
Research Article
Austin J Pharmacol Ther. 2016; 4(2).1084.
“Antiarthritic Activity of Whole Plant Extract of Pentatropis capensis on Wistar Rats”
Shah SK, Rao NJ, Patel TH and Patel BV*
Department of Pharmacology, Sardar Patel College of Pharmacy, India
*Corresponding author: Patel BV, Department of Pharmacology, Sardar Patel College of Pharmacy, Bakrol, 388315, India
Received: May 26, 2016; Accepted: July 18, 2016; Published: July 29, 2016
Abstract
Arthritis is an autoimmune disorder characterized by synovial proliferation & inflammation, cartilage destruction and deformity of joints. It can be a disabling and painful condition, which may lead to substantial loss of functioning and mobility if not adequately treated. The study was carried out to evaluate the antiarthritic effect of Methanolic Extract of Pentatropis Capensis (MEPC) in arthritic rats. Adult Wistar rats were used in the study. Dose selection was done by performing Formaldehyde (0.1ml i.d. of 2% v/v formaldehyde) induced arthritis model. 140mg/kg MEPC dose was obtained as ED50 and was used in Complete Freund’s Adjuvant (CFA) arthritis model. In CFA model, 0.1ml CFA was injected in left hind paw on 0 day only. Arthritic rats were administered with standard drug (indomethacin, 0.3mg/kg) and MEPC (140mg/kg) from 1st to 12th day. Physical parameters like change in body weight, paw volume, paw diameter and arthritic index were measured during experiment. On 21st day, blood was collected and various hematological parameters and Rheumatoid Factor were performed. The ankle joints were collected for histopathological and radio graphical examinations. MEPC markedly reduced paw volume, paw diameter, arthritic index, ESR (Erythrocyte Sedimentation Rate) and Total WBC (White Blood Cell) count, while markedly increased RBC (Red Blood Cell) and Hb (Hemoglobin) as compared to arthritic animals. Rheumatoid Factor was significantly (P< 0.05) reduced in MEPC treated group when compared to arthritic animals. Histopathological and radio graphical studies of joints showed protective effect of MEPC. MEPC possess protective effect against CFA induced arthritis that can be attributed towards its antiarthritic activity.
Keywords: Arthritis; Pentatropis capensis; Complete freund’s adjuvant; Formaldehyde; Rheumatoid factor
Abbreviations
MEPC: Methanolic Extract of Pentatropis Capensis; ESR: Erythrocyte Sedimentation Rate; WBC: White Blood Cell; RBC: Red Blood Cell; Hb: Hemoglobin, CFA: Complete Freund’s Adjuvant
Introduction
Joint is location where two or more bones are connected to each other. The joints of the skeletal system contribute to homeostasis by holding bones together in ways that allow the movement and flexibility [1].
Arthritis is a joint disorder featuring inflammation of one or more joints [2]. It is an autoimmune disorder characterized by synovial proliferation and inflammation, cartilage destruction and deformity of joints also a systemic inflammatory disease associated with generation of oxidative stress that produced vascular dysfunction [3].
It is caused by pro-inflammatory molecules released by macrophages including reactive oxygen species and eicosanoids such as prostaglandins, leukotrines and cytokines [4]. It affects about 1% of the population of world in a female and male ratio 2.5:1 [2].
The currently used allopathic medicines cannot cure arthritis and these medicines are often associated with several side effects. Ayurvedic medicines are popular in India and commonly used for treatment of arthritis which alleviates pain in people with arthritis [5].
One of the herbal plants is Pentatropis capensis Linn. Bullock belongs to Asclepiadaceae family which is geographically well distributed in West Bengal, Gujarat, Delhi, Rajasthan, Pakistan, and Sri Lanka [6]. It exerts anti-bacterial, keratolytic, antiseptic, hydrogen peroxide radical scavenging activity [7]. Its ethanolic extract and alcoholic extract exerts antiinflammatory activity by inhibition of inflammatory effect 5 HT & Histamine and analgesic activity respectively [8,9].
Several herbal plants containing various phytoconstituents like n-octacosanol, α- amyrin, friedelin, β- sitosterol, salicylic acid, cardiac glycosides, steroids, flavonoids, tannins have shown antiinflammatory as well as antiarthritic activity [10-13]. Pentatropis capensis Linn. Bullock also contains some of these phytoconstituents. Hence, it may be possible that it may have protective effect against arthritis.
