
Mini Review
Austin Therapeutics. 2015;2(1): 1013.
How to Deliver Therapeutics or Imaging Agents to the Infracted Heart?
Minghui Li 1†, Guangtian Wang11†, Haichun Li1 and Haisheng Peng1,2*
1Department of Pharmaceutics, Daqing Campus, Harbin Medical University, China
2Department of Chemical and Biological Engineering, Iowa State University, USA †These authors contributed equally to this work
*Corresponding author: Haisheng Peng, Department of Pharmaceutics, Daqing Campus of Harbin Medical University, 1 Xinyang Rd Daqing, 163319, China
Received: September 17, 2014; Accepted: February 13, 2015; Published: February 16, 2015
Abstract
Heart, as a pump, provides the motive power and drives the blood containing nutrients and cytokines to the whole body. There are three challenges for the heart-targeting drug delivery. Firstly, the ratio of heart mass to the body is about 0.5 % while the infarcted foci should be smaller than the whole heart. Secondly, heart always moves to push blood ahead and keep rapidly rinsing the tissue. The last but the most important, lack of specific valuable markers always stresses the enthusiasm of researchers and impedes the progressive of heart-targeting drug delivery. In this review, we summarize several antibodies that have been used in the heart targeted drug delivery system such as anti-myosin antibody, anti-cTnI antibody, anti-P-selectin antibody, and anti-collagen homing peptide antibody. We also summarize several peptides that can direct the drug to the infarcted myocardium such as heart-homing peptide with a sequence of CRPPR and transactivating transcriptional activator peptide (TATp, 11 amino acids) from HIV-1. We hope that such a review will help researchers who are exploring heart targeted drug delivery systems.
Keywords: Heart-targeting nanoparticles; Ischemic heart disease; miRNA;Apoptosis; in vivo imaging
Introduction
Ischemic Heart Disease (IHD) is the most common type of heart disease. The disease with high morbidity and mortality endangers the life quality of people and it has become a severe social issue threatening the public health. Many data have confirmed that local injection of some drugs can treat the condition [1]. However, the limitation of administration route is a major challenge in the clinic application. Heart-targeted drug delivery system not only can relieve the symptoms of the IHD, but decrease the reverse effects [2,3]. The screening and discovery of pathophysiologic marker in the affected cardiac muscle will be the most important task for the design of active heart targeting drug delivery system. The markers will be employed to direct the nanocarriers to the affected tissue and apoptotic cells, even the organelle such as mitochondria. When cardiomyocytes undergo the hypoxemia or experimental ischemic occlusion, the apoptosis or necrosis of the cells occurs [4]. In the tissue level, specific protein or peptide that leaks out of the cardiomyocytes can be used to develop drug delivery systems and improve the accumulation of nanoscale carriers in the ischemic region [5]. Normally, all intracellular macromolecules always stay in the myocardial cells. Only when the intact cell membrane disrupts, will the components, such as myosin, traverse the cell membrane. Khaw and Torchilin designed antimyosin antibody modified liposomes that coated with or without Polyethylene Glycol (PEG) and studied the targeted accumulation in ischemic myocardium [5,6]. Torchilin et al. found that the antimyosin antibody can direct the carrier to the infarcted myocardium. Additionally, PEG can enhance the accumulation of liposomes in the foci due to the prolonged circulation time, Enhanced Permeability and Retention (EPR) effect [6]. No matter what kinds of liposomes people used, they just delivered the drug to the extracellular matrix around the affected cells, but not into these cells. The Transactivating Transcriptional activator peptide (TATp, 11 amino acids) from HIV- 1 can efficiently introduce large macromolecular molecules, such as DNA and proteins, into mammalian cells in vitro and in vivo. They used both TATp and anti-myosin antibody to modify on the surface of the low cationic liposomes–plasmid DNA complexes (lipoplexes) to realize the gene therapy for the myocardial infarction [7].
In the myocardiocyte, the counterpart of myosin that regulates the movement of cardiac muscle is cardiac Troponin (cTn) including three domains cTnT, cTnI, and cTnC [8]. Yang et al. have confirmed that microRNA-1 (miR-1) is a potential anti-arrhythmic target which can be down-regulated by the Antisense Oligonucleotides (AMO- 1) to relieve arrhythmia [4]. Based on this, our group developed anti-cTnI antibody modified liposomes that loaded with AMO-1 to treat the rat arrhythmia from ligation of left anterior descending branch of coronary artery. We found that the immunoliposomes accumulated in the ischemic region and penumbra where the cTnI leaked out of the necrotic cells and had an elevated expression by immunohistochemistry staining. The affected cells internalized the immunoliposomes, then the released AMO-1 repressed the function of miR-1 and relieved the arrhythmia. Interestingly, the carriers just stayed in the plasma where matured miR-1 should exist due to the interaction of cTnI with its antibody. In the literature, ischemia apoptosis of cells will happen 4h after ligation. In our studies, the liposomes reached the ischemic region 5min after intravenous injection by in vivo imaging. The data have confirmed that our carriers are a potential drug delivery system for the IHD [9] (Schematic 1).
