
Research Article
Austin J Vet Sci & Anim Husb. 2015;2(1): 1004.
Subclinical Mycobacterium Infections in Wild Delta Smelt
Baxa DV, Javidmehr A, Mapes SM and Teh SJ
1Department of Anatomy, Physiology and Cell Biology, University of California, Davis, USA
2Department of Medicine and Epidemiology, University of California, Davis, USA
*Corresponding author: Baxa DV, Department of Anatomy, Physiology and Cell Biology, University of California, Davis, School of Veterinary Medicine, 1089 VM3B, USA
Received: September 30, 2014; Accepted: February 11, 2015; Published: February 13, 2015
Abstract
This study describes the occurrence and magnitude of latent mycobacterial infections and other pathogens in delta smelt (Hypomesus transpacificus), an endemic fish in the San Francisco Estuary (SFE). Our current knowledge on subclinical infections is characterized by the presence of mycobacterial DNA and absence of clinical signs and Mycobacterium. In our previous studies however, we showed that handling and increased water temperature, stress factors frequently encountered in captivity, have altered infections from benign to severe mycobacteriosis. In the current study, sub adult and adult smelt captured from August 2011 to May 2012 in the SFE were analyzed for presence of bacterial and viral agents (n=741) using standard isolation and culture techniques and Mycobacterium qPCR (n=237). Although mycobacteriosis, Mycobacterium, and virus were not observed in all of the fish examined, 96% of the smelt were positive for mycobacterial DNA. Higher pathogen scores and mycobacterial gene copy numbers were observed in smelt from anthropogenically impacted sites, isolated tributaries, and adult fish. Smelt captured in low salinity zone (1-6 practical salinity units, psu), the preferred habitat in summer and fall, had more infections (higher pathogen scores and mycobacterial gene copy numbers) compared to other salinity regions. Harboring mycobacterial DNA and bacterial pathogens are robust indicators of the cumulative effect of multiple stressors on delta smelt health and the ecological conditions of habitats in the SFE. These findings may provide a foundation in our current understanding of microbial infections in smelt and their potential contribution to health and conservation efforts of this threatened species.
Keywords: Delta smelt; Mycobacterium; San Francisco Estuary
Abbreviations
SFE: San Francisco Estuary; FMWT: Fall Midwater Trawl; SKT: Spring Kodiak Trawl; AHP: Aquatic Health Program; MTC: Mycobacterium tuberculosis complex
Introduction
The upper San Francisco Estuary (hereafter SFE) is the largest estuary in the U.S. Pacific Coast. The estuary provides habitat for ecologically and economically important fish species and is the largest source of municipal and agricultural water in California [1]. Multiple stressors, invasive species, and anthropogenic activities have been implicated in the degradation of the SFE, viewed as one of the most altered ecosystems in the world [2,3]. These factors have contributed to the decline of fish populations [3,4].
Although the cumulative impact of various stressors has been the major hypothesis explaining fish population declines [2-4], the contribution of pathogens and diseases is one potential yet an understudied factor that may affect pelagic fishes in the SFE. The Delta smelt, Hypomesus transpacificus, endemic to the Sacramento San Joaquin Delta [5], is currently protected under the federal and state Endangered Species Acts [6]. Extensive occurrence of latent mycobacterial infections (non-lethal DNA carrier) has been reported in wild smelt populations [7]. Furthermore, recent findings in our laboratory showed that although Mycobacterium and the disease, mycobacteriosis, have not been observed among smelt captured in their natural habitats in the SFE, smelt harboring Mycobacterium DNA are predominant [8a-8e]. In captivity, infections can progress from dormant to disease when wild cohorts are subjected to intensive culture conditions and increased water temperature (≥16°C) [8].
Mycobacteriosis affects a wide range of freshwater and marine aquatic organisms [9,10] to the extent that all fish species are vulnerable to the disease [11]. Epizootics of mycobacteriosis have impacted many resident fish species in the Chesapeake Bay [12,13], an estuarine ecosystem altered by anthropogenic inputs as the SFE. The purpose of this study is to evaluate the nature and extent of subclinical mycobacterial infections and other bacterial and viral pathogens in delta smelt within the SFE and its tributaries. In cooperation with ongoing fish monitoring surveys, subadult and adult delta smelt were captured across salinity regions and habitats and analyzed by sampling months, location, and salinity zones to determine for potential association of these factors with infection occurrence and severity.
