Review Article
Austin J Vasc Med. 2014;1(1): 3.
Angiogenesis and Pseudo-Vasculogenesisin Human Placenta: Early Trophoblast Invasion and Spiral Artery Remodelling
Sivasubramaniam SD*
School of Science and Technology, Nottingham Trent University, UK
*Corresponding author: Sivasubramaniam SD, School of Science and Technology, Placental Research Group, Nottingham Trent University, Clifton Lane, Nottingham NG11 8NS, UK
Received: October 02, 2014; Accepted: October 30, 2014; Published: November 03, 2014
Abstract
Placental trophoblasts have a heterogeneous group of immature cell populations including stem cells. These cells undergo a series of molecular events such as proliferation, migration, and metastasis to produce blood vessels. The cytotrophoblast cells undergo two different but inter-connected pathways to establish a functional placenta in humans. The first one, the invasive pathway, leads tospiral artery remodelling (psudo-vaculogenesis) and thereby increases the placental blood flow; and the other, non-invasive pathway, produce villous circulation (angiogenesis). Both processes are tightly controlled by several internal and external factors; many of which, the functions and the control mechanisms are yet to be elucidated. Although these steps resemble the events in tumorigenesis, unlike tumour, the trophoblast cells proliferate and then invade. Therefore their tumour-like behaviour is kept at check. Since placentation resembles tumour invasion, understanding the factors/mechanisms that control trophoblast invasion may help us to identify target molecules for cancer chemotherapy.
Keywords: Cytotrophoblast; Syncytiotrophoblast; Invasion; Pseudo-vasculogenesis
Introduction
The formation of new blood vessels is brought about by two main processes; (a) Vasculogenesis-de novo synthesis of blood vessels and (b) angiogenesis-creation of new vessels from already existing vessels. Interestingly the normal development of human placenta is dependent on both processes. Placental trophoblast cells have a heterogeneous group of immature cell populations including stem cells (trophoblast stem cells TSC) and progenitor cells [1]. These cells undergo a series of molecular events such as proliferation, migration, and metastasis to produce (in case of vasculogenesis) new blood vessels or modify already existing ones (in case of angiogenesis). Although these steps resemble the events in tumorigenesis, unlike tumour cells, the trophoblast cells proliferate and then invade. Therefore their tumour-like behaviour is kept at check [2]. This mini review provides a general overview of the events (and molecules) involved in the controlled trophoblast invasion resulting in pseudo-vasculogenesis and spiral artery remodelling.
Placental vasculogenesis and angiogenesis
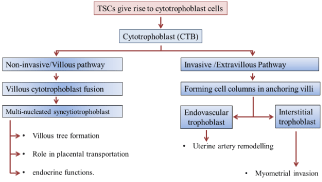
The differentiation mechanism of human Cytotrophoblast (CTB) progenitor cells can be categorised into two general pathways: (a) non-invasive villous trophoblasts and (b) invasive extravillous trophoblast [3]. These two pathways are mutually co-exist during trophoblast differentiation. However, it is found that during first trimester CTB follow the invasive pathway; whereas, later in pregnancy they follow the non-invasive pathway. This suggests that trophoblastic differentiation process continues until the end of pregnancy in a dynamic way [4].
Invasive pathway
The invasive pathway is also known as invasive extravillous trophoblast pathway. This actually involves the development of interstitial or endovascular cytotrophoblastcells (iEVT and enEVT respectively) [4]. Initially, the cytotrophoblastic stem cells present at the villous basement membrane start to proliferate. They then form cell columns of anchoring villi to support the placental perfusion [5]. A group of rapidly proliferating CTBs can be found at the proximal ends of anchoring villi. On the other hand, the CTBs located at the distal part are capable of invasion as they contain integrin α-5β- 1 and fibronectin rich matrix. In order to form the Extra Villous Cytotrophoblast (EVTs), the CTBs from the distal ends of columns start to exit their cell contacts. That is they stop proliferating and undergo differentiation for angiogenesis [4]. They detach themselves from columns and differentiate into invasive iEVTs and enEVTs [6]. The differentiation process of heterogeneous trophoblast cells is unclear. However in-vitro studies suggest this process may be intrinsic by switching of adhesion molecules; also several proteases may play a large part in this differentiation process [4].
Next the iEVTs migrate and invade into uterine tissue where it can penetrate the one-third of the adjacent myometrium. Similarly, enEVTs migrate towards uterine arteries and replace all the endothelial cell lining. This process is termed as conversion (or spiral artery remodelling), which helps in the lowering vascular resistance to allow increased blood flow during pregnancy [7]. The iEVT cells also take part in spiral artery remodelling by destroying smooth muscles of the artery. As the result of invasion by enEVTs and iEVTs, uterine spiral arteries are reconstructed during the early pregnancy.
