
Mini Review
Austin Tuberc Res Treat. 2017; 2(1): 1007.
Natural and Synthetic Quinoline Derivatives as Antituberculosis Agents
Casal JJ1,2 and Asís SE2*
¹CONICET-Center of Investigations in Bionanciencias, Polo Scientific Technological, Argentina
²Department of Organic Chemistry, University of Buenos Aires, Argentina
*Corresponding author: Assis SE, Department of Organic Chemistry, Faculty of Pharmacy and Biochemistry, University of Buenos Aires. Junín 956. C1113AAD Autonomous City of Buenos Aires, Argentina
Received: July 20, 2017; Accepted: September 26, 2017; Published: October 03, 2017
Abstract
Tuberculosis (TB) is a contagious and an often severe airborne disease caused by Mycobacterium tuberculosis (Mtb). TB typically affects the lungs and is usually treated with a regimen of drugs taken for 6 months to 2 years, depending on the type of infection. There were an estimated 1.4 million TB deaths in 2015, and an additional 0.4 million deaths resulting from TB disease among people living with HIV. Although the number of TB deaths fell by 22% between 2000 and 2015, TB remained one of the top 10 causes of death worldwide in 2015.
Quinoline nucleus occurs in several natural compounds and synthetic derivatives displaying a broad range of biological activity including antituberculosis. Many quinoline derivatives isolated from natural products were reported to exhibit moderate antitubercular activity. The quinoline ring was shown to confer anti-TB activity and confirms that quinoline-based scaffolds are promising leads for new TB drug developments. Many quinolines recognized as antimalarial agents showed activity as anti-TB drugs. Bedaquiline, a clinically important anti-TB drug, is the inspiration and model for designing novel antitubercular structures.
Keywords: Quinolines; Synthesis; Tuberculosis; Mycobacterium tuberculosis
Abbreviations
TB: Tuberculosis; Mtb: Mycobacterium tuberculosis; MDRTB: Multi Drug-Resistant TB; CQ: Chloroquine; MIC: Minimal Inhibitory Concentration; FQ: Fluoroquinolones; FQ: Ferroquine; DARQ: Diarylquinoline
Introduction
Tuberculosis (TB) is a contagious and an often severe airborne disease caused by Mycobacterium tuberculosis (Mtb). TB typically affects the lungs and is usually treated with a regimen of drugs taken for 6 months to 2 years, depending on the type of infection.
In 2015, there were an estimated 480,000 new cases of Multidrug- Resistant TB (MDR-TB) and an additional 100,000 people with Rifampicin-Resistant TB (RR-TB) who were also newly eligible for MDR-TB treatment. There were an estimated 1.4 million TB deaths in 2015, and an additional 0.4 million deaths resulting from TB disease among people living with HIV. Although the number of TB deaths fell by 22% between 2000 and 2015, TB remained one of the top 10 causes of death worldwide in 2015 [1].
Quinoline nucleus occurs in several natural compounds and synthetic derivatives displaying a broad range of biological activity, such as antimalarial, anti-bacterial, antifungal, antihelmintic, cardiotonic, anticonvulsant, anti-inflammatory, and analgesic activity [2]. Quinine, as a component of the bark of the cinchona (quinaquina) tree, was used to treat malaria from as early as the 1600s in South America and was first isolated in 1820 in Europe [3]. The systematic modification of this alkaloid, which is a 4,6-substituted quinoline, led to diverse quinoline antimalarial drugs. The first synthesized one was the potent and inexpensive chloroquine (CQ), a 7-chloroquinoline with an amino substituent at position 4 [4].
The quinoline alkaloids 4-methoxy-2-phenylquinoline (1), graveolinine (2), and kokusagine (3), isolated from lunasiaamara, displayed significant activity towards M. tuberculosis H37Rv with MICs of 16 μg/mL. The known quinoline alkaloids dictamnine (4) and ?-fagarine (5), isolated from roots of Zanthoxylumwutaiense, were reported to exhibit moderate antitubercularactivity (H37Rv strain) with MICs of 30 μg/mL (Figure 1) [5].
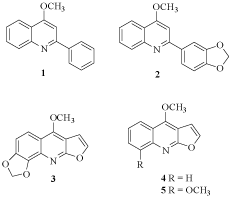
Figure 1: Quinoline alkaloids isolated from Lunasiaamara and
Zanthoxylumwutaiens.