Methods
Fish sampling and processing
This study is part of an ongoing project with a broader goal of evaluating the health of delta smelt inhabiting the low salinity zone (1-6 psu, practical salinity units) of the SFE. In collaboration with the California Department of Fish and Wildlife (CDFW), smelt were collected in 2011-2012 from ongoing surveys: the Fall Mid Water Trawl (FMWT) in September to December and the Spring Kodiak Trawl (SKT) from January to May. The surveys collect fish annually across sites in the SFE to determine the abundance and distribution of delta smelt and other pelagic species (FMWT) and to follow movements of reproductively mature smelt (SKT). Smelt were collected once or twice a month according to CDFW monitoring schedules across discreet locations in the upper SFE (Figure 1). This study used water temperature and salinity measured at 1 m below the water surface using YSI 6600 (YSI Inc.). Fish captured from each site were counted, assigned individual ID numbers, flash-frozen in liquid nitrogen, and brought to the Aquatic Health Program (AHP) laboratory at UC Davis for analysis.
Validation of bacterial and viral isolation using nitrogen flash frozen tissues
A validation test was conducted to ensure the diagnostic accuracy of bacterial isolation in tissues of fish flash frozen in nitrogen after field collections. Flash freezing in liquid nitrogen and then transporting to the lab for dissection was the only practical option to maintain the integrity of the tissues for various analyses where disease was an integral component. For the validation test, laboratory grown smelt were used to determine if bacteria in kidney and spleen were viable after flash freezing whole fish in liquid nitrogen. Standard methods require fresh tissues for bacterial and viral isolation [14], however under extenuating circumstances, freezing samples are useful for pathogen isolation [15,16]. Common bacterial fish pathogens remained viable at 20-60 days post freezing [17]. The World Health Organization [18] suggests preserving clinical specimens in liquid nitrogen or in -20 or -70°C for viral analysis.
In the validation test, parallel tissues (pooled spleen and kidney) of smelt (n=10) that were fresh or nitrogen frozen for 7 days, the average number of days for storing fish samples in liquid nitrogen prior to necropsy, were compared for bacterial isolation. Fresh samples were processed as follows: kidney and spleen were dissected from each smelt, pooled, suspended in 350 μl Minimum Essential Medium Eagle (MEM, Sigma Aldrich), and homogenized for 1 min. After thorough mixing, a 50 μl homogenate was inoculated onto each of the three media as indicated below. The remaining homogenate from each pooled tissue were stored in liquid nitrogen for 7 days, referred to as the frozen samples. Prior to inoculation, samples were briefly thawed on ice at room temperature, and inoculated (50 μl) onto each medium. Results showed that the types and number of bacterial colonies, and overall pathogen scores were similar between fresh and 7-day post frozen tissues after incubation at 15°C for 7 days (Table 1), suggesting that flash freezing is an acceptable method for preserving field samples of delta smelt. Among 4 of 10 fish that showed colonies, the overall pathogen scores were similar in fresh and frozen tissues (Table 1).
Fish number
Blood Agar General isolation
Tryptone Yeast Extract (TYES) Flexibacteria
MiddleBrook 7H10 Mycobacteria
Overall Pathogen Score
Fresh
Frozen
Fresh
Frozen
Fresh
Frozen
Fresh
Frozen
1
1 *
1
3
2
0
0
1
1
2
0
0
1
1
0
0
1
1
3
0
0
0
0
0
0
0
0
4
0
0
0
0
0
0
0
0
5
0
0
0
0
0
0
0
0
6
0
0
0
0
0
0
0
0
7
0
0
2
2
0
0
1
1
8
0
0
0
0
0
0
0
0
9
0
0
2
2
0
0
1
1
10
0
0
0
0
0
0
0
0
*Number of colonies/50 ml of tissue homogenate. Bacterial types are similar in fresh or frozen tissues. Aeromonas sp. was isolated on Blood Agar while flexibacteria were recovered on TYES. Frozen tissue homogenate was stored in liquid nitrogen for 7 days.
Pathogen Score: 0=absence of bacterial growth; 1=minimal number of colonies (N=1-10); 2=moderate number of colonies (N=11-20); 3=high number of colonies (>21-200); 4=colonies are too numerous to be counted (>201). Pathogen scores were assigned based on the range of colony numbers that grew on plates.
Table 1: Number of bacterial colonies and overall pathogen score from fresh or frozen tissue (kidney + spleen) of delta smelt.