Observing the world wide prevalence of the disease, the side effects and poor efficacy of the currently available medicines and increasing uses of herbal plants in the treatment, the study was designed to screen antiarthritic activity of methanolic extract of whole plant of Pentatropis capensis Linn. Bullock by using Formaldehyde and Complete Freund’s Adjuvant induced arthritis models.
Materials and Methods
Collection of plant material
Arial parts of whole plant of Pentatropis capensis were collected from Himalaya Naturals, Dehradun, Uttarakhand and were authenticated by G. C. Jadeja at Anand Agriculture University, Anand, Gujarat, India (SPCP/Herbarium/005/2013).
Extraction procedure
Collected plant was cleaned, washed and dried under the shed. Then it was coarsely powdered in blender and passed through sieve #40. Then, 200 g of Pentatropis capensis powder was subjected to extraction by a simple maceration process using 70% methanol for 48 hrs. Then the extracted liquid has evaporated at 45 °C, the solvent free mass thus obtained was weighed and coded as MEPC (Methanolic Extract of Pentatropis capensis). It was stored in airtight container in refrigerator for further use. Obtained semi solid-sticky plant extract was subjected to phytochemical screening tests.
Experimental procedure
Females are more prone to arthritis as compared to males [2]. Thus, Female Wistar rats were used as experimental animals for the study. The animals were housed in a group of 6 rats per cage under well-controlled conditions of temperature (22 ± 2°C), humidity (55 ± 5%) and 12hrs/12hrs light-dark cycle. Animals had free access to conventional laboratory rat chow diet and water ad libitum. The protocol SPCP/IAEC/RP-003/2012-13 of the experiment was approved by Institutional Animal Ethics Committee (IAEC) as per the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India.
Following models were used in study
- Formaldehyde induced arthritis model: This model was used to find out ED50 value of MEPC.
- Complete Freund’s Adjuvant model: Model was used to find antiarthritic activity of MEPC.
Formaldehyde induced arthritis [2]: Animals were randomly allocated in six groups, with n=6 animals in each group in each group, as follows: Group-I Disease control received 0.1 ml formaldehyde (2% v/v), Group-II, III, IV and V received MEPC 100, 200, 300 and 400 mg/kg, p.o. Thirty minutes after oral administration of drugs, arthritis was induced by subplantar administration of 0.1 ml formaldehyde (2% v/v) into the left hind paw of all the animals. This was designated as day 1. Drug treatment was continued for the duration of 9 more days.
Formaldehyde (0.1 ml 2% v/v) was again injected into the same paw on the third day. The paw volume was measured by a Plethysmograph on the first day of the experiment. Group II, III, IV, and V received 100, 200, 300 and 400 mg/kg of MEPC respectively for 10 days. After 30 min of administration of drug, 0.1 ml of 2% v/v formaldehyde was injected in sub-plantar region of left hind paw on 1st and 3rd day. On the 10th day paw volumes of all animals were measured and a graph was plotted of percentage reduction in paw volume vs. doses of MEPC to measure ED50 of MEPC. The dose of ED50 obtained, was used in CFA induced arthritis model (Dose of ED50 was found to be was 140 mg/kg).
Complete freund’s adjuvant induced arthritis model [14]: Female Wistar rats weighing 150-200 g were selected for the experiment. They were divided into four groups each group contained six animals as follows: Group-I Normal control, Group-II received Complete Freund’s adjuvant (0.1 ml/rat, i.d.) and served as Diseased control, Group-III received Indomethacin (0.3 mg/kg, p.o.) and served as Standard treated and Group-IV (MEPC 140 treated) received MEPC (140 mg/kg, p.o.). Thirty minutes after oral administration of drug, 0.1 ml of CFA (0.05% Mycobacterium butyricum in mineral oil) was injected into the subplantar surface of the left hind paw by a 26 gauge needle. This was designated as day 0. Drug treatment was continued for duration of 11 more days. Physical parameters like change in body weight [15], paw volume [15], paw diameter [15] were measured on days 0, 4, 7, 14 and 21. On day 21, Arthritic Index [15] was found and blood was collected from Retro-orbital sinus of rats for measurement of hematological parameters like RBC [16], Hb [17], ESR [18] and Total WBC [17,19] count and specific indicator of arthritis i.e. Rheumatoid Factor [20]. The synovial joints were also collected from the same day for histopathological [15] and radio graphical examinations [21] by sacrificing rats.