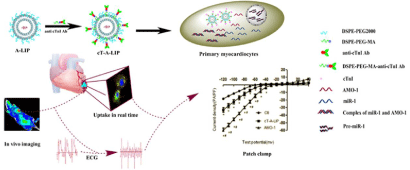
Schematic 1 Overexpression of cardiac troponin I (cTnI) is an obvious pathological change in ischemic cardiac tissues, which can be used to design cardiactargeting
liposomes. Down-regulation of miR-1 could relieve arrhythmogenesis by the anti-miR-1 antisense oligonucleotides (AMO-1). Anti cTnI antibody modified
liposomes can enhance delivery of the AMO-1 to the ischemic cardiac tissues and relieve the arrhythmias in rats [9].
Professor Thomas published one article and reported regulation of miR-21 in heart disease on Nature [10] Small RNA has become the hottest field in the therapy of heart diseases. Angiogenesis in
the ischemic region will be initiated three days after myocardium infarction. Hwang et al. confirmed that Vascular Endothelial Growth Factor (VEGF) peptides promoted angiogenesis in ischemic myocardium [11]. After the treatment of cytokine and antioxidants, those cells that destined to die within the area of myocardial ischemiareperfusion may survive. Based on the data of increased expression of endothelial cell adhesion molecules in the Myocardial Infarction (MI) region, Scott et al. encapsulated Vascular Endothelial Growth Factor (VEGF) into the anti-P-selectin-conjugated liposomes. They confirmed that the immunoliposome changed cardiac function and vasculature post-MI in rat [12].
The peptide with a sequence of CRPPR is a heart-homing peptide by in vivo phage display and Cysteine-Rich Protein-2 (CRIP-2) was proposed as the receptor. The CRPPR-modified liposomes cross the intact murine cardiac endothelium to deliver the drug to the affected tissue [13]. The S. typhimurium defective in the synthesis of ppGpp (ΔppGpp S. typhimurium) can selectively accumulate in the infarcted myocardium without the off-target effects. Uyenchi et al. utilized the defective bacteria, as a vector, to deliver gene/protein to the infarcted myocardium, which didn’t induce the serious local or systemic immune reactions [14].
3-{4-[2-hydroxyl-(1-methyl ethylamine) propyl oxygen] phenyl} Propionic Acid Cetylester (PAC), selective ligand of β1- adrenoreceptor, has been used to navigate the internalization of liposomes by cells under normoxia or hypoxia conditions. Chen et al. found that the uptake of modified liposomes by cells suffered from hypoxemia was more than 8-fold than that of common vesicles. Lisinopril is in a group of drugs called ACE inhibitors [15]. Ghann et al. synthesized the lisinopril-conjugated gold nanoparticles and observed the feasibility of diagnosis for cardiovascular diseases by CT. The image highlighted the tissue such as heart, and lung with upregulation of ACE [16].
People hope to deliver the drug to the affected tissue immediately once their heart suffered from ischemic conditions. Unfortunately, some time the patients are not conscious of their heart under the hypoxemic condition. Therefore, they lose the best time to treat the detrimental changes. At the same time, the long-term hypoxemia induces collagen formation in the ischemic tissue. The collagenhoming peptide, Collagen Adhesin (CNA35), becomes a candidate for drug delivery system targeting to the infarcted myocardium. Danila et al. synthesized collagen-targeting gold nanoparticles, which could highly distribute in the myocardial scar. The high accumulation of the metal nanoparticles enhanced the contrast for CT imaging [17].
Microbubble is another drug delivery system for therapy and diagnosis. Yan et al. prepared anti-Matrix Metalloproteinase 2 (MMP2) antibody modified Cationic Microbubble (CMB) to deliver Timp3 plasmid to the ischemic myocardium in rat. Three days after administration, the animals intravenously injected microbubbles had significant increase of TIMP3 protein levels in the infarct scar and peri-infarct area [18]. The initiation of myocardiocyte apoptosis plays a vital role in the progressive weakening of the peri-infarct zone myocardium after MI. In this phase, cells that underwent sublethal injury may survive if they received right treatment. Phosphatidylserine is normally localized to the inner surface of the cellular membrane. However, when sublethal cell injury occurs, the phospholipids will translocate to the external layer of the cell membrane. Annexin V in the matrix could conjugate with phosphatidylserine in the existence of calcium ion. Lam et al. found that the Superparamagnetic Iron Oxide (SPIO) nanoparticles modified with annexin V could detect the early apoptotic cell populations. They doubted whether the treated cells could internalize the nanoparticles through Annexin V binding. A61603 (A6), a1adrenergic receptor agonist, could rescue the affected cells from apoptosis through extracellular signal-regulated kinase (ERK) pathway. The data further confirmed that Annexin V conjugated SPIO highly bound on the surface of rescued cells and enhanced the contrast of image after A6 treatment [19]. This study reminds us that Annexin V can be used to design the ischemic heart targeted nanoparticles.
