0.7 |
iTPR |
0.58 ± 0.15 |
0.86 ± 0.26 |
SP |
116.2 ± 11.7 |
133.5 ± 14.1 |
DP |
68.1 ± 6.6 |
86.4 ± 8.7 |
BRS |
19.4 ± 11.6 |
7.3 ± 3.5 |
Post ingestion changes from pre-ingestion values in z scores (preingestion means = 0, standard deviations = 1).