Statistical analysis
All the parametric data obtained from the various parameters were statistically evaluated by one way Analysis of Variance Test (ANOVA) followed by Dunnett’s post test while non parametric data were analysed by Wilcoxon sign rank test. Data were expressed as Mean ± SEM and P values less than 0.05 (p<0.05) were used as the significant level. Statistical analysis was done using Graph Pad Prism software, version 5.03.
Results
Phytochemical screening outcomes of MEPC
The Methanolic Extract of Pentatropis Capensis (MEPC) was subjected to phytochemical investigation. The results revealed the presence of steroids, alkaloids, carbohydrates, flavonoids, tannins and glycosides in the extract.
Results of formaldehyde induced arthritis model
Effect of MEPC on Paw volume in formaldehyde induced arthritis: Rat paw volume decreased with rise in dose of MEPC showing the decline in paw volume in dose dependent manner. Graph of percentage reduction in paw volume vs. dose of MEPC was plotted and ED50 was found to be 140 mg/kg and it was used in CFA model (Table 1, Figure 1).
Groups
Average of Paw Volume (ml)
Reduction in Paw Volume (ml)
Percentage reduction in Paw volume
Disease control
0.60 ± 0.02
-
-
MEPC (100 mg/kg)
0.48 ± 0.02
0.12
40
MEPC (200 mg/kg)
0.39 ± 0.02
0.21
70
MEPC (300 mg/kg)
0.36 ± 0.03
0.24
80
MEPC (400 mg/kg)
0.30 ± 0.01
0.30
100
All values are expressed as Mean ± SEM (n=6).
Table 1: Effect of MEPC on percentage reduction in Paw volume in Formaldehyde model.
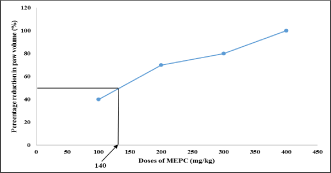
Figure 1: Effect of MEPC on percentage reduction in paw volume in
formaldehyde model.
Results for complete freund’s adjuvant induced arthritis model
Effect of MEPC on Physical parameters in CFA induced arthritis: Body weight of rats before & after induction and their mean difference is portrayed in (Table 2). Mean difference in Body weight of disease control group was decreased as compare to normal control group. While, the reduction in body weight was prevented with the treatment with standard MEPC group.
Groups
Mean Body Weight (g)
Before induction
After induction
Mean difference in Body Weight
Normal Control
183± 4.85
230±3.87
47±3.95
Disease Control
212±13.21
221±31.48
9.33±1.26#
Standard treated
189±6.14
215±1.96
26±4.69*
MEPC 140 treated
191±4.27
203±4.03
12±0.68
All values are expressed as Mean ± SEM (n=6), #P<0.05, significant as compared to normal control, *P<0.05, significant as compared to disease control. Statistical analysis was carried out by One-way ANOVA followed by Dunnett’s post hoc test.
Table 2: Effect of MEPC on Body Weight in CFA model.
There was significant increase in rat paw volume in CFA injected disease control group when compared to normal control group on 4th, 7th, 14th and 21st days (Figure 2). While, standard and MEPC 140 treated groups showed forbade the rise in paw volume when compared to disease control group. On day 21, there was 63.66% and 58.18% reduction in joint swelling in rats treated with standard indomethacin and 140 mg/kg MEPC, respectively.
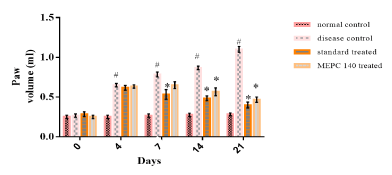
Figure 2: Effect of MEPC on Paw Volume in CFA model.
All values are expressed as Mean ± SEM (n=6), #P<0.05, significant as
compared to normal control, *P<0.05, significant as compared to disease
control. Statistical analysis was carried out by One-way ANOVA by Dunnett’s
post hoc test.