Bacterial and viral isolation from wild smelt
A total of 741 delta smelt collected between 2011 and 2012 across sites in the SFE were used for pathogen isolation (Table 2, Figure 1). Following necropsy of each fish, the kidney and spleen pool was placed in sterile tubes containing 2 ml MEM and homogenized for 1 min. Subsamples of the homogenate were inoculated (ca. 20 μl/ plate) onto three media: Blood agar for general bacterial isolation, Tryptone Yeast Extract Salt (TYES) for fastidious flexibacteria, and Middlebrook 7H10 agar (Difco) for mycobacteria. The plates were incubated at 15°C and examined for bacterial growth at 7 days post inoculation. Bacterial isolation followed the procedures of the AFS-Fish Health Section [14]. The severity of anomaly was assessed according to Goede and Barton [19] where values were assigned (0-4) based on the number and identity of colonies that grew on plates, referred to as "pathogen score": 0=absence of bacterial growth, 1=minimal number of colonies (N=1-10), 2=moderate number of colonies (N=11-20), 3=high number of colonies (21-200), and 4=colonies are too numerous to be counted (>201). Pathogen scores were assigned based on these range of colony numbers that grew on plates; known fish pathogens (e.g. Flavobacterium) were assigned higher scores.
Survey/Month
No. fish for Mycobacterium qPCR
No. fish with Mycobacterium DNA
No. fish for bacterial isolation
No. fish with bacteria**
FMWT 2011 - Subadult
August
4
4
4
4
September
17
17
43
14
October
14
13
44
0
November
22
20
41
30
December
27
26
122
87
Total in FMWT
84
80 (95%)
254
135 (53%)
SKT 2012 - Adult
January
63
59
151
105
February
27
26
105
76
March
26
26
95
41
April
30
30
108
79
May
7
7
28
13
Total in SKT
153
148 (97%)
487
314 (64%)
Total number of samples examined or % infected
237
96%
741
61%
* FMWT=Fall Midwater Trawl; SKT=Spring Kodiak Trawl
** Bacteria were isolated from spleen and kidney pool/fish by culture on plate media and identified by phenotypic characterization and representative isolates by 16S rDNA sequencing (see supplemental data).
Table 2: Number of delta smelt examined and proportion of infected smelt (Mycobacterium DNA or growth of other bacteria) from the San Francisco Estuary.
After bacterial inoculation, the supernatant from the remaining homogenate from each of the 741 fish was used for viral isolation by tissue culture following standard methods [14]. Cell lines including EPC (Epithelioma papulosum Cyprinid carp), CHSE-214 (Chinook Salmon Embryo), and BF2 (Bluegill Fry) were inoculated with each supernatant sample. In addition, specific cell lines that we developed from delta smelt gill and threadfin shad gill following standard methods [14] were also used for virus isolation. Cell cultures were incubated at 15°C for a minimum of 21 days and sub cultured for 14 days.
Identification of bacterial isolates and pathogen scores
Colonies on Blood agar, TYES, and MiddleBrook 7H10 agar were examined for basic morphologic and biochemical characteristics [14]. Dominant bacterial isolates were identified presumptively by phenotypic characterization and verified using 16S ribosomal RNA (rRNA) gene sequencing [20]. The universal bacterial primers EUBA/ EUBB were used for initial PCR to amplify the 16S ribosomal DNA (rDNA) regions. The amplified products were submitted to Davis Sequencing Inc. (https://www.davissequencing.com/index.htm) to determine the DNA sequences of representative isolates and then compared to known sequences in the GenBank database. BLAST search results are provided as supplementary data.
Mycobacterium qPCR
Frozen tissues are routinely used for standard qPCR analysis of fish pathogens [21] hence were not validated in this study. For qPCR analysis, fish (n=237) were randomly selected to represent a sample population of 741 fish (Table 2) using Excel 2010 simple random sampling method. DNA was extracted from the remaining supernatant (virus inoculum) plus the pellets from the kidney and spleen homogenate (bacterial inoculum) using the DNeasy blood and tissue kit (Qiagen). The DNA samples were tested by qPCR for Mycobacterium tuberculosis complex (MTC) using a proprietary assay available at the UC Davis Real-time PCR Research and Diagnostic Core Facility (https://www.vetmed.ucdavis.edu/vme/taqmanservice/). The assay was designed on the conserved region of the rpoB gene based on multiple alignment generated from a previous study (GenBank accession number: GQ293224) [22,23]. The rpoB gene encodes the beta subunit of RNA polymerase, well conserved within the Mycobacterium genus [22]. The assay used a hydrolysis probe with 5 prime FAM and 3 prime MGB (Life Technologies). A synthesized DNA plasmid containing a portion of 16S rRNA of Mycobacterium marinum, the species commonly found in fish, was used to establish standard curves for estimating the number of Mycobacterium gene copy numbers present in smelt tissues. The MTC qPCR detects the DNA of several Mycobacterium species as causative agents of human and animal tuberculosis and has been successfully used for evaluating mycobacterial infections in delta smelt [8a-8e,24].