Synthetic Quinoline Derivatives
In 2004, Jain et al. reported a series of substituted quinolines with high anti-TB activity. Many of these compounds were initially synthesized as the precursors for targeted antimalarials. The most effective compound, 2,8-dicyclopentyl-4-methylquinoline exhibited activity against both drug-sensitive and drug-resistant M. tuberculosis. To optimize previously identified lead 4-(adamantan- 1-yl)-2-quinolinecarbohydrazide, two new series of 2-substituted quinolines containing 4-(adamantan-1-yl) group were synthesized. Four analogs of the 4-adamantan-1-yl-quinoline-2-carboxylic acid N´-alkylhydrazides exhibited promising anti-TB activity (99% inhibition) at 3.125 μg/mL. The most potent compound 4-adamantan- 1-yl-quinoline-2-carboxylic acid (2-chlorobenzylidene) hydrazide inhibited drug-sensitive M. tuberculosis H37Rv at 1.00 μg/mL (99% inhibition) and was equipotent to standard drug isoniazid. [6a,b].
On the other hand, synthetic fluoroquinolones (FQ) possessing broad-spectrum anti-mycobacterial activity as ciprofloxacin (6) and ofloxacin (7) were used as part of multi drug regimens to cure patients infected with M. tuberculosis and M. avium. Structural modifications of FQ produced candidates as moxifloxacin (8) and analogs that are more efficacious than earlier FQ (Figure 2). Their bactericidal effects involve an interaction of the drugs with DNA-gyrase and DNAtopoisomerase IV [7-10].
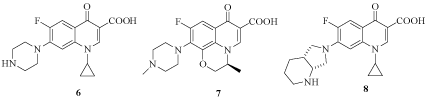
Figure 2: Synthetic fluoroquinolone derivatives.
The quinoline ring was shown to confer anti-TB activity and confirms that quinoline-based scaffolds are promising leads for new TB drug developments [11]. Furthermore, ferrocene core is an attractive pharmacophore for drug design in the area of medicinal organometallic chemistry because of its unique properties such as stability, aromaticity, low toxicity and redox activity. In 2011 was reported the synthesis and antitubercular activity of a new series of derivatives where the ferrocene core is connected to heterocyclic moieties via a hydrazone linker. The quinoline-ferrocene hybrid 9 showed potent activity in a concentration range comparable to the reference drug ethambutol. Based on these findings, was evaluated the antimycobacterial activity of Ferroquine (FQ, SSR97193, 10), a recognized antimalarial drug candidate because of its structural similarity to hybrid compound 9 (Figure 3). FQ also exhibited potent activity against M. tuberculosis, albeit its effect was more modest [12].
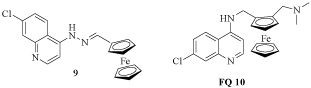
Figure 3: Quinoline-ferrocene hybrid 9 and Ferroquine (FQ).
Bihalogenated 8-hydroxyquinolines (quinolin-8-ols) are a group of known drugs with antimicrobial activities. The commonly used ones include broxyquinoline, clioquinol, chlorquinaldol, and iodoquinol. The anti-tuberculosis activities of a monohalogenated 8-hydroxyquinoline, cloxyquin (5-chloroquinolin-8-ol), against 150 clinical M. tuberculosis isolates, including multidrug-resistant strains, were reported in 2007 [13]. Clioquinol (5-chloro-7-iodo-8- hydoxyquinoline) was employed to prepare new Cu(II) complexes with anti-tuberculosis activity [14]. In recent years, drug repositioning has gained considerable attention in drug discovery and development [15].
Our research group reported the Friedländer synthesis of twelve new acridine derivatives and fused quinolines inspired in natural occurring antitubercular alkaloids and three terms exhibited inhibition activity against M. tuberculosis (Mtb) H37Rv. The cyclopenta[b]quinoline derivative 11 and the acridine derivative 12 had remarkable MIC values against the rifampin resistant strain but only compound 11 exhibited bactericidal activity at 50 μg/mL (Figure 4) and its intracellular activity is similar to rifampin and it was not cytotoxic at low concentrations [16]. Moreover, a new series of polycyclic quinolines and acridine analogs have been synthesized and are under evaluation against M. tuberculosis (Mtb) H37Rv.