Administration of CFA produced an increase in the joint diameter of all the animals, which was persistent throughout the observation period (Table 3). Maximum joint swelling was observed on day 7, after which there was a gradual decrease in paw diameter except in the disease control group.
Groups
Mean Paw Diameter (mm)
0 day
4th day
7th day
14th day
21st day
Normal Control
7.50±0.22
7.58±0.20
7.60±0.24
7.60±0.24
7.53±0.16
Disease Control
8.50±0.22
11.00±0.18#
13.16±0.30#
15.0±0.25#
17.08±0.32#
Standard treated
8.00±0.25
10.83±0.30
12.66±0.33
10.33±0.42*
9.50±0.22*
MEPC 140 treated
8.50±0.02
10.91±0.27
12.83±0.33
12.50±0.34*
11.00±0.36*
All values are expressed as Mean ± SEM (n=6), #P<0.05, significant as compared to normal control, *P<0.05, significant as compared to disease control, Statistical analysis was carried out by One-way ANOVA by Dunnett’s post hoc test.
Table 3: Effect of MEPC on Paw Diameter in CFA model.
The pattern of amelioration in arthritis was evaluated according to scoring system. Arthritic rats of disease control group showed significant (P< 0.05) elevation in arthritic index score as compared to normal control animals. While, the standard indomethacin and MEPC 140 treated groups showed significant (P< 0.05) decline in arthritic index score when compared to disease control group (Figure 3).
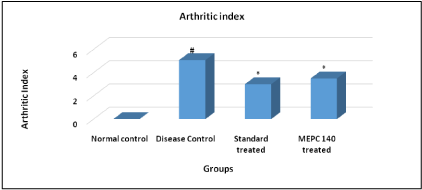
Figure 3: Effect of MEPC on Arthritic Index in CFA model.
All values are expressed as Mean ± SEM (n=6), #P<0.05, significant as
compared to normal control, *P<0.05. Statistical analysis was carried out by
Wilcoxon sign rank test.
Effect of MEPC on Hematological parameters in CFA model: The effect of MEPC on various hematological parameters are shown in (Table 4). There was significant decrease in RBC count and Hb level, and steep rise in ESR and Total WBC count in disease control group when compared to normal control group. While treatment with MEPC 140 and standard indomethacin significantly (P<0.05) prevented the decrease in RBC count & Hb level and elevation in ESR & WBC counts as compare to disease control group.
Groups
RBC (millions/mm3)
Hb (g %)
ESR (mm/hr)
Total WBC Count (cells/mm3)
Normal Control
9.59±0.68
14.14±0.67
2.56±0.74
7000.00±294.87
Disease Control
4.44±0.84#
7.51±0.58#
10.85±0.80#
15040.00±727.40#
Standard treated
9.07±0.69*
13.75±0.58*
5.28±0.70*
6828.00±478.47*
MEPC 140 treated
8.38±1.00*
12.61±0.52*
7.55±0.74*
8300±364.29*
All values are expressed as Mean ± SEM (n=6), #P<0.05, significant as compared to normal control, *P<0.05, significant as compared to disease control. Statistical analysis was carried out by One-way ANOVA by Dunnett’s post hoc test.
Table 4: Effect of MEPC on Hematological parameters in CFA model.
Effect of MEPC on Rheumatoid Factor in CFA model: Level of Rheumatoid factor was significantly (P<0.05) elevated in disease control group when compared to normal control group. While animals treated with standard indomethacin and MEPC 140 mg/kg showed significant decline Rheumatoid factor level (Figure 4).
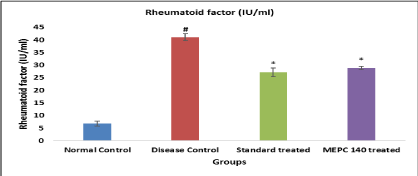
Figure 4: Effect of MEPC on Rheumatoid Factor in CFA model.
All values are expressed as Mean ± SEM (n=6), #P<0.05, significant as
compared to normal control, *P<0.05, significant as compared to disease
control. Statistical analysis was carried out by One-way ANOVA by Dunnett’s
post hoc test.