Statistical analysis
Smelt collected across locations from August 2011 to May 2012 were grouped by sampling months to evaluate seasonal trends and by salinity gradient (<1, 1-6, >6 psu) to test for associations between presence of infections (mycobacterial DNA and other pathogens) and respective salinity zones. To determine if smelt from certain habitats differed in infection severity, collection sites were clustered into five distinct geographic groups based on the following habitat characteristics: close to anthropogenic inputs, upstream for spawning and migration of adult smelt, and isolated tributaries (Table 3, Figure 1). One way analysis of variance (ANOVA) followed by an all pairs Tukey's test was used for the various analyses: mean number of mycobacterial gene copy numbers and pathogen scores among fish across sampling months, habitats, and three salinity regions. All data handling and analyses were performed using Microsoft Excel 2010 and JMP®Pro11.
Results
The prevalence of smelt harboring Mycobacterium DNA was high in both subadults (95%) and adults (97%) (Table 2). Mycobacterium gene copy numbers varied each month from August to May (Figure 2A). Although gene copy numbers were relatively higher among samples in September, they were not statistically different across smelt captured between August to December (P>0.05); (Figure 2A). Gene copy numbers were relatively lower among samples examined from January to March (8-12°C), however fish caught from April to May (16-22°C) showed a marked increase in gene copy numbers (Figure 2A). Stress associated with upstream migration and increased water temperature may be contributing to significantly higher gene copy numbers among smelt examined in April compared to January (Figure 2A), (P=0.0415).
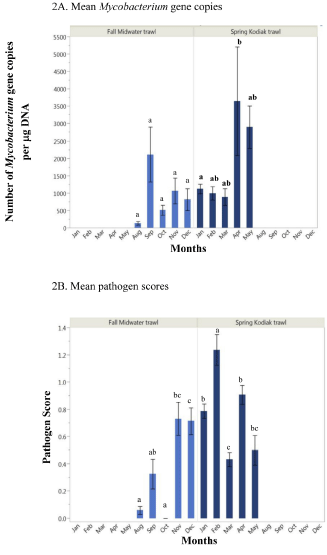
Figure 2: Mean Mycobacterium gene copy numbers (A) and pathogen scores (B) of subadult (Fall Midwater Trawl) and adult (Spring Kodiak Trawl) delta smelt collected across months in the San Francisco Estuary. Mean with the same letters are not statistically significant.
Overall, the frequency of smelt harboring bacterial pathogens was lower in FMWT subadults (53%) compared to SKT adults (64%); (Table 2). Although pathogen scores varied among fish across months, smelt harbored fewer pathogens beginning in August with no pathogens from smelt in October (Figure 2B). In contrast, higher pathogen scores were observed among fish in November and December (Figure 2B) where the pathogen scores were significantly higher than August (P=0.0002). The highest mean pathogen score (1.23) was observed in February; this average pathogen score was significantly higher compared to January (0.78), March (0.43), April (0.90), and May (0.50) (P<0.0001), (Figure 2B). Pathogen scores were significantly lower in March compared to January (P=0.004) and April (P=0.0001). An inverse relationship was observed between pathogen score and mycobacterial gene copy number across sampling months that varied in water temperature. For example, pathogen scores were generally higher among smelt sampled in cooler months (November- March at 8-16°C) while in warmer months such as May (>16°C), smelt showed lower pathogen scores (Figure 2B) and increased gene copy numbers (Figure 2A).
