
Research Article
J Stem Cell Res Transplant. 2016; 3(1): 1021.
Characterization of Cyclosporine Concentrations and Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation
May MB¹*, Hogan KE², Costa LJ³ and Glode AE4
¹Oncology Clinical Pharmacy Specialist, Baptist Health Lexington, USA
²Hematology/Oncology Clinical Pharmacy Specialist, Medical University of South Carolina, USA
³University of Alabama School of Medicine, USA
4Oncology Clinical Pharmacy Specialist, University of Colorado Anschutz Medical Campus, USA
*Corresponding author: Megan Brafford May, Oncology Clinical Pharmacy Specialist, Baptist Health Lexington, USA
Received: March 11, 2016; Accepted: May 18, 2016; Published: May 21, 2016
Abstract
Background: Approximately 20-50% of allogeneic hematopoietic stem cell transplantation (HSCT) patients develop acute graft-versus-host disease (GVHD) post-transplant. A commonly used immunosuppression regimen in reduced-intensity conditioning transplants includes a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil). Cyclosporine is dosed based on avoidance of toxicities and maintaining therapeutic drug concentrations (175 ng/mL – 225 ng/mL measured by highperformance liquid chromatography).
Objective: The primary objective is to assess whether the time and percent within therapeutic range of trough cyclosporine levels within the first 30 days has a correlation to the incidence and severity of acute GVHD.
Methods: This is a single-center retrospective review of patients 18 years of age or older who received their first allogeneic HSCT with reduced-intensity conditioning and cyclosporine as a component of GVHD prophylaxis between January 1, 2008 and July 31, 2012. The comparisons in this study are the average cyclosporine levels within the first 30 days to the development and severity of acute GVHD.
Results: A total of 94 patients were included in the analysis. Overall grade I-IV acute GVHD developed in 75% of patients with 30% of those patients experiencing grade III-IV acute GVHD. Patients with grade III-IV acute GVHD had mean Standard Deviation (SD) cyclosporine concentrations from day 0 to 30 of 187 ± 28 ng/mL, which was lower than 194 ± 25 ng/mL in patients without acute GVHD (p = 0.240).
Conclusion: We did not detect a statistically significant correlation between subtherapeutic cyclosporine exposure within the first 30 days and occurrence of severe acute GVHD, suggesting variation in the cyclosporine levels do not play a major role in the occurrence of severe acute GVHD or the range of the cyclosporine trough concentration is above what is needed to prevent GVHD.
Keywords: Cyclosporine; Graft-versus-host disease; Hematopoietic stem cell transplantation
Background
Approximately 20-50% of allogeneic hematopoietic stem cell transplantation (HSCT) patients develop acute graft-versus-host disease (GVHD) post-transplant. In HSCT, the donor-derived cells of the graft can recognize non-self minor or major histocompatibility antigens on recipient (host) cells and mount an immune response of GVHD. Similarly, the graft may recognize non-self transplantation or tumor-associated antigens on remaining host tumor cells and mount an anti-tumor response called a graft-versus-tumor (GVT) effect. GVT effect is readily demonstrated for some malignancies and contributes significantly to a decreased rate of relapse [1]. Reducedintensity conditioning regimens use significantly lower doses of conditioning treatment which lessens the intensity of the toxicities associated with the preparative regimens [2].
Due to the lack of intensity in bone marrow ablation prior to stem cell infusion, unique immunosuppression strategies are required to prevent GVHD. A commonly used immunosuppression regimen in reduced-intensity conditioning transplants includes a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil). Cyclosporine is dosed based on avoidance of toxicities and maintaining therapeutic drug concentrations (175ng/mL – 225ng/mL measured by high-performance liquid chromatography) [2]. Toxicities associated with cyclosporine include hypertension, tremor, hepatotoxicity, seizures, hemolytic uremic syndrome and nephrotoxicity. Not monitoring the levels of cyclosporine closely could lead to subtherapeutic or supratherapeutic dosing in the patient.
Previous studies have shown that higher concentrations of immunosuppressives were not associated with lower acute or chronic GVHD rates; however, it did protect from higher grades of GVHD [3-5]. The study by Ram et al. concluded that higher cyclosporine concentrations relatively early after reduced-intensity HSCT did confer protection against acute GVHD and reduced the risk of nonrelapse and overall mortality [3].
Methods
This a single-center retrospective review of patients 18 years of age or older who received their first allogeneic HSCT with reducedintensity conditioning between January 1, 2008 and July 31, 2012 at a 704 bed tertiary academic medical center. Patients had to receive a cyclosporine based immunosuppressive regimen to be included. This study was initiated after approval from the Medical University of South Carolina’s (MUSC) Institutional Review Board. Demographic data including age, race, gender, and type of malignancy was collected in addition to the following: cyclosporine concentrations on days 0-30, if acute GVHD developed, organ site where acute GVHD developed, stage of GVHD and overall grade of GVHD, and cumulative prednisone dose for treatment of GVHD.
Specific statistical tests were selected based on the type and distribution of the data being compared. Data analysis was performed using descriptive statistics such as proportions, means, and medians along with Standard Deviations (SD) or interquartile ranges depending on the type and distribution of the data collected. Nominal data was analyzed using the Fishers Exact test. All continuous data was found to be nonparametric and therefore was analyzed using the Mann-Whitney U test. The level of statistical significance for tests was determined to be a p-value< 0.05. Inferential statistics were utilized to test differences between patient groups with the potential for performing multi-variate regression analysis to assess the influence of confounding variables.
Results
A total of 94 patients were included in the analysis (Table 1).
Characteristic
Value (%)
Mean age
51 (21-67 years)
Male gender
58 (61.7)
Caucasian
77 (81.9)
Transplant Type
Matched unrelated donor/mismatched unrelated donor
58 (61)
Matched related donor
36 (39)
Diagnosis
Acute lymphoblastic leukemia (ALL)
12 (12.7)
Acute myeloid leukemia (AML)
50 (53.2)
Aplastic Anemia
1 (1.1)
Chronic lymphocytic leukemia (CLL)
3 (3.2)
Chronic myelogenous leukemia (CML)
9 (9.6)
Myelodysplastic syndrome
13 (13.8)
Multiple myeloma
1 (1.1)
Myelofibrosis
2 (2.1)
Non-Hodgkin’s Lymphoma
3 (3.2)
Table 1: Demographics (n = 94).
Two patients were excluded; 1 patient died before day 30 and 1 patient discontinued cyclosporine before day 30 due to development of thrombotic microangiopathy. Overall grade I-IV acute GVHD developed in 71(75%) patients with grade III-IV acute GVHD documented in 28 (30%) of those patients (Table 2). Except for day 0, the mean cyclosporine levels were within the appropriate drug level range in all patients (175ng/mL – 225ng/mL measured by high-performance liquid chromatography) (Table 3). The mean cyclosporine concentrations within the first 10 days post-transplant were consistently lower in patients that did not develop acute GVHD, although the difference was not statistically significant (Table 4). Patients with grade III-IV acute GVHD had mean concentrations from day 0 to 30 of 187±28 ng/mL, which was lower than 194±25 ng/ mL in patients without GVHD (p = 0.240) (Table 4).
Grade
n (%)
I
16 (17)
II
27 (28.7)
III
20 (21.3)
IV
8 (8.5)
Table 2: Overall Grade of Acute GVHD at Development (n = 71).
Days post-transplant
Mean cyclosporine level (ng/mL) ± SD*
Day 0
132 ± 66
Days 0-10
197 ± 41
Days 0-30
193 ± 27
*Mean cyclosporine level (ng/mL): Measured by liquid chromatography/mass spectrometry.
Table 3: Average Cyclosporine Levels at Specified Time Points.
Days post-transplant
Overall grade III or IV acute GVHD* (n = 28)
No acute GVHD*
(n = 66)
p value
Day 0
141 ± 88
127 ± 57
0.429
Days 0-10
197 ± 47
196 ± 38
0.851
Days 0-30
187 ± 28
194 ± 25
0.240
*Mean cyclosporine level (ng/mL): Measured by liquid chromatography/mass spectrometry.
Table 4: Average Cyclosporine Levels in Patients With and Without Acute GVHD Development.