Histopathological examination of ankle joints in CFA model: Photomicrograph of ankle joint of normal control had not shown inflammation, bone destruction, articular cartilage destruction and presence of connective tissue structure with the absence of infiltration of cells. While, Photomicrograph of ankle joint of disease control group, showed severe inflammation with marked edema associated with granuloma formation as compared to normal control group. It also showed higher degree of articular cartilage destruction and bone erosion, marked infiltration of cells and bone destruction.
Photomicrograph of ankle joint of Standard treated group had shown mild inflammation as compared to disease control group. It showed mild infiltration of cells. It did not show boney erosion or destruction due to its effect on arthritis and that MEPC 140 treated group, showed moderate inflammation with edema as compared to disease treated group. It showed mild articular cartilage destruction, infiltration of cells and little bone erosion and destruction (Figure 5).
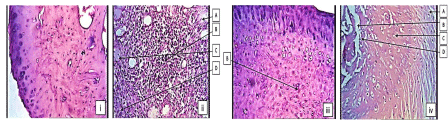
Figure 5: Histopathology of ankle joints in CFA model (i) normal control, (ii)
disease control, (iii) standard treated and (iv) MEPC 140 treated groups:
A- Articular cartilage destruction, B- Infiltration of cells, C- Bone erosion, DBone
destruction
Radiographical examination of ankle joints in CFA model: As shown in the (Figure 6), ankle joints of disease control group animals had moderate erosion, joint space narrowing and marked joint destruction. Standard treated group had showed mild erosion. MEPC 140 treated group had showed mild erosion, joint space narrowing and joint space destruction.
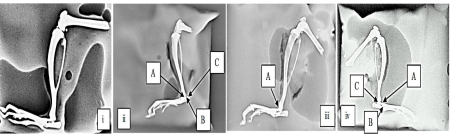
Figure 6: Effect of MEPC on Radiographs of joints in CFA model (i) normal
control, (ii) disease control, (iii) Standard treated and (iv) MEPC 140 treated
groups:
A- Erosions, B- Joint space narrowing, C- Joint space destruction
Discussion
Arthritis is a systemic chronic autoimmune disease associated with multiple inflammatory mediators that lead to joint damage, synovial inflammation and cartilage and bone damage [22]. Other frequently occurring forms of arthritis include Osteoarthritis, Rheumatoid arthritis, Fibromyalgia, Gout and Lupus. Common symptoms include pain, aching, stiffness, and swelling in or around the joints. Some forms of arthritis, such as rheumatoid arthritis and lupus, can affect multiple organs and cause widespread symptoms [23]. The major medications that are available in the present time include NSAIDs, corticosteroids, DMARDs and cytokine inhibitors like etanercept, infliximab and anakinra. The search is still going on for discovering new drugs which can treat the disease in a safer way [15].
Formaldehyde induced arthritis is one of the most commonly used acute model for assessing antiarthritic activity. In present study, Formaldehyde model was used for measuring ED50 of MEPC (Methanolic Extract of Pentatropis capensis). The development of edema in rat paw after injection of formaldehyde is due to the release of histamines, serotonins and prostaglandins. Similar results were observed in present study and arthritic rats of disease control group showed rise in paw volume. While, there was decrease in paw volume with the increase in MEPC doses, which may be due to protective effect of MEPC.
Complete Freund’s Adjuvant (CFA) induced arthritis is well established model and has been widely used from many years for evaluation of anti-inflammatory and anti-arthritic agent [24,25]. After administration of CFA in left hind paw of rats, there is joint swelling, infiltration of inflammatory cells, bone destruction, joint cartilage erosion and remodeling which results in destruction of joint integrity and function disability occurs in legs [25].
CFA is inactivated and dried mycobacteria which are responsible for stimulation of cell mediated immunity which ultimately increases the production of certain immunoglobulin. CFA induced arthritis is a primary and secondary chronic arthritis [25]. Prostaglandins are generated in primary inflammatory phase and auto antibodies are generated in secondary immunological state. Release of various inflammatory mediators including cytokines (IL-1B and TNF-α), interferons and PDGF are responsible for the initiation of pain along with swelling of the limbs and joints, bone deformations and disability of joint function [26]. Results of present study supported the fact and showed significant increase in paw volume and paw diameter after administration of CFA which reflected the status of arthritis.