In conclusion, the cardioprotective drug should go to the right place at the right time. Who, however, can assist them to the place where they are needed in deed? The biological and biochemical changes which occur after myocardium infarction should be well known before we can go.
Acknowledgement
This study was supported by grants from the National Natural Science Foundation of China (No. 81402865), and Scientific Research Fund of Heilongjiang Provincial Education Department (No. 12541348 and No. 12511322).
References
- Thum T. MicroRNA therapeutics in cardiovascular medicine. EMBO molecular medicine 2012; 4: 3-14.
- Jain KK. Applications of biotechnology in cardiovascular therapeutics. Springer. 2011.
- Liu M, Li M, Wang G, Liu X, Liu D, Peng H, et al. Heart-targeted nanoscale drug delivery systems. Journal of Biomedical Nanotechnology. 2014; 10: 2038-2062.
- Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nature medicine. 2007; 13: 486-491.
- Khaw B, Mattis JA, Melincoff G, Strauss HW, Gold, HK, Haber E, et al. Monoclonal antibody to cardiac myosin: imaging of experimental myocardial infarction. Hybridoma. 1984; 3: 11-23.
- Torchilin V, Klibanov A, Huang A, Donnell S, Nossiff N, Khaw B. Targeted accumulation of polyethylene glycol-coated immunoliposomes in infarcted rabbit myocardium. The FASEB journal. 1992; 6: 2716-2719.
- Ko Y, Hartne W, Kale A, Torchilin V. Gene delivery into ischemic myocardium by double-targeted lipoplexes with anti-myosin antibody and TAT peptide. Gene therapy. 2008; 16: 52-59.
- Dong WJ, Robinson JM, Xing J, Cheung HC, Umeda PK. An interdomain distance in cardiac troponin C determined by fluorescence spectroscopy. Protein Science. 2000; 9: 280-289.
- Liu M, Li M, Sun S, Li B, Du D, Sun J, et al. The use of antibody modified liposomes loaded with AMO-1 to deliver oligonucleotides to ischemic myocardium for arrhythmia therapy. Biomaterials. 2014; 35: 3697-3707.
- Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008; 456: 980-984.
- Hwang H, Kwon J, Oh PS, Lee TK, Na KS, Lee CM, et al. Peptide-loaded Nanoparticles and Radionuclide Imaging for Individualized Treatment of Myocardial Ischemia. Radiology. 2014; 273: 160-167
- Scott RC, Crabbe D, Krynska B, Ansari R, Kiani, MF. Aiming for the heart: targeted delivery of drugs to diseased cardiac tissue. 2008; 5: 459-470
- Zhang H, Li N, Sirish P, Mahakian L, Ingham E, CurryFR, et al. The cargo of CRPPR-conjugated liposomes crosses the intact murine cardiac endothelium. Journal of Controlled Release. 2012; 163: 10-17.
- Le UN, Kim HS, Kwon JS, Kim MY, Nguyen VH, Jiang, SN, et al. Engineering and visualization of bacteria for targeting infarcted myocardium. Molecular Therapy. 2011; 19: 951-959.
- Chen Y, Deng Y, Hao Y. Surface modification of liposomes for cardiomyocytes targeting in vitro. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2005; 60: 238-240.
- Ghann WE, Aras O, Fleiter T, Daniel MC. Syntheses and characterization of lisinopril-coated gold nanoparticles as highly stable targeted CT contrast agents in cardiovascular diseases. Langmuir. 2012; 28: 10398-10408.
- Danila D, Johnson E, Kee P. CT imaging of myocardial scars with collagen-targeting gold nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine. 2013; 9: 1067-1076.
- Yan P, Chen KJ, Wu J, Sun L, Sung HW, Weisel RD, et al. The use of MMP2 antibody-conjugated cationic microbubble to target the ischemic myocardium, enhance Timp3 gene transfection and improve cardiac function. Biomaterials. 2014; 35: 1063-1073.
- Lam J, Gong Y, Robbins RC, Simpson PC, Yang PC, Dash, R. Restorative effects of alpha-1A adrenergic are detectable using T2* and targeted nanoparticles in a mouse myocardial infarction (MI) model. Journal of Cardiovascular Magnetic Resonance. 2013; 15: P177.