The pathogen scores were based on the number and identity of bacterial flora found in the kidney and spleen of delta smelt. Aeromonas, Pseudomonas, Microbacterium, Sphingopyxis, Zooglea, and Bacillus that grew on Blood agar plates and Flavobacterium on TYES were identified by phenotypic characterization. Sequencing of 16S rDNA confirmed the identity of most of these species including Methylibium, Brevundimonas, and Luteimonas (see supplemental data). These organisms are commonly found in the aquatic environment and some species of Flavobacterium are pathogenic in fish. From all of the 741 smelt examined, viral agents (cytopathic effect) were not observed from any of the cell lines inoculated.
Acid-fast colonies consistent with mycobacteria did not grow on Middle Brook 7H10 agar. However, 96% of the fish showed mycobacterial DNA and across sampling sites, higher mycobacterial gene copy numbers were observed among smelt captured near anthropogenically altered naval shipyards, among migrating adults, and among fish sampled from isolated tributaries (Table 3).
Across salinity regions (<1, 1-6, and >6 psu), results showed that mean Mycobacterium gene copy numbers did not differ statistically among FMWT subadults (P=0.477) or SKT adults (P=0.077); (Figure 3A). Pathogen scores of subadult smelt varied across salinity regions while adult collected at 1-6 psu showed significantly higher mean pathogen scores (1.2) compared to fish at <1 psu (0.64, P<0.0001) and >6 psu (0.74, P=0.0004) (Figure 3B).
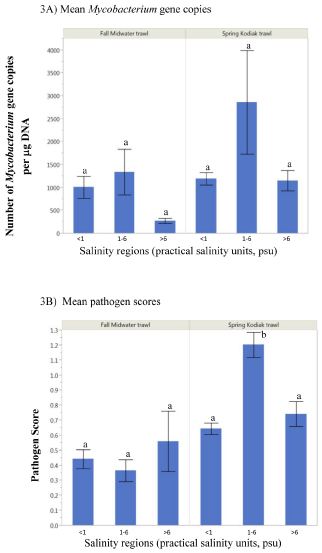
Figure 3: Mean Mycobacterium gene copy numbers (A) and pathogen scores (B) of subadult (Fall Midwater Trawl) and adult (Spring Kodiak Trawl) delta smelt collected across salinity regions. Mean with the same letters are not statistically significant.
Discussion
Our findings demonstrate that latent mycobacterial infections are widespread among delta smelt collected across sampling months and locations in the SFE. Smelt captured near anthropogenically impacted sites such as the US Naval weapons station at Concord and the US Naval shipyard at Suisun Bay, California showed relatively higher mycobacterial gene copy numbers. The latter is a potential point source of contaminants corroding from the ships [25]. Exposure of delta smelt to contaminated waters in these habitats could enhance their vulnerability to environmental mycobacteria, as fish exposed to polluted environments are generally more susceptible to infections [26-29]. While relatively higher mycobacterial gene copy numbers were also observed among fish captured in isolated tributaries (i.e. sites 606, 609, 610, Figure 1), it is unknown if gene copy numbers were associated with mycobacterial community habitat niches [30] or with the physicochemical attributes of the habitats. Organic content and pH can affect the growth of environmental bacteria [31]. In the SFE, the magnitude and range of biotic and abiotic factors and contaminants [32, 33] could contribute in the vulnerability of smelt to pathogen exposures in the environment. As frequency and severity of infections may depend on ecosystem health [34], the degraded state of the SFE ecosystem has been generally associated with overall stress effect on resident fishes.
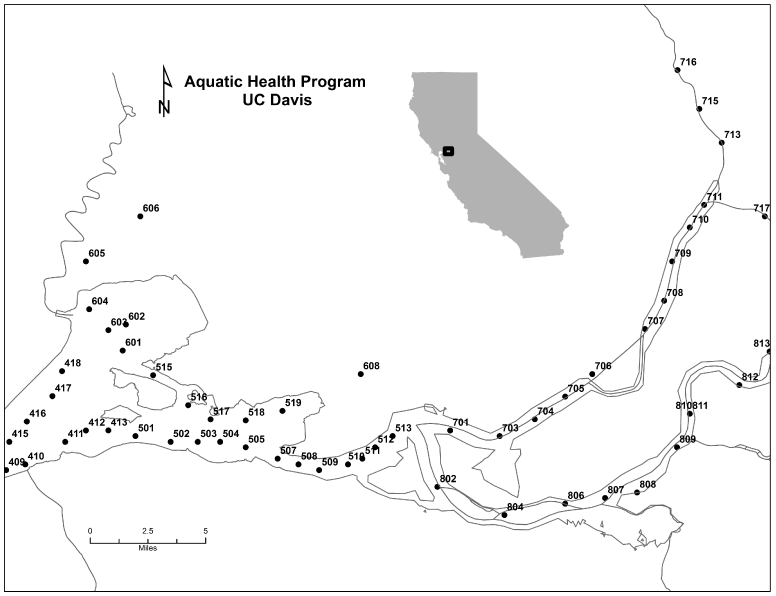
Figure 1: Upper San Francisco Estuary: Sampling locations for delta smelt used in the current study as monitored by the California Department of Fish and Wildlife.
The SKT survey captures smelt migrating upstream or developing into fully mature adults in their natural spawning grounds in the SFE [4, 6]. Smelt examined from SKT showed relatively higher mycobacterial gene copy numbers and pathogen scores compared to subadults from the FMWT survey. Furthermore, adult smelt collected in February showed the highest pathogen scores from among all of the samples examined across months and life stages in this study (Figure 2B). While it is unknown if the bacterial species that were isolated are harmful to delta smelt, it is important to note that susceptibility to bacterial exposures may be amplified at critical stages of smelt maturity as shown in our results. In addition to various stressors, exposure to pathogens may also affect migration and spawning of smelt in the SFE. Although Mycobacterium was not isolated from any of the delta smelt (n=741) that we examined, 96% of the fish were positive for mycobacterial DNA. Our results corroborate previous findings in smelt from the lower Sacramento River where Mycobacterium DNA was detected in 54% of the fish despite the lack of pathogen isolation [7]. As Mycobacterium is ubiquitous [9-11,35], it is highly likely the smelt contracted the mycobacteria in the water column through ingestion of contaminated food and contact with other infected fishes [35].
Delta smelt captured from the 1-6 psu habitats showed significantly higher pathogen scores and relatively increased mycobacterial gene copy numbers than other salinity regions. As smelt residency is centered in the low salinity zone (ca. 2 psu isohaline) in the summer and fall [4,36], smelt distribution in summer may coincide with enhanced exposures to environmental pathogens through warm temperature effects on pathogen fitness in a weakened host [37]. However, the relationship between infection prevalence and salinity, including potential interaction with water temperature, need to be evaluated within a controlled environment to support this hypothesis. We have shown in captive smelt that water temperature (=16°C) altered infections with Mycobacterium from benign to lethal [8]. The current study also demonstrated higher mycobacterial gene copy numbers among smelt captured at >16°C suggesting that water temperature can regulate the severity of subclinical mycobacterial infections of delta smelt in their natural habitat in the SFE. Although water temperature may exceed 16°C in the estuary during summer, smelt may move to other sites and therefore not subjected continuously to high water temperature that may support clinical infections with Mycobacterium as those observed in controlled laboratory conditions [8]. Alternatively, exposure of smelt to high water temperatures in the SFE may cause clinical infections with Mycobacterium, however, we are only able to collect relatively healthy fish as infected fish may have been dead or eaten by predators. For this reason, investigating the factors that trigger the active replication of Mycobacterium in field populations of smelt is critically important. The role of physico- chemical stressors in promoting infections in the field is intricately tied to complex interactions governing the host, the pathogen, and the environment [26-29,34]. Other critical factors that affect disease onset and severity in fish are the biology of the pathogen in the host, the host response, and the epidemiology of the causative organism [37]; these have yet to be assessed in delta smelt.
Mycobacterial infections have occurred among fish species with direct or indirect origin in the SFE. Subclinical infections with Mycobacterium were first reported three decades ago in wild adult striped bass in the Sacramento and San Joaquin rivers [38]. Chronic mortalities due to M. marinum were subsequently observed in cultured yearling striped bass that were progeny of striped bass from the SFE [39]. In 2000, we observed the first case of mycobacteriosis and isolation of M. chelonae and Mycobacterium sp. in wild delta smelt following a one month holding at 16°C-well water [8]. Chronic mortalities have occurred in smelt culture facilities including acute infections in reconditioned broodstock [24]. As demonstrated in these studies, mycobacterial infections may be latent in wild or cultured smelt populations but the presence of stressors may trigger infections from subclinical to severe infections. To date, M. marinum, M. fortuitum, and M. chelonae have been isolated from clinically infected delta smelt [8,24].
Our concept of latent mycobacterial infections in delta smelt draws certain similarities with other species. Latent or chronic tuberculosis in humans is a state where the agent (M. tuberculosis) persists in affected individuals without causing any symptomatic disease [40,41]. Latently infected individuals persistently harbor M. tuberculosis DNA in their lungs [42]. In mice models of latent Mycobacterium infections, tubercle bacilli were lacking but immune suppression using steroid induced active pathogen replication and overt infections [43]. In zebra fish, latent mycobacterial infections were experimentally established and reactivated by irradiation to demonstrate the mechanisms associated with subclinical and clinical mycobacterial infections [44]. Latent mycobacterial infections in delta smelt is characterized by the absence of pathogen but presence of mycobacterial DNA [7,8a-8e] and infections may progress from subclinical to clinical in the presence of stressors such as water temperature and handling [8]. We hypothesize that the immune system of the smelt could be compromised due to the immunosuppressive effects of the stressors that rendered activation to disease as observed in the zebra fish model [44]. Also unknown in delta smelt is whether the phenomenon (pathogen negative but DNA positive) is tied to regulation of certain genes associated with the pathogen or the host. In humans, latent tuberculosis is linked with gene regulation and metabolic pathways of the etiologic agent [40] including the complex association of mycobacterial niches and metabolic states [44]. Assessing the mechanisms and pathogenesis of latency that prevents or allows progression to mycobacteriosis in the annual life cycle of the delta smelt [6,45] will be challenging to study.
Pathogens and diseases are one of the least studied stressors affecting threatened species in the SFE. It is important to note that although mixed bacteria were isolated from delta smelt, these may have originated from ubiquitous bacteria that are normally present in wild fish. For this reason, the recovered bacteria may not be pathogenic in delta smelt. Pathogens are natural components of ecosystems such that pathogens and hosts may evolve in response to each other and to conditions in the ecosystem [46]. The current study encompassed non-lethal, subclinical mycobacterial infections among a significant number of smelt at different stages of development. The potential risk of harboring subclinical mycobacterial infections in smelt is currently unknown. Based on initial observations in our laboratory, we have detected mycobacterial gene copy numbers ranging from 5,800-439,000/μg DNA in tissues of smelt that succumbed to mycobacteriosis in captivity. The implication of the range in gene copy numbers (Table 3) in latent infections that currently predominate in smelt populations in the SFE compared to clinical infections [8,24] remain to be determined. Whether the ranges of mycobacterial gene copy numbers in wild smelt are harmful will be investigated.
Habitat
Sampling sites
Number of fish
Mean Mycobacterium gene copy numbers/mg DNA**
Subadult - FMWT *
A - US Navy weapons station, Concord
405, 407, 411, 412, 413,502, 503, 507, 509, 510,512
21
842.14
B - isolated tributaries
606, 609
8
1113.26
C - migratory region
703,704,705,706, 802, 807
28
1035.98
D - upstream
716, 719, 721, 797
10
643.84
E - US Navy Shipyard, Suisun
418, 516, 517, 518, 519, 601, 602
16
1638.26
Adult - SKT*
A - US Navy weapons station, Concord
501, 504, 508, 513
16
1333.65
B - isolated tributaries
606, 609, 610
30
3474.95
C - migratory region
704, 706, 707, 801, 804
34
838.34
D - upstream
713, 715, 716, 719
47
1388.94
E - US Navy Shipyard, Suisun
340, 411, 418, 519, 520, 602
21
1250.56
* FMWT=Fall Midwater Trawl survey; SKT=Spring Kodiak Trawl survey
** Kidney + spleen DNA were used for qPCR assays
Table 3: Comparison of Mycobacterium gene copy numbers in delta smelt collected from various locations in the San Francisco Estuary.
Although the underlying drivers or reservoirs of the mycobacterial infections in the current study are unknown, disease prevalence and severity in wild fish represent an intricate interaction among pathogens, hosts, and the environment of which the latter is the least understood [34]. Exposure of delta smelt to mycobacteria across habitats in the SFE is highly likely because of the ubiquity of the pathogen [9-11,35] combined with the smelt's extreme vulnerability to local environmental calamity [6]. As Mycobacterium is an opportunistic organism [35], it may only cause disease in smelt under certain environmental conditions as shown in our previous study [8]. It is important to consider that infected smelt may have died in the wild or been eaten by predators, and the fish that are captured and analyzed are relatively "healthier" fish. This potential scenario may fundamentally explain, in part, the subclinical nature of the mycobacterial infection, absence of viral agents, and lack of overt clinical infections. Mycