
Review Article
Austin J Vet Sci & Anim Husb. 2023; 10(1): 1110.
Review on the Role of Biofilm Formation in Bacterial Pathogenesis
Umer AA*
Animal Health Institute (AHI), Bacteriology Laboratory, Ethiopia
*Corresponding author: Abdi Ahmed Umer Animal Health Institute (AHI), Bacteriology Laboratory P.O. Box 04, Sebeta, Ethiopia
Received: December 12, 2022; Accepted: January 31, 2023; Published: February 07, 2023
Abstract
Biofilms are defined as a accumulation of microorganisms living in a self-assembled matrix of polymeric substances that adheres to a variety of surfaces. Depending on the interaction between the surface and constituent cells, biofilms can be monolayer or multilayer. Initial attachment, irreversible attachment, micro colony growth, biofilm maturation, and biofilm dispersal are the five steps of the biofilm formation process. The processes that contribute tobiofilm formation are a dynamic and complicated process. Biofilm Formation Causes many bacteria have the ability to transition between planktonic and biofilm forms. The biofilm state appears to be a natural and prevalent condition of bacteria. Biofilm-forming bacteria are important for animal health. The risk of infection in animal species is likely to be higher than in humans. Because of the differences in animal housing and living conditions.
Microorganisms capable of generating biofilms include P. aeruginosa, S. epidermidis, E. colispp, S. aureus, E. cloacae, and K. pneumonia. Bacterial pathogenesis of biofilms occurs frequently on medical devices and bits of dead tissue, such as sequestra of dead bone; nevertheless, they canals of or nonliving tissues. Biofilms in bacterial infections can improve the pathogenicity of the organism while also protecting it from external treatment. UV radiation, pH stress, chemical exposure, phagocytosis, dehydration, and antibiotics are all threats to cells that biofilms protect them. The Role of Signaling in Biofilm Formation is the bond between environmental stimuli and the bacteria’ reciprocation of the relevant signaling is required for the formation of a biofilm. Two-component systems and Quorum sensing can both be induced by these sensing systems. Tissue culture plate, cong red agar, and bioluminescent assay are some of the laboratory tests used to diagnose biofilm. The emergence and spread of antibiotic resistance among bacteria is one of the world’s most serious health issues. One of the implications of the bacterial biofilm communities that lead to persistent illnesses is antibiotic resistance.
Keywords: Biofilm; Bacterial; Pathogenesis
Introduction
Bacterial cells can proliferate in two ways: planktonic cells and sessile aggregates, which are known as biofilms. Bacterial biofilms are clumps of bacteria adhered to surfaces that are encased in a matrix of extracellular polymeric compounds [8]. Antonie Van Leeuwenhoek, a Dutch researcher, was the first to identify ‘animalcule’ on teeth surfaces using a basic microscope, and this was considered the first microbial biofilm discovery [10]. Over 99 percent of bacteria on Earth are considered to reside in biofilm communities [53].
Microbes, which is primarily self-produced, improves survival in harsh settings, including antibiotic tolerance, and gives the biofilm structure [31]. Biofilms can be found in both natural and human-made environments. Biofilms can grow on a range of surfaces, including inert and alive materials like tissues and cells [13].
Microbes produce biofilms to help them resist unpredictable environmental stressors such as temperature variations, desiccation, UV radiation, cleans in agents like biocides and disinfectant pressure, and host immune systems. Biofilms can be found in industrial settings, hotels, waste water channels, toilets, labs, and medical settings, and they most typically form on hard surfaces that are submerged in or exposed to an aqueous solution.
It can form on living as well as non-living surfaces [45] Antibiotics are protected by the Extracellular Polymeric Substances (EPS) matrix, which prevents drug entry at bactericidal concentrations. When compared to planktonic bacteria, bacteria within a biofilm are several orders of magnitude more resistant to antibiotics [37].
Bacterial Biofilm
Biofilms are bacterial populations that are embedded in a matrix of extracellular polymeric substances that are bonded to a surface. Bacteria exist as separate creatures in a ‘planktonic state,’ according to a popular misperception about microbial life. Microorganisms, on the other hand, naturally aggregate on a wide range of surfaces, where they form sessile, sedentary populations. Biofilms protect bacteria from harmful conditions, and biofilm development appears to be a key role in the disease cycle of bacterial pathogens in animals, humans, and plants. In most bacterial species, bacterial surface components and extracellular molecules [mainly flagella, Lipopolysaccharides (LPSs), and Exopolysaccharides (EPSs), in conjunction with environmental and quorum-sensing cues, are critical for auto aggregation and biofilm growth.
Environmental signals initiate biofilm development, and flagella are required for the biofilm community to approach and move across the surface [2]. These surfaces include household and industrial pipes, biological materials such as contact lenses, medical devices such as implants and catheters, and human and animal tissues. These single or multiple microbial aggregates are generally called biofilms and can be made up of different bacterial and fungal communities. The proximity of microorganisms allows the exchange of substrates, the distribution of metabolites, and the elimination of toxic end products, so that different species can support each other. In addition, the structure of the biofilm community can protect the bacteria in it from antibacterial agents, shear forces, and the immune system [11].
Types of Bacterial Biofilm
Biofilms can be monolayer or multilayer depending on how the surface interacts with the constituent cells. Interactions between constituent cells are less important in a single-layered biofilm than interactions between cells. In the creation of a mono layer microbial biofilm, there are several types of sticky structures. Preformed adhesion features, such as flagellum or pilus, boost transient attachments to the surface and hence speed up the production of monolayer biofilms in one kind. The microbial adhesin is generated in one type while the shift to permanent attachment is occurring in another [27].
When microorganisms can cling to a surface as well as to one other, they form a multilayer biofilm. It has been shown that the surface features of bacteria cause repulsion in many circumstances. For example, the O antigen, which is normally negatively charged in nature, determines the chemical characteristics of the cell wall of gram-negative bacteria. This repulsive force caused by comparable charge among bacteria must be negated in order to build a multilayer biofilm. The addition of divalent cations, the formation of Extracellular Polymeric Substances (EPS), and the mutation, downregulation, or silence of the O antigen producing genes may disguise this negative charge [14].
Biofilm Formation Process
Biofilm is a group of microorganisms that are securely adhered to a biotic or abiotic surface, encased in an Extracellular Polymeric Substance (EPS) matrix, and can exhibit novel gene expression, protein synthesis, growth rate, and metabolic activities. The production of biofilms follows a stage-by-stage process. The biofilm grows more firmly adhered as it progresses, and the microorganisms within it become more protected against cleaners and sanitizers [18].
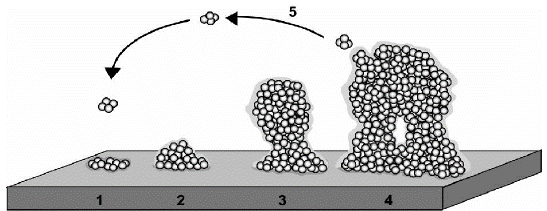
Figure 1: The development of a biofilm, depicted as a five-stage process.
Source: (Lasa, 2006) [30]
Stage1: Reversible or Initial Attachment: 2. Irreversible Attachment: 3. Microcolony Formation. 4. Biofilm Maturation. 5. Biofilm Dispersal.
Reversible or initial attachment: The transition from planktonic to biofilm mode is marked by bacterial surface adhesion [54]. Planktonic microorganisms interact with a conditioned surface to form reversible adhesion. However, the interaction, which comprises van der Waals forces, electrostatic forces, and hydrophobic contacts, is relatively weak. Surfaces that are rough, hydrophobic, and coated with various organic compounds will work best for the attachment [12] The link between bacteria and the surface of attachment is strengthened by bacterial features such as fimbriae, pili, and flagella. In general, cell appendages engaged in reversible attachment and bacteria commit tothe biofilm lifestyle or depart the surface and return to a planktonic lifestyle at this stage [54].
Irreversible attachment: Loosely bound organisms consolidate the attachment process at this stage by producing extracellular polymeric substances that complex with surface materials and/or receptor-specific ligands located on pili, fimbriae, and fibrillae, or both. After microorganisms are attached to preconditioned and permissive surfaces, the cell begins an irreversible adhesion and accumulates as a multilayered cell. The creation of a biofilm begins with a layer of polymeric substances (EPS) on which microbial cells swarm, followed by the biofilm's expansion. A number of physiological and anatomical changes have happened during this step, including nonmotility of the attached cells [49].
Microcolony formation: Micro colonies are formed when microbial cells anchored within the extracellular matrix proliferateina coordinated community. Micro colony formation, according to Dunne, is caused by the simultaneous aggregation and growth of microorganisms, as well as the creation of EPS [13].
Micro colonies, which are the fundamental units of biofilm, are divided into channels with varied microenvironments. After cells are securely connected to conductive surfaces, a slew of bacteria emerges and exudes polymeric compounds that can act as a "glue" to keep microorganisms attached to various surfaces. Micro colonies are formed as a result of these consecutive occurrences [59].
Biofilm maturation: A biofilm may develop into spatially well-arranged, three-dimensional mature biofilm structures such as mushroom or tower-like structures interspersed with fluid-filled channels in which nutrients, oxygen, and essential substances can be diffused and circulated if conditions are suitable for sufficient growth and differentiation [41].
The establishment of biofilm is governed by density-dependent chemical signals released by bacterial populations embedded in a self-produced extra cellular matrix in each micro environment. Quorum sensing is a signaling mechanism that is utilized to communicate and orchestrate group behaviors like as virulence factor secretion and biofilm formation. Quorum sensing triggers a coordinated maturation and breakdown of the biofilm. Cell-to-cell signaling is important for cell attachment and dissociation from biofilms in general [46].
Biofilm dispersal: Biofilm development is a cyclical process in which bacterial cells are detached from the mature biofilm and revert to their prior state of life, planktonicity. Disconnected bacterial cells will look for new surfaces to cling to and begin a fresh round of biofilm formation. Microbial cells will select whether to live together or “fall apart” at this step, based on environmental cues [29]. This stage is critical for disseminating bacteria into food products from the standpoint of food contamination. Biofilm cells can be separated from actively growing cells or the impoverished environment, and aggregates can be removed. Nutrient scarcity has been shown to push microbes to seek out new habitats [59].
Biofilm Formation Factors
Biofilm creation is a dynamic and complicated process that comprises initial bacterial cell attachment to the substrate, physiological changes within the microbe, multiplication of adherent cells to form micro colonies, and finally biofilm maturation. Biofilm-associated bacteria have different physiology and greater tolerance to the immune system and drugs than their free-living planktonic counterparts, making biofilm a source of chronic and persistent infections [42].
Biofilm Formation Causes
Many bacteria have the ability to transition between planktonic and biofilm forms. Planktonic bacteria have a fast rate of cell development and reproduction. The biofilm state, on the other hand, appears to be a natural and prevalent condition of bacteria. A bacterial biofilm condition may be required for a variety of reasons [24]. For starters, biofilm can improve bacteria's endurance to hostile environmental circumstances. Bacteria cancelling to a surface or tissue to avoid being was headway by water move mentor blood flow. Oral biofilms may withstand repeated high shear stresses. Second, the EPS matrix protects bacterium cells in deeper levels from antimicrobial agents, most likely by reducing antimicrobial agent diffusion. Biofilms limit bacterial mobility and enhance cell density, creating an ideal environment for eDNA (plasmid) exchange (through conjugation), which encodes for antibiotic resistance in some cases [44].
Role of Biofilm on Bacterial Pathogenesis
Biofilms originate on inert surfaces or dead tissue, and are usually found on medical equipment and fragments of dead tissue like sequestra of dead bone; nevertheless, they can also form on living tissues, as in the case of endocarditis [56]. Antigens are released by sessile bacterial cells, which trigger antibody formation. However, antibodies are in effective at killing bacteria within biofilms, and they may cause immunological complex harm to near by tissues. Biofilm infections are rarely healed by the host defensive mechanisms, even In people without standing cellular and humoral immune responses [7].