Multi-variant analysis of race, gender, donor type, age, diagnosis, and average cyclosporine concentrations for days 0 to 30 were not associated with development of grade III or IV acute GVHD. Age was the only factor associated with increased development of acute GVHD (p = 0.029). The number of days of sub therapeutic cyclosporine concentrations (< 175ng/mL) within the first 30 days was not correlated with development of acute GVHD (p = 0.421) (Figure 1). Mean days of subtherapeutic cyclosporine levels within the first 10 days was 4±3 days and 12±6 days within the first 30 days. Median overall survival for all patients was 428 ±200 days (Figure 2). Median overall survival for patients with no acute GVHD (737±270 days) was longer in comparison to patients with any grade acute GVHD development (191±109 days) (p = 0.164).
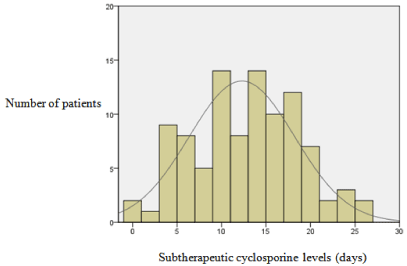
Figure 1: Days of subtherapeutic cyclosporine levels.
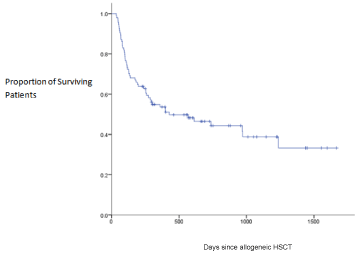
Figure 2: Survival Curve.
Discussion
This study demonstrated that 75% of patients developed acute GVHD at MUSC who received a cyclosporine containing immunosuppressive regimen for a reduced-intensity allogeneic HSCT, which is above the average range reported in the literature. This study evaluated both intrapatient and interpatient variability as it relates to cyclosporine concentrations. Intrapatient variability was determined by comparing cyclosporine levels within the first 30 days and interpatient variability was determined by calculating the SD of the average cyclosporine levels across all patients.
The results suggest that cyclosporine concentrations are similar in allogeneic HSCT patients whether they develop acute GVHD or not; therefore, sub therapeutic cyclosporine concentrations appear to not be associated with the development of acute GVHD. One explanation for the results that low cyclosporine levels did not affect acute GVHD could be because the effect was over shadowed by the higher use of corticosteroids in this population.
Limitations
There were some limitations to this study. First, the data in regards to the day acute GVHD developed and the stage of acute GVHD were collected retrospectively and were reliant upon appropriate documentation. The staging of GVHD was also a limitation as this was completed by differing providers also reliant upon retrospective review of documentation. There was not a consistent time point for measuring cyclosporine concentration or standard method for cyclosporine dosage adjustments. This study also did not take into consideration concomitant medications used for prevention of acute GVHD.
This study did not identify factors that contribute to the development of acute GVHD due to the small sample size compared to the Ram et al. study which included 1,181 patients. The goal of this study was to improve the quality and safety of the immunosuppression regimen protocol which has the potential to benefit many patients and the organization. This study did generate hypotheses for future studies and areas for clinical improvement.
Conclusion
Cyclosporine concentrations were not associated with the development of acute GVHD in this study. Risk factors were also not identified. Institutions should consider reviewing use of this agent for the prevention of acute GVHD after an allogeneic HSCT due to the high incidence of GVHD seen in our study.
References
- Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. ASBMT. 2012; 18: 1150-1163.
- Solomon SR, Nakamura R, Read EJ, Leitman SF, Carter C, Childs R, et al. Cyclosporine is required to prevent severe acute GVHD following T-celldepleted peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003; 31: 783-788.
- Ram R, Storer B, Mielcarek M, Sandmaier BM, Maloney DG, Martin PJ, et al. Association between calcineurin inhibitor blood concentrations and outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012; 18: 414-422.
- Ghalie R, Fitzsimmons WE, Weinstein A, Manson S, Kaizer H. Cyclosporine monitoring improves graft-versus-host disease prophylaxis after bone marrow transplantation. Ann Pharmacother. 1994; 28: 379-383.
- Yee GC, Self SG, McGuire TR, Carlin J, Sanders JE, Deeg HJ. Serum cyclosporine concentration and risk of acute graft-versus-host disease after allogeneic marrow transplantation. N Engl J Med. 1988; 319: 65-70.