Thus, the events in remodelling process can be divided into following synchronised activities. With decidual activity, the early vascular remodelling is initiated. This is followed by (a) iEVT invasion which destroys the spiral artery smooth muscles and (b) migration and incorporation of enEVT into the vessel (spiral artery). Finally re-endothelisation (replacement of endothelial lining of spiral arteries) and sub-intimal thickening happens. At the end of this invasive process the spiral arteries are transformed from “high resistant low capacity” to “low resistant high capacity” vessels; satisfying the high demand of the growing foetus. Interestingly, these iEVT and enEVT cells have different gene expression profile which makes them divergent from their other trophoblast cell lineage and provides the capacity to invade(see below) [8].
Non-invasive or villous pathway
The non-invasive villous pathway is also known as syncytial pathway. Here the trophoblast cells fuse to differentiate in multinucleated Syncytiotrophoblast (STBs). This syncytial layer covers the placental villous tree and intimately takes part in the materno-foetal exchanges [9]. Initially syncytial layer invades the uterine epithelium and shows its involvement in the formation of primary villous (the invasive pathway – see below). By 21 days post conception the mesenchymal cells inside the villi transform into hemangiogenic precursor cells and migrate toward the periphery to develop into hemangioblasticcell cords forming the first vessels (angiogenesis) within the villous. The same cells later give rise secondary and tertiary villi by more extra embryonic mesenchymal cell invasion, including villous branching and vascularisation mechanism [10]. The absorption capacity of this layer is enhanced due to the presence of microvilli by increasing the surface area. The outer STB layer, despite its non-mitotic nuclei, are continuously replenished throughout pregnancy via the fusion of the underlying CTBs layer; and by discarding the aged portions of STBs in the form of syncytial knots [11].
Another cellular event which is also started immediately after the implantation is the formation of floating villi and the anchoring villi. The floating villi are covered with Syncytiotrophoblast (STB) cells (generated by the core villous CTB cells through fusion mechanism). These floating villi help in the transport of nutrients, oxygen by passing through endothelial layer. The sequence of events in this invasion are summarised in (Figure 1) [slightly modified from [1]]. The mechanisms that are present to orchestrate these events are not entirely clear. The possible factors involved in placental angiogenesis and psudo-vaculogenesis are briefly explained below.
Schematic representation of the two different pathways of trophoblast differentiation.
Both invasive and non-invasive cells are generated from the Cytotrophoblast (CTB). The Extravillous Cytotrophoblasts (EVT) at the proximal end of anchoring column take non-invasive pathway; whilst the EVTs at the distal end generate the invasive pathway.Figure 1:Schematic representation of the two different pathways of trophoblast differentiation.
Both invasive and non-invasive cells are generated from the Cytotrophoblast (CTB). The Extravillous Cytotrophoblasts (EVT) at the proximal end of anchoring column take non-invasive pathway; whilst the EVTs at the distal end generate the invasive pathway.
Factors regulating trophoblast differentiation and invasion
Trophoblast invasion is controlled by many regulatory factors such as Metalloproteinase (MMPs), cytokines, surface integrins, and other Major Histocompatibility Complex (MHC) [12]. Also the differentiation process of trophoblast cells is influenced by various other factors which exert their effect in paracrine or autocrine manner. Most importantly, angiogenic proteins such as Vasculoedotheial Growth Factor (VEGF) [and Placental Growth Factor (PGF)] are also found to be involved in spiral artery re-modulation. VEGF also inhibits the maturation of antigen-presenting Dendritic Cells (DC) and therefore helps to maintain immature pro-angiogenic DC [13]. These factors are secreted not only by trophoblast cells and villous mesenchymal cells, but also by the uterine stromal/glandular myometrial/and endothelial cells, and various other immune cells present at maternal-foetal interface [3].
As mentioned above, the iEVTs that invades deeply in the decidua express high level of collagen IV, integrin, and α-1-β-1 [14]. Their invasion mechanism also influenced by the down-regulation of integrin α-6-β-4 and the up-regulation of fibronectin integrin α-5-β-1. Down-regulation of E-cadherin also contributes in the loss of cell-to-cell contact in order to increase their invasiveness. There are also several proteases secreted from iEVTs to help in this invasion mechanism by degrading extracellular matrix. For example iEVTs are reported to express Urokinase Type Plasminogen Activator (UPA), several Matrix Metalloproteinase (MMP) and most notably gelatinases MMP-2 and MMP-9 [3]. Most surprisingly the inhibitors of these enzymes [such as plasminogen inhibitor1/2 (PAI 1/2) and Tissue Inhibitor Metalloproteinase-1 (TIMP-1)] are also co-localised in iEVTs showing the invasive proves is tightly controlled. There are several other factors involved in the trophoblast invasion, differentiation and migration. They also regulate different stages of placental development. These factors and their involvement in human placentation process have been summarised in (Table 1).
Factors
Expression/Production site
Functions
VEGF family
CTBs, STBs, EVTs, and perivascular cells
Vasodilation, angiogenesis, syncytialization
TGF-β family
Decidual NK cells, decidual macrophages
Inhibition of early trophoblast differentiation anddown regulation of invading CBTs. Promoting syncytialization,
Hepatocyte growth factor
Decidua, villous syncytium, extra villous cytotrophoblast
Rapid trophoblast cell proliferation
Epidermal growth factor
Decidua, syncytiotrophoblast cells
STBs differentiation, placental endrocrine function modulation
Insulin like growth factors
Trophoblast cells
Foetal/placental growth
Angiopoietin
Decidua, endothelial cells, STBs, CTBs
Angiogenesis
Placental growth factor
Trophoblast cells
Angiogenesis
Colony stimulating factor
Placenta, decidua
Growth stimulation of invasive trophoblast cells.
Table 1: Factors involved in trophoblast invasion and vasculogenesis.
Apart from these extra cellular controls there are several intra-cellular factors that are up- or down-regulated. Studies have suggested, transcription factor OCT-4 as one of the crucial controller for maintenance of TS cells totipotency [15,16]. Other transcription factors such as, TEAD4, CDX2 and EOMES are the controllers of trophoblast lineage development. Recent comparative microarray study carried out within our laboratory has identified several other transcription related factors and other proteins may have a role in trophoblast invasion and psudo-vaculogenesis. For example, Aurora Kinase (AURK) family of proteins which control the mitosis/meiosis processes duringembryogenesis may also regulate the invasion. Other intracellular proteins such as Jagged (involved innotch signalling pathway) have also been implicated in this highly controlled angiogenesis. Interestingly these newly identified “targets” are found to be differently expressed in pre-eclampsia, a pregnancy related disease where the trophoblast invasion is low. Exploring the functions of these new proteins in trophoblast invasion would help to understand the controlled invasive process.
Concluding Remarks
Trophoblast invasion is a highly controlled event during early pregnancy which results in angiogenesis and pseudo-vasculogenesis. The fact that the placentation resembles tumour invasion, understanding the factors/mechanisms that controlled trophoblast invasion would help us to identify target molecules for cancer chemotherapy.
References
- Tarrade A, Lai Kuen R, Malassiné A, Tricottet V, Blain P, Vidaud M, et al. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Invest. 2001; 81: 1199-1211.
- Soundararajan R, Rao AJ. Trophoblast 'pseudo-tumorigenesis': significance and contributory factors. Reprod Biol Endocrinol. 2004; 2: 15.
- Chen JZ, Sheehan PM, Brennecke SP, Keogh RJ. Vessel remodelling, pregnancy hormones and extravillous trophoblast function. Mol Cell Endocrinol. 2012; 349: 138-144.
- Pollheimer J, Husslein P, Knöfler M. Invasive trophoblasts generate regulatory collagen XVIII cleavage products. Placenta. 2005; 26: S42-45.
- Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology. 1997; 138: 4977-4988.
- Menkhorst E, Winship A, Van Sinderen M, Dimitriadis E. Human extravillous trophoblast invasion: intrinsic and extrinsic regulation. Reproduction, Fertility and Development. Reproduction Fertility and Development 2014 [In press].
- Aplin JD. Embryo implantation: the molecular mechanism remains elusive. Reprod Biomed Online. 2006; 13: 833-839.
- Soares MJ. Embryo implantation - coordination of maternal and embryonic adaptations. Int J Dev Biol. 2014; 58: 71-74.
- Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004; 25: 114-126.
- Huppertz B, Peeters LL. Vascular biology in implantation and placentation. Angiogenesis. 2005; 8: 157-167.
- Heazell AE, Moll SJ, Jones CJ, Baker PN, Crocker IP. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007; 28: S33-40.
- Lunghi L, Ferretti ME, Medici S, Biondi C, Vesce F. Control of human trophoblast function. Reprod Biol Endocrinol. 2007; 5: 6.
- Holtan SG, Creedon DJ, Haluska P, Markovic SN. Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin Proc. 2009; 84: 985-1000.
- Damsky CH, Fisher SJ. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol. 1998; 10: 660-666.
- Roberts RM, Fisher SJ. Trophoblast stem cells. Biol Reprod. 2011; 84: 412-421.
- Pfeffer PL, Pearton DJ. Trophoblast development. Reproduction. 2012; 143: 231-246.