Animal Health Importance of Biofilm Forming Bacteria
Quorum sensing, swarming, and biofilm formation are examples of collaborative group behaviors used by bacteria to adapt to a variety of settings, including human hosts [50]. In general, quorum sensing during host tissue colonization is linked to virulence gene expression and acute-phase infections, whereas biofilm formation aids the establishment of chronic infections, immune evasion, and treatment tolerance [16]. The role of biofilm in human and animal disease processes is now generally acknowledged. The risk of infection in animal species is likely to be higher than in humans. Because of the differences in animal housing and living conditions [57]. The natural bacterial microflora in the mouths of dogs and cats is made up of a variety of aerobic, facultative, and totally anaerobic bacteria. Teeth provide continual dampness and adhesive surfaces in the oral cavity, allowing vast bacterial deposits to form [40].
Biofilm Forming Microorganisms
Almost all microorganisms (99.9%) have the ability to produce biofilm on a variety of surfaces, including biological and inert surfaces. Micro organisms create Extracellular Polymeric Substance (EPS) and form biofilm when they bind to a surface. Due to its antibiotic resistance and sickness associated within dwelling medical devices, biofilm poses
Significant threat to public health [48]. H. influenza has been discovered to have the ability to develop biofilm in the human body and to evade the immune system.
P. aeruginosa, S. epidermidis, E. coli spp, S. aureus, E. cloacae, and K. pneumoniae are among the bacteria that can produce biofilms [17]. Several gram-positive (e.g.Bacillus spp., Listeria monocytogenes, Staphylococcus spp., and Lactobacillus plantarum and Lactococcuslactis) and gram-negative (e.g. Escherichia coli, Pseudomonas aeruginosa). In aquatic conditions, cyano bacteria also create biofilms [53].
a. Escherichiacoli
E. coli is a Gram-negative rod-shaped bacteria that causes a wide range of nosocomial and community diseases, including UTIs and prostatitis. It can secrete toxins and polysaccharide, as well as produce biofilm. In vitro, it can also create biofilm [38]. E. colicapsules are large molecules with a high molecular weight that bind to the cell surface. E. colicapsules play an indirect function in biofilm formation by preventing bacterial adherence tothe surface. The ability of E. coli to generate biofilm is affected by a variety of environmental factors. Because of the existence of exopolymers, E. coli biofilms can be hundreds of microns thick, making antibiotic therapy challenging [33].
b. Staphylococcusaureus
Staph aureus is a multi drug-resistant bacteria that causes a variety of nosocomial infections. As a biofilm, it grows on catheters and chronic wounds [39] S. aureus recycles proteins in the cytoplasm to produce the extracellular matrix. The ability of cy toplasmic proteins to act as matrix proteins provides for more flexibility and adaptation to S. aureus in the creation of biofilms in infectious settings, and it may favor the production of mixed-species biofilms in chronic wounds (Lucy et al. 2014).
Diseases Related Biofilm
Microorganisms cling to surfaces and form biofilms, which have been linked to a number of diseases of major public health relevance (Mahami and Adu-Gyamfi 2011). Biofilms have been connected to a number of diseases. Many bacterial infections, such as chronic lung, wound, and ear infections, are caused by biofilms. Bioflms can colonize medical equipment like catheters and implants as well. Biofilms are responsible for more than 80% of all microbial illnesses in bacterial infections; the existence of biofilms can increase the pathogenicity of the bacteria while also protecting it from being killed by external treatment.
In adverse conditions, bacteria have long used biofilm production to survive. UV radiation, pH stress, chemical exposure, phagocytosis, dehydration, and antibiotics are among threats that biofilms protect cells from (Gupta et al., 2015). Biofilm-associated illnesses, unfortunately, are resistant to both conventional biocides and the host immune system. As a result, diagnosing and treating the condition is difficult [19].
a. Cysticfibrosis
The lung disease Cystic Fibrosis (CF) involves the creation of thick, sticky mucus, which restricts airways and makes it difficult for patients to breathe. P.aeruginosa infects 80% of CF patients on a long-term basis. P. aeruginosa can also be found on medical apparatus, devices, and tools [26], but unfortunately, there are few effective antibiotics for treating persistent P.aeruginosa infections.
b. Dental plaque
Biofilms play a significant function in dentistry, and many studies on dental biofilms have been conducted [35]. In dental plaque, about 700 species of bacteria and archaea have been identified [58]. Many oral cavity disorders, such as dental caries, periodontitis, gingivitis, and others, are caused by dental plaque. For initial colonization and subsequent biofilm formation on the enamel surfaces, bacteria must communicate with one another. If bacteria do not stick to the tooth surface, they are rinsed away with saliva [52].