Changes in body weight have also been used to assess the course of the disease and the response to therapy of anti-inflammatory drugs. As the incidence and severity of arthritis increased, the changes in the body weights of the rats also occurred during the course of the experimental period. The loss of body weight may be due to alterations in the metabolic activities of diseased rats [4]. Similarly, disease control rats showed decrease in body weight. Some of findings suggest that absorption of 14C-glucose and 14C-leucine in rat’s intestine was reduced in the case of inflamed rats but on the treatment with anti-inflammatory drugs, the decrease in absorption was nullified and it shows that the anti-inflammatory drugs correct the decreased/ deranged absorption capacity of intestine during inflammation [4]. In present study, standard indomethacin and MEPC 140 treated group forbade the decrease in body weight as compared to disease control group.
Prajapati et al (2011) reported that inflammation at the site of injection of CFA is the first visible sign of the initiation of the disease and injection of CFA in the hind paw of rats resulted in to sustained increase in paw volume and paw diameter [15]. The data in present study supported the research, where disease control rats showed elevation in paw volume and paw diameter. Arthritis induced rats were treated with MEPC there was significant reduction in paw volume on 7th, 14th and 21st days and in paw diameter on 14th and 21st days which may be due to inhibition of the release of inflammatory mediators. Showing anti-inflammatory activity of MEPC.
Arthritic index includes the combined index of inflammation, formation of nodules and extent of spread of the disease to other organs. This gives the full picture of the disease [15]. There was also selective reduction in arthritic index in MEPC treated rats which showed immunosuppressive effect of Pentatropis capensis.
Decreased level of RBC & Hb represents anemic condition in arthritic rats. The significant increase in Total WBCs count in disease control group may be due to the stimulation of immune system against the invading antigens [27]. ESR is measure of inflammation and allergy. ESR is an index of suspension stability of RBCs in plasma. The number and size of RBC is associated with ESR. It also involved in the accelerated formation of endogenous proteins including plasma proteins such as fibrinogen, α and β globulins. ESR is elevated during the inflammation, stress and cell necrosis [28]. In this investigation, treatment with MEPC significantly restored decreased level of RBC & Hb, elevated level of ESR & Total WBC count. Rheumatoid Factor is specific indicator of arthritis and MEPC at the dose of 140 mg/kg tried to normalize the hematological parameters and rheumatoid factor as compared to disease control group.
Patel et al (2013) reported that histopathology study of disease control animals showed severe inflammation with marked edema, bone destruction and highest necrosis of ankle joint as compared to normal control group [29]. The findings of present study supported the fact where disease control group showed similar results. While standard indomethacin and MEPC treated rats showed protective effect against bone destruction.
Radiographic changes in arthritis conditions are useful diagnostic measures which indicate the severity of the disease. Soft tissue swelling is the earlier radiographic sign, whereas prominent radiographic changes like boney erosions and narrowing of joint spaces can be observed in developed stages of arthritis [30]. Similar results were seen in present study where rats treated with CFA in disease control group showed highest joint damage was seen in radiograph of ankle joint. While, Standard and MEPC 140 treated groups showed inhibitory effect on it. Hence, MEPC may be useful in treatment of arthritis.
Conclusion
In the light of above findings, methanolic extract of Pentatropis capensis Linn. Bullock at specified dose level of 140 mg/kg, p.o. showed significant reduction in paw volume, paw diameter and arthritic index. It also normalized the hematological parameters and rheumatoid factor in arthritic rats. Histopathologiacal and radiographical studies confirmed the protective effect of methanolic extract of Pentatropis capensis. Thus, it can be concluded that methanolic extract of Pentatropis capensis showed significant protective effect on arthritic rats and Pentatropis capensis can be used as an adjuvant therapy for arthritis.
References
- Ellis H, Standring S, Gray and Henry D. Gray’s anatomy: the anatomical basis of clinical practice. Elsevier Churchill Livingstone. 2005; 38.
- William CS. “Arthritis”.
- Vyas AS, Patel NG, Panchal AH, Patel RK, Patel MM. Anti-arthritic and vascular protective effects of Fenugreek, Boswellia Serrata and Acacia Catechu alone in the combinations. An Int J Pharm Sci. 2010; 1: 95-111.
- Kore KJ, Shete RV, Desai NV. Anti-arthritic activity of hydro alcoholic extract of Lawsonia Innermis. Int J Drug Dev & Res. 2011; 3: 217-224.