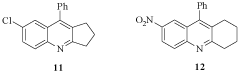
Figure 4: Cyclopenta[b]quinoline derivative 11 and the acridine derivative 12.
Bedaquiline, previously known as TMC207 (13), was developed by Johnson & Johnson pharmaceutical company. This Diarylquinoline (DARQ) was approved in 2012 by the United States FDA for the treatment of MDR-TB in adults. It acts by a novel mechanism by targeting proton pump of Adenosine Triphosphate (ATP) synthesis, leading to inadequate synthesis of ATP [17,18].
A quinoline moiety is its essential pharmacophoric feature and the bedaquiline performance inspired several authors for the design and synthesis of new drugs, as shown in (Figure 5). Several compounds showed MICs with low values. The most potent compounds 14 and 15 exhibited MICs of 0.2 and 0.39 μg/mL, respectively [19].
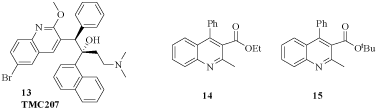
Figure 5: Bedaquiline and other active quinoline derivatives.
The design concept of quinoline derivatives and the introduction of the ethambutol pharmacophore, a first line TB drug, in the expectation to improve its activity, possible by the incorporation of a different mode of action through a different target, led to the synthesis of substituted 4-amino-7-chloroquinolines which were active against M. tuberculosis [20]. At the same time, Chattopadhyaya et al. have divided DARQ molecule into four hemispheres and have synthesized new series of compounds based on North-East (NE) and South-East (SE) hemispheres and several 2,3,6-trisubstituted quinolines were found to be active. Docking calculations and analysis have shed light on their knowledge of pharmacophoric contribution from different hemispheres of DARQ and their importance [21,22].
More recently, taking in account 2-(quinolin-4-yloxy)acetamides described as potent in vitro inhibitors of Mycobacterium tuberculosis growth, new chemical modifications were carried out, yielding highly potent antitubercular agents with MIC values as low as 0.05 μM. In addition, the synthesized compounds 16 (Figure 6) showed potent and selective activity against drug-sensitive and drug-resistant Mtb strains as well as activity in a macrophage-infected model [23].
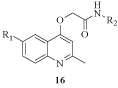
Figure 6: General structure of 2-(quinolin-4-yloxy)acetamides.
Conclusions
Natural occurring quinoline derivatives possess antitubercular activity. Several antimicrobial quinolones and antimalarial quinolines also demonstrated activity against M. tuberculosis. Bedaquiline, a diarylquinoline under clinical studies acts by a novel mechanism of action. Many authors inspired in bedaquiline have been preparing new series of quinoline derivatives which could exert their activity at the same target or not. The replacement of substituents or other functional groups attached at different regions of the DARQ led to promising candidates for the development of novel drugs for TB treatment. These results also encourage our research group to continue the developing of new quinoline derivatives as antituberculosis agents.
References
- World Health Organization. 2017.
- Marella A, Tanwar OP, Saha R, Ali MR, Srivastava S, Akhter M, et al. Quinoline: A versatile heterocyclic. Saudi Pharmaceutical Journal. 2013; 21: 1-12.
- Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malaria Journal. 2011; 10: 144.
- Kumar A, Paliwal D, Saini D, Thakur A, Aggarwal S, Kaushik D. A comprehensive review on synthetic approach for antimalarial agents. Eur J Med Chem. 2014; 147-178.
- García A, Bocanegra-García V, Palma-Nicolás JP, Rivera G. Recent advances in antitubercular natural products. Eur J Med Chem. 2012; 49: 1-23.
- a) Vangapandu S, Jain M, Jain R, Kaur S, Singh P. Ring-substituted quinolines as potential anti-tuberculosis agents. Bioorg Med Chem. 2004; 12: 2501-2508. b) Nayyar A, Monga V, Malde A. Coutinho E, Jain R. Synthesis, anti-tuberculosis activity, and 3D-QSAR study of 4-(adamantan-1-yl)-2- substituted quinolones. Bioorg Med Chem. 2007; 15: 626-640.
- Tripathi RP, Tewari N, Dwivedi N, Tiwari VK. Fighting tuberculosis: an old disease with new challenges. Medicinal Research Reviews. 2005; 25: 93- 131.
- Neu HC. Clinical use of the quinolones. The Lancet. 1987; 1319-1322.
- Hans H, Gadebusch HH, Shungu DL. Norfloxacin, the first of a new class of fluoroquinolone antimicrobials, revisited. International Journal of Antimicrobial Agents. 1991; 1: 3-28.
- Dinakaran M, Senthilkumar P, Yogeeswari P, China A, Nagaraja V, Sriram D. Novel ofloxacin derivatives: Synthesis, antimycobacterial and toxicological evaluation. Bioorg Med Chem Lett. 2008; 18: 1229-1236.
- Candéa ALP, Ferreira MDL, Pais KC, Cardoso LN, Kaiser CR, Henriques MDG, et al. Synthesis and antitubercular activity of 7-chloro-4-quinolinylhydrazones derivatives. Bioorg Med Chem Lett. 2009; 19: 6272-6274.
- Mahajan A, Kremer L, Louw S, Guéradel Y, Chibale K, Biot C. Synthesis and in vitro antitubercular activity of ferrocene-based hydrazones. Bioorg Med Chem Lett. 2011; 21: 2866-2868.
- Hongmanee P, Rukseree K, Buabut B, Somsri B, Palittapongarnpim P. In vitro activities of Cloxyquin (5-Chloroquinolin-8-ol) against Mycobacterium tuberculosis. Antimicrobial Agents and Chem. 2007; 51: 1105-1106.
- Patel KS, Patel JC, Dholariya HR, Patel KD. Multiple heating rate kinetic parameters, thermal, X-ray diffraction studies of newly synthesized octahedral copper complexes based on bromo-coumarins along with their antioxidant, anti-tubercular and antimicrobial activity evaluation. Spectrochimica Acta Part A Mol Biomol Spectrosc. 2012; 96: 468-479.
- Cherdtrakulkiat R, Boonpangrak S, Sinthupoom N, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. Derivatives (halogen, nitroandamino) of 8-hydroxyquinoline with highly potent antimicrobial and antioxidant activities. Biochemistry and Biophysics Reports. 2016; 6: 135-141.
- Muscia GC, Buldain GY, Asís SE. Design, synthesis and evaluation of acridine and fused-quinoline derivatives as potential anti-tuberculosis agents. Eur J Med Chem. 2014; 73: 243-249.
- Andries K, Verhasselt P, Guillemont J, Göhlmann HWH, Neefs JM, Winkler H, et al. A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium tuberculosis. Science. 2005; 307: 223-227.
- Rustomjee R, Diacon AH, Allen J, Venter A, Reddy C, Patientia RF, et al. Early Bactericidal Activity and Pharmacokinetics of the Diarylquinoline TMC207 in Treatment of Pulmonary Tuberculosis. Antimicrob Agents Chemother. 2008; 52: 2831-2835.
- Tanwar B, Kumar A, Yogeeswari P, Sriram D, Chakraborti K. Design, development of new synthetic methodology, and biological evaluation of substituted quinolines as new anti-tubercular leads. Bioorg Med Chem Lett. 2016; 26: 5960-5966.
- de Souza MVN, Pais KC, Kaiser CR, Peralta MA, Ferreira M, Lourenço MCS. Synthesis and in vitro antitubercular activity of a series of quinoline derivatives. Bioorg Med Chem. 2009; 17: 1474-1480.
- Upadhayaya RS, Vandavasi JK, Vasireddy NR, Sharma VV, Dixit SS, Chattopadhyaya J. Design, synthesis, biological evaluation and molecular modelling studies of novel quinoline derivatives against Mycobacterium tuberculosis. Bioorg Med Chem. 2009; 17: 2830-2841.
- Upadhayaya RS, Kulkarni GM, Vasireddy NR, Vandavasi JK, Dixit SS, Sharma V, et al. Design, synthesis and biological evaluation of novel triazole, urea and thiourea derivatives of quinoline against Mycobacterium tuberculosis. Bioorg Med Chem. 2009; 17: 4681-4692.
- Pissinate K, Villela AD, Rodrigues-Junior V, Giacobbo BC, Grams ES, Abbadi BL, et al. 2-(Quinolin-4-yloxy)acetamides are active against drug-susceptible and drug-resistant Mycobacterium tuberculosis Strains. ACS Med Chem Lett. 2016; 7: 235-239.