c. Wounds
Biofilms are commonly found in chronic wounds. Compared with an acute wound, which is usually not associated with biofilm, a biofilm-related chronic wound persists and only slowly heals. Biofilms usually form on the outer layer of wound but some biofilm are also embedded in the deep layers of wounds [23].
d. Urinaryinfection
Biomaterials in the urinary tract, such as catheters, enhance the likelihood of bacterial biofilm formation, which can result in urine infection. Bacteria can cling to the synthetic foreign body's surfaces. Bacterial biofilms infect almost all urinary catheters (both the inner and exterior surfaces). P. mirabilis biofilms, for example, which can be crystalline in nature, can block catheters, requiring patients to replace the clogged catheters [22].
e. Prosthetic joint infection
Gram-positive bacteria, such as staphylococci, are commonly seen in prosthetic joint infections [36]. Bacteria (from the blood or lymph) bind to the surface of prosthetic joints to develop biofilms almost immediately after surgery. Unlike conventional bacterium infections, which cause symptoms such as fever, biofilm infections on these implants may take time to manifest symptoms such as pain [55].
f. Cardiac Valve Infection
Bacterial biofilm on mechanical cardiac valve causes a disease called prosthetic valve endocarditis. The species involved in endocarditis are S.epidermidis, S.aureus, Streptococcus spp., Coryne bacterium spp., Enterococcus spp. and Candida spp [9] Accumulated biofilm can disrupt or block the artificial cardiac valve, resulting in diminished flow, turbulence or even leaking. Detached biofilm cells can migrate along with the blood stream and cause infection in other organs. Biofilm fragments in the blood circulation can block blood at the terminal points, such as the brain and kidney.
Signaling in Biofilm Formation
The connection between environmental stimuli and the microorganisms' reciprocation of the appropriate signaling events is required for the formation of a biofilm. Many sensor systems are capable of incorporating environmental cues into signaling pathways. Two-component systems and Quorum sensing can be induced by these Signaling systems (Jonas et al.2009).
Quorum Sensing
At high cell density, a process of cell-to-cell communication regulates gene expression. The concentration of an auto inducer, which is produced by bacteria into the environment, exceeds a particular threshold at high cell density, resulting in gene expression and regulation of many physiological activities. Bacteria must synchronize their gene expression in order to build a biofilm, and some use quorum-sensing systems to doit. QS appears to be a process through which bacteria manage collective activities and monitor cell density [32].
Two-Component System
Histidine kinase and response regulator proteins make up the two-component signaling system. HK is a sensor protein with a ligand-binding domain on the N-terminus and a kinase domain on the C-terminus. The transfer of phosphoryl groups from Adenosine Triphosphate (ATP) to a particular conserved histidineresi due in HK causes signal transduction. Following that, HK moves the phosphoryl group from histidine to RR's as part at eresi due [51]. This phosphate activate RR, which regulates transcription, In general, GacS (HK)/GacA (RR) two-component systems are implicated in the production of Pseudomonas aeruginosa biofilms [43]. The rsm genes, which code for RsmY and RsmZ, which control the transition between planktonic and sedentary forms, are induced by this system [3]. A progressively coordinated network of gene expression is essential for the creation of biofilm. As a result, these signaling events play a critical role in the creation of microbial biofilms by producing adaptive responses to external and internal stimuli [4].
Materials and Methods
Biofilm Laboratory Diagnosis is Test
Tissue Culture Plate: The TCP assay is most widely used and is considered a standard test for detection of biofilm formation. The microorganisms are grown in polystyrene tissue culture plates for 24 hours then after washing fixed with sodium acetate (2%) and stained with crystal violet (0.1% w/v). Biofilm formation is detected by measuring optical density with ELISA reader [6].
Congo Red Agar: Biofilm generation by Staphylococcus isolates is tested using a specially prepared solid medium called brain Heart Infusion Broth (BHI), which is supplemented with 5% sucrose andCongo red. BHI (37 gms/L), sucrose (50 gms/L), agar no. 1 (10 gms/L), and congo red stain (0.8 gms/L) made up the medium. Congo red was made as a concentrated aqueous solution that was autoclaved at 121°C for 15 minutes, separate from the other medium elements, and then added after the agar had cooled to 55°C. Plates were inoculation and aerobically incubated at 37°C for 24 to 48 hours. Black colonies with a dry crystalline quality indicated a positive result. Weak slime producers usually remained pink, though occasional darkening at the centers of colonies [15].
Bioluminescent Assay: To analyze the bio-activity of the bacterial biofilm, the ATP content of the biofilm was assayed using the ATP bioluminescence assay. A linear relationship between the ATP content and the number of the planktonic Pseudomonas aeruginosa cells. Attenuated Total Reflecting Spectros copy used to monitor the conditioning films that are an early harbinger of biofilm formation [15].
Antimicrobial Resistance
The emergence and spread of antibiotic resistance among bacteria is one of the world's most serious health issues [5]. One of the outcomes of bacterial biofilm communities that lead to chronic illnesses is antibiotic resistance. Biofilm-forming Klebsiella Pneumoniae is a Multidrug-Resistant (MDR) bacteria that affects people and is a leading cause of hospital infections with high morbidity and mortality due to restricted treatment choices [21]. Biofilm development is away for bacteria to with stand harmful environmental impacts like antibiotics and anti microbial drugs. Biofilm, according to Verderosa et al., is resistant to antibiotic therapy and is a primary source of persistent and recurring infections by clinically important pathogens over the world. This is due to the fact that biofilm formation and subsequent encasement of bacterial cells in a complex matrix can increase resistance to antimicrobials and sterilizing treatments, making these organisms difficult to eliminate and manage [28]. The host immune system responds to bacterial infections by activating a number of signaling pathways, cytokines, and genes involved in stress management [20]. Many bacterial diseases that are initially thought to be strictly extracellular can remain inside the host by forming biofilms as a result of a process of adaptation that allows the bacteria to evade the host's innate defense. The escape of biofilms from the host's innate response is damaging to the host since the inflammatory influx released by the body in response to the bacterial infection may cause tissue damage [1]. Antibiotics are protected by the Extracellular Polymeric Substances (EPS) matrix, which prevents drug entry at bactericidal concentrations. When compared to planktonic bacteria, bacteria within a biofilm are several orders of magnitude more resistant to antibiotics. Biofilms, for example, can withstand antimicrobial doses 10–1000 times higher than those required to inactivate genetically similar planktonic bacteria [37].
Conclusions
Biofilms are microbial communities that live in close proximity to one another and can cling to both biotic and a biotic surfaces. They biofim build structures that function as a barrier to sanitizer. Sanitizer, cleaner, and disinfectant disturb microbial cells and their attachment on surfaces and environments enables bacterial pathogens to colonize in harsh situations. It is preferable to develop appropriate materials with technology that will decrease bacterial attachment and make cleaning easier. It is also crucial to understand their genes, which are involved in encoding bacterial cell surfaces that are important for adhesion. The release of signaling molecules that might alert others for survival in hazardous situations is another important aspect of bacterial biofilm formation. To disrupt their communication networks, effective methods should be created. In the medical field, biofilm-forming microbes are a major issue. Biofilm-forming bacteria are wrapped in a matrix that protects them from antibiotics and the immune system of the host.
Biofilm formation by bacteria and their subsequent resistance to antibiotic and bactericidal is a slow but serious threat to health. Biofilm formation has become a ubiquitous phenomenon not only for human and animal infections, but also on non biological aspects. Biofilms are formed on food items and water which are considered as the basic necessities of daily life. Current therapeutic approaches for prevention of biofilms is limited to use of antimicrobial agents and post infection remedy lies in surgical removal of the biofilm followed by continued antibiotic administration. But nonetheless novel strategies are also being used to combat the problem. Option of vaccination against specific biofilm-associated bacteria is also being explored and one can hope that prevention and inhibition of biofilms by bacteria can be achieved in near future.
References
- Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. Staphylococcus aureus biofilms properties, regulation and roles in human disease. Virulence. 2011; 2: 445–459.