- “Ayurvedic treatment of arthritis”.
- Khare CP. Indian Medicinal Plants. An illustrated dictionary. 8th edn. Springer. 2007: 471.
- Korukola N, Bonthu MG, Vegesna KR, Varma H, Naidu S. Phytochemical, anti fungal, anti microbial and anti oxidant studies on whole plant extract of Pentatropis Capensis. Int J Pharm Chem and Bio Sci. 2012; 24: 453-463.
- Chandrasekaran K, Suseela L. Anti inflammatory activity of ethanolic extracts of Pentatropis Capensis and Sarcostemma Secamone. Int J Pharma and Bio Sci. 2011; 2: 44-80.
- Chandrasekaran K, Suseela L. Analgesic activity of ethanolic extract of pentatropis capensis and sarcostemma secamone. Int J Pharm Chem and Bio Sci. 2012; 2: 73-76.
- Gupta, “Vanda roxburghii”. La-Medicca (India) Private Limited. Indian J. med. Res. 1946; 34, 253; Basu, Curr. Sci. 1971; 40: 86.
- “Chemical land”, November 2012.
- Jose MP, Maria CR, Rosa MG. Anti-inflammatory activity of beta-sitosterol in a model of oxazalone-induced contact-delayed-type hypersensitivity. BLACPMA. 2006; 5: 57-62.
- Fadeyi OO, Obafemi CA, Adewunmi CO and Iwalewa EO. Antipyretic, analgesic, anti-inflammatory and cytotoxic effects of four derivatives of salicylic acid and anthranilic acid in mice and rats. African J. Biotech. 2004; 3: 426-431.
- Vogel HG. Drug Discovery and Evaluation. Pharmacological Assays, 2nd edn. 2002; 802-803.
- Prajapati DS, Shah JS, Sen DJ. The Modulatory effect of telmisartan on antiinflammatory effect of rosiglitazone in adjuvant arthritis model. Int. J. Res. in Pharm. and Biomed. Sci. 2011; 2: 554-566.
- Basic haematology.
- Kale SR, Kale RR. Haematology, Practical human anatomy and physiology. 7th edn. Nirali prakashan, Pune. 2007; 5–31.
- Hematocrit, erythrocyte sedimentation rate, haemoglobin.
- Manual WBC.
- Rheumatoid Factor.
- Joosten LAB, Lubberts E, Helsen MMA, Van Den Berg WB. Dual role of IL-12 in early and late stages of murine collagen type II arthritis. J. Immunol. 1997; 159: 4094–4102.
- Ono Y, Inoue M, Mizukami H, Ogihara Y. Suppressive effect of Kanzo-bushito, a Kampo medicine, on collagen-induced arthritis. Biol Pharm Bull. 2004; 27: 1406-1413.
- Marks JS. Arthritis meeting the challenge, National center for chronic disease prevention and health promotion, Center for disease control and prevention. 1-4.
- Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE. Oral Anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn- Schmiedeberg Arch Pharmacol. 2004; 369: 294–299.
- Walz DT, Dimartino MJ, Misher A. Adjuvant-induced arthritis in rats. II. Drug effects on physiologic and biochemical and immunologic parameters. J Pharmacol Exp Ther. 1971; 178: 223–231.
- Eric GB, Lawrence JL. Rheumatoid arthritis and its therapy, the textbook of therapeutics drug and disease management, Baltimore: Williams and Wilkins company, 1996; 579–595.
- Ekambaram S, Perumal SS, Subramaniam V. Evaluation of antiarthritic activity of Strychnos potatorum Linn seeds in Freund’s adjuvant induced arthritic rat model. BMC Complementary and Alternative med. 2010: 1-9.
- William JK, Arthritis, Allied Condition. A Textbook of Rheumatology, Baltimore, Tokyo: Waverlay Company, 1996: 1207.
- Patel SS, Shah PV. Evaluation of anti-inflammatory potential of the multidrug herbomineral formulation in male Wistar rats against rheumatoid arthritis. J. Ayurveda Integr Med. 2013; 4:86-93.
- Harris ED. Rheumatoid arthritis. Pathophysiology and implications for therapy. N Eng J Med 1990; 322: 1277-1289.