Case Report
J Stem Cells Res, Rev & Rep. 2014;1(3): 1013.
A New Medical Device, Based on Rigenera Protocol, in the Management of Complex Wounds
Giaccone M1,2, Brunetti M1,2, Camandona M1,2, Trovato L3* and Graziano A3
1Division of General Surgery, A.O.U. Cittàdella Salute e della Scienza, Turin, Italy
2Department of Surgical Sciences, University of Turin, Italy
3Human Brain Wave srl, Italy
*Corresponding author: Trovato L, Human Brain Wave, srl 10128 Turin, Italy
Received: September 22, 2014; Accepted: October 22, 2014; Published: October 24, 2014
Abstract
Injury to the skin provides a unique challenge, as wound healing is a complex and intricate process. Acute wounds have the potential to move from into to chronic wounds, requiring the physician to have a thorough understanding of outside interventions to bring these wounds back into the healing cascade. The development of new and effective interventions in wound care remains an area of intense research. For this purpose, we aimed to report the use of a new medical device, called Rigeneracons® (Human Brain Wave srl, Turin), in the management of complex wounds of different etiology in two subjects where the wound healing process was very difficult. Rigeneracons® devices are based on Rigenera protocol that consists in the use of a cell population enriched of progenitor cells able to improve a tissue repair. By these case reports, we demonstrated the efficacy of Rigenera protocol in improving wound healing process through application of a cell suspension rich of progenitors cells in two different subjects that are donor and acceptor of these micro-grafts.
Keywords: Wound healing; Rigenera protocol; Rigeneracons®
Introduction
A wound may be defined as the interruption of the normal structure and function of the skin and underlying tissues. Currently, are well-established physiological processes implicated in the tissue repair including hemostasis, where the clot represents the basis for repair mechanism, and inflammation, where vessels dilation and monocytes activation lead to digestion of necrotic tissue [1- 2]. This phase can be prolonged by several factors which block the subsequent phases of repair, transforming acute wound into a chronic [3]. If repair process is not hindered, begins proliferative process where Mesenchymal cells have the potential to reconstitute tissues under the skin, while basal epithelial cells grow around the wound [4]. The final step of tissue repair is represented by remodeling process, characterized by a stage of maturation leading to the final appearance of the scar. Several factors can interfere with this process as cardiovascular diseases, diabetes and infections [5]. For this reason, the comprehension of this process is critical to develop more effective medications. As described above, Mesenchymal cells play a key role during proliferative phase occurring in the wound healing [6]. Mesenchymal Stem Cells (MSCs) are multi-potent cells having trophic and support functions, and therapeutic effects in regenerative medicine. In addition are able to release anti-inflammatory cytokines, trophic and anti-apoptotic molecules, and to promote the protection and repair of damaged tissues [7-8]. In addition to MSCs, Stem-like cell subpopulations, referred to as “Side Population” (SP) cells have been identified in several tissues and tumors. After their identification, has been demonstrated that these subpopulations are at least 1000 fold enriched in multipotent stem cells or progenitor cells [9]. From the original discovery in mouse bone marrow, SP cells have been identified in several other organs and tissues including skin, lung, liver, heart, brain, mammary gland and skeletal muscle but they can probably be isolated in all the tissues of the body [10]. For example, has been reported that Dental Pulp Stem Cells (DPSCs) are capable of differentiate into osteoblasts secreting abundant Extracellular Matrix (ECM) and that can build a woven bone in vitro [11-13]. The quality and quantity of regenerated bone formed by DPSCs was demonstrated in in vitro and in vivo experiments using stem cells and biomaterials [14-16]. Thus, dental pulp could be considered as an interesting and potentially important source of autologous stem/ progenitor cells that are ready for use for therapeutic purposes, such as the repair/regeneration of craniofacial bones.
Based on this evidence, we showed that Rigenera protocol is able to successfully isolate these subpopulations by the use of a new medical device called Rigeneracons® (Human Brain Wave, Turin, Italy) [17]. In fact, the goal standard to Rigeneracons® is to disaggregate a small piece of tissue and opportunely select a cell population with a size of 50 micron. These cell populations are suitable to form autologous micro-grafts, which can be used alone or in combination with biomaterials to obtain a biocomplex, ready to be implantable in the subjects needing of such intervention.
In this case report, we aim to describe two clinical cases where Rigenera protocol was successfully used in the management of complex wounds through application of autologous micro-grafts.
Clinical case 1
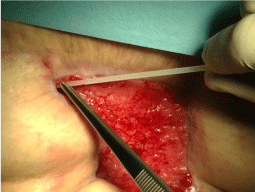
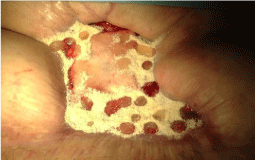
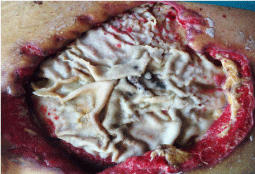
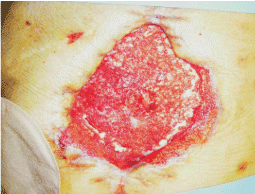
A woman, 50 year old, with no comorbidities, after two years from laparoscopic gastric bypass surgery and with good results in terms of weight loss, has undergone to abdominoplasty bariatric. As complication, was observed necrosis at the ends of the flaps. After 10 days from surgery, the patient was examined at our center and to the first medical examination the necrotic area was between 150 and 200 cm2. The first intervention was necrosectomy in outpatient, evidencing a wide loss of tissue. On this wound, was placed a VAC® therapy that patient has performed during its recovery for one week and then at home. The outpatient controls showed a progressive improvement of the wound with a progressive cleansing and the appearance of granulation tissue on the bottom of the same. However, after 2 months of VAC® therapy, the margins were still undermined, while all area was not in axis with respect to the skin surface (Figure 1). On this wound, we decided to apply the Rigenera protocol, after informed consent by patient. To this purpose, we collected a sample of skin about of 3 cm2 from the patient, in order to obtain the cell suspension that was directly injected in the granulation tissue (Figure 2). Subsequent medications consist in a simple management, based on the cleansing of the wound and the replacement of sterile gauze enriched of Vaseline. Subsequent outpatient visits showed a gradual improvement of the wound with the gradual disappearance of undermined area and a gradual leveling of the wound to the skin surface. After about 2 months, the wound was completely smoothed and reduced to a small size (Figure 3). Even the scar was mild compared to the starting condition.
Figure 1: The wound after two month of VAC® therapy.
Figure 2: Suspension of Mesenchymal cells obtained by Rigeneraprotocol, directly injected in the granulation tissue.
Figure 3: The wound, after about 2 months, was completely smoothed and reduced to a small size.
Clinical case 2
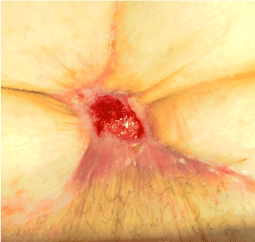
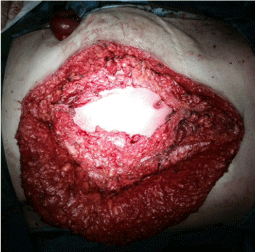
A man, 78 years old, diabetic, affected by liver cirrhosis and hiatal hernia. The patient was subjected to a complex surgery but hiatal hernia was not reduced into the abdomen and was performed distal esophagectomy. During abdomen intervention, the presence of adhesions, correlated to previous abdominal operations, leaded opening of the colon and the necessary resection of a portion of this. The postoperative course was complicated by the appearance of an entero-cutaneous fistulas related to a colonic anastomosis dehiscence. The patient was subjected to a second intervention where was packed ileostomy protection and repackaged the colonic anastomosis. To close the laparotomy, was used a biological prosthesis (Figure 4). The postoperative course was further complicated by the ascetic failure, which required a period of treatment in intensive care hepatology. The production of as cites, poor liver synthesis, the general condition of the patient and local factors have affected on the primary healing of the surgical wound. The skin overlying the biological prosthesis gradually became necrotic, making it essential a necrosectomy, after which the biological prosthesis appeared almost completely exposed (Figure 5).
Figure 4: Closure of laparotomy through a biological prosthesis.
Figure 5: Necrosectomy, after which the biological prosthesis appeared almost completely exposed.
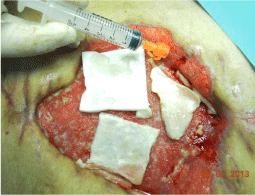
The wound was treated with advanced medications until complete coverage of the biological prosthesis with granulation tissue (Figure 6). The patient was also evaluated by plastic surgeons, however, did not considered appropriate to perform a rotation flap. On the wound, has been used for about 15 days the VAC® therapy. This device, however, not proved to be effective, cause of the inability to maintain good suction, given the presence of the ileostomy. Then, the patient was treated with Rigenera protocol when the dimension of wound was about 250 cm2, right margin near to ileostomy resulted undermined and, despite to be a better tissue granulation, small areas appeared still covered by fibrin. It was performed a skin drawn of about 4 cm2 and after processing with Rigenera system; we obtained about 10 ml of cell suspension, which was injected on wound surface (Figure 7).
Figure 6: Wound treated with advanced medications until complete coverage of the biological prosthesis by the granulation tissue.
Figure 7: Wound treated with Rigeneraprotocol.
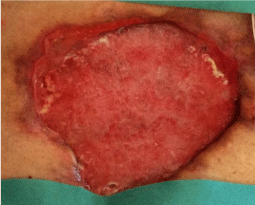
The wound was medicated and renewed every 48 hours. Despite the general and local factors of the patient, including the colonization of the wound by pseudomonas aeruginosa, we observed a progressive improvement in terms of good surface and reduction of size of the wound (Figure 8).
Figure 8: After 1 month, the wound is still wide, but in axis with respect to the skin surface, cleansed and easy to manage with simple dressings.
Discussion & Conclusion
In this report for the first time, we reported preliminary data on the effectiveness of Rigenera protocol in the management of complex wounds. The gold standard to Rigenera protocol is in fact to obtain viable cells that can be used as micro-grafts, in turn injectable in the patients. Moreover, using Rigenera protocol, the donor and acceptor are the same individual, preventing the possible complications linked to implants of no autologous micro-grafts. Rigenera protocol can be used easily and safely during the time of intervention both in operating room and in ambulatory. Furthermore, Rigenera protocol is currently used in the oro-maxillo-facial field as showed by very recent studies [18,19] but it can be potentially applied in others clinical field including plastic surgery, dermatology and orthopedics.
In conclusions, this report clearly demonstrates that Rigenera protocol provides an useful instrument for the management of complex wounds, even if, although our experience has been extremely positive, its efficacy needs to be validate by perspective clinical trials on suitable number of subjects. In front of our results, we trust that this medical device may provide a new therapeutic approach to improve wound healing process on the basis of several positive features, including ethical approach given the use of autologous tissue in the obtaining of cell suspension, facility of procedure, possibility to repeat the procedure for lesions particularly wide, and cheap cost of Rigeneracons® device, thus allowing to reduce the employment of more expansive devices or advanced medications.
In summary, our experience displays a good efficacy of Rigenera protocol on wound healing, opening the way for its employment in the clinical practice or in the management of both acute and chronic wounds but also in other clinical field needing of an instrument able to repair a tissue lesion.
References
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008; 16: 585-601.
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003; 83: 835-870.
- Montfrans CV, Stok M, Geerkens M. Biology of chronic wounds and new treatment strategies. Phlebology. 2014; 29: 165-167.
- Zahorec P, Koller J, Danisovic L, Bohac M. Mesenchymal stem cells for chronic wounds therapy. Cell Tissue Bank. 2014.
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007; 25: 9-18.
- Hu MS, Rennert RC, McArdle A, Chung MT, Walmsley GG, Longaker MT, et al. The Role of Stem Cells During Scarless Skin Wound Healing. Adv Wound Care (New Rochelle). 2014; 3: 304-314.
- Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014; 54: 1418-1437.
- Baghaban Eslaminejad M, Malakooty Poor E. Mesenchymal stem cells as a potent cell source for articular cartilage regeneration. World J Stem Cells. 2014; 6: 344-354.
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996; 183: 1797-1806.
- Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008; 268: 1-9.
- Laino G, d'Aquino R, Graziano A, Lanza V, Carinci F, Naro F, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005; 20: 1394-1402.
- Laino G, Carinci F, Graziano A, d'Aquino R, Lanza V, De Rosa A, et al. In vitro bone production using stem cells derived from human dental pulp. J Craniofac Surg. 2006; 17: 511-515.
- Laino G, Graziano A, d'Aquino R, Pirozzi G, Lanza V, Valiante S, et al. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol. 2006; 206: 693-701.
- d'Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007; 14: 1162-1171.
- d'Aquino R, De Rosa A, Laino G, Caruso F, Guida L, Rullo R, et al. Human dental pulp stem cells: from biology to clinical applications. J Exp Zool B Mol Dev Evol. 2009; 312: 408-415.
- Graziano A, d'Aquino R, Laino G, Proto A, Giuliano MT, Pirozzi G, et al. Human CD34+ stem cells produce bone nodules in vivo. Cell Prolif. 2008; 41: 1-11.
- Zanzottera F, Lavezzari E, Trovato L, Icardi A, Graziano A. Adipose Derived Stem Cells and Growth Factors Applied on Hair Transplantation. Journal of Cosmetics, Dermatological Sciences and Applications. 2014; 4: 268-274.
- Graziano A, Carinci F, Scolaro S, D’Aquino R. Periodontal tissue generation using autologous dental ligament micro-grafts: case report with 6 months follow-up. Annals of Oral & Maxillofacial Surgery. 2013; 1: 20.
- Brunelli G, Motroni A, Graziano A, D'Aquino R, Zollino I, Carinci F. Sinus lift tissue engineering using autologous pulp micro-grafts: A case report of bone density evaluation. J Indian Soc Periodontol. 2013; 17: 644-647.