- Branda SS, Vik S, Friedman L, Kolter R. Biofilms: The matrix revisited. Trends Microbiol. 2005; 13: 20–26.
- Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, et al. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol Microbiol. 2009; 73: 434–445.
- Bordi C, de Bentzmann S. Hacking into bacterial biofilms: a new therapeutic challenge. Ann Intensive Care. 2011; 1: 19.
- Cepas V, L´opez Y, Muñoz E, Rolo D, Ardanuy C, Marti S, et al. Relationship between Biofilm formation and antimicrobial resistance in gram negative bacteria. Microbial Drug Resistance. 2019; 25: 72-79.
- Christensen GD, Simpson WA, Yonger JA, Baddour LM, Melton DM, et al. Adherence of coagulase negative staphylococci to plastic tissue cultures: a quantitative model for the adherence of staphylococcito medical device. J Clin Microbiol. 1985; 22: 996-1006.
- Cochrane DMG. Immune response to bacterial biofilms. Med Micro boil J. 1988; 27: 255.
- Caruso G. Microbial Colonizationin Marine Environments: Overview of Current Knowledge and Emerging Research Topics. Journal of Marine Science and Engineering. 2020; 8: 78.
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial Biofilms. Annu Rev Microbiol. 1995; 49: 711–745.
- Costerton J, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Sci. 1999; 284: 1318-1322.
- Davey ME, O’Toole GA. Microbial biofilms: From ecology to molecular genetics. Microbiol Mol Biol Rev. 2000; 64: 847–86.
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 2002; 15: 167–193.
- Dunne W. Bacterial adhesion: seen any good biofilms lately? Clinical Microbiology Reviews. 2002; 15: 155-166.
- Feldman MF, Wacker M, Hernandez M, Hitchen PG, Marolda CL, et al. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichiacoli. Proc Natl Acad Sci USA. 2005; 102: 3016–3021.
- Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989; 42: 872-4.
- Furukawa S, Kuchma SL, O’Toole GA. Keeping their options open: Acute versus persistent infections. J Bacteriol. 2006; 188: 1211–1217.
- Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005; 13: 34-40.
- Garrett TR, Bhakoo M, Zhang Z. Bacterial adhesion and biofilms on surfaces. Prog Nat Sci. 2008; 18: 1049–1056.
- Hall MR, Mc Gillicuddy E, Kaplan LJ. Biofilm: basic principles, pathophysiology, and implications for clinicians. Surg Infect (Larchmt.) 2014; 15: 1–7.
- Hartmann A, Rothballer M, Hense BA, Schroder P. Bacterial quorum sensing compounds are important modulators of microbe plant interactions. Front Plant Sci. 2014; 5: 131.
- Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. International Journal of Antimicrobial Agents. 2010; 35: 322–332.
- Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev. 2008; 21: 26–59.
- James GA, Swogger E, Wolcott R, Pulcini EL, Secor P, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008; 16: 37–44.
- Jefferson KK. What drives bacteria to produce a biofilm. FEMS Microbiol Lett. 2004; 236: 163–173.
- Jonas K, Melefors O, Romling U. Regulation of c-di-GMP metabolism in biofilms. Future Microbiol. 2009; 4: 341–358.
- Jones AM, Govan JR, Doherty CJ, Dodd ME, Isalska BJ, et al. Spread of a multiresistant strain of Pseudomonas aeruginosa in an adult cystic fibrosis clinic. Lancet. 2001; 358: 557–558.
- Karatan E, Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol MolBiol Rev. 2009; 73: 310–347.
- Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018; 4: e01067.
- Kostakioti M, Hadjifrangiskou M, Hultgren SJ. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harbor Perspectives in Medicine. 2013; 13: a010306.
- Lasa I, Penades J. Bap: a family of surface proteins involved in biofilm formation. Res Microbiol. 2006; 157: 99-107.
- Limoli DH, Jones CJ, Wozniak DJ. Bacterial extracellular polysaccharidesin biofilm formation and function. Microbiol Spectr. 2015; 3: 10.
- Lyon GJ, Muir TW. Chemical signaling among bacteria and its inhibition. Chem Biol. 2003; 10: 1007–1021.
- Larson EL, Gomez-Duarte C, Lee LV, Latta PD, Kain DJ, et al. Microbial flora of hands of homemakers. American J Infect Control. 2003; 31: 72-79.
- Foulston L, Elsholz AKw, DeFrancesco AS, Losick R. The Extracellular Matrix of Staphylococcus aureus Biofilms Comprises Cytoplasmic Proteins That Associate with the Cell Surface in Response to Decreasing pH. MBio. 2014; 5: e01667-14.
- Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004; 38: 204–211.
- Matthews PC, Berendt AR, McNally MA, Byren I. Diagnosis and management of prosthetic joint infection. BMJ. 2009; 29: b1773.
- Myszka K, Czaczy K. Bacterial biofilms on food contact surfaces – a review. Polish Journal of Food and Nutrition Sciences. 2011; 61: 173–180.
- Naves P, Prado D, Huelves L, Cerrato VR, Ruiz V, et al. Effects of human serum albumin, ibuprofen and N-acetyl-l-cysteine against biofilm formation by pathogenic Escherichia coli strains. J Hospital Infect. 2010; 76: 165-170.
- Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008; 42: 541-564.
- Pavlica Z. Biofilm, Microbial communities and periodontal disease, World Congress World Small Animal Veterinary Association. 2006.
- Petrova OE, Sauer K. Sticky situations: key components that control bacterial surface attachment. Journal of Bacteriology. 2012; 194: 2413–2425.
- Puttamreddy S, Cornick NA, Minio FC. Genome-wide transposon mutagenesis reveals a role for pO157 genes in biofilm development in Escherichia coli O157:H7 EDL933. Infection and Immunity. 2010; 78: 23772384.
- Rasamiravaka T, Labtani Q, Duez P, El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Bio Med Res Int. 2015; 1–17.
- Rasmussen TB, Givskov M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol. 2006; 296: 149–161.
- Rao V, Ghei R, Chambers Y. Biofilms Research-Implications to Biosafety and Public health. 2005.
- Donlan RM. Biofilms: microbial life on surfaces. Emerging Infectious Diseases. 2002; 8: 881–890.
- Roy R, Tiwari M, Donelli G, Tiwari V. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018; 9: 522–554, 2018.
- Sekhar S, Kumar R, Chakraborti A. Role of biofilm formation in the persistent colonization of Haemophilus influenzae in children from northern India. J Med Microbiol. 2009; 58:1428-1432.
- Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiplephenotypes during development as a biofilm. Journal Of Bacteriology. 2009; 184: 1140–1154.
- Solano C, Echeverz M, Lasa I. Biofilm dispersion and quorum sensing. Curr Opin Microbiol. 2014; 18: 96–104.
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000; 69: 183–215.
- Sbordone L, Bortolaia C. Oral microbial biofilms and plaque-related diseases: microbial communities and their role in the shift from oral health to disease. Clin Oral Investig. 2003; 7: 181–188.
- Tasneem U, Yasin N, Nisa I, Faisal Shah, Ubaid Rasheed, et al. Biofilm producing bacteria: A serious threat to public health in developing countries. Journal of Food Science and Nutrition. 2018; 1: 25-31.
- Toyofuku MT, Inaba T, Kiyokawa N, Obana Y, Yawata, Nomura N. Environmental factors that shape biofilmformation, Bioscience, Biotechnology, and Biochemistry. 2016; 80: 7–12.
- Trampuz A, Zimmerli W. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly. 2005; 135: 243–251.
- Ward KH, Olson ME, Lam K, Costerton JW. Mechanism of persistent infection associated with peritoneal implant. J Med Microbiol. 1992; 36: 406-13.
- Zambori C, Tirziu E, Nichita I, Cumpanasoiu C, Gros R, et al. Biofilm Implication in Oral Diseases of Dogs and Cats. Anim Sci Biotechnol. 2012; 45: 208-212.
- Zaura E, Keijser B, Huse S, Crielaard W. Defining the healthy “core microbiome” of oralmicrobial communities. BMC Microbiol. 2009; 9: 259.
- Zhao X, Zhao F, Wang J, Zhong N. Biofilm formation and control strategiesof foodborne pathogens: food safety perspectives. RSC Advances. 2017; 7: 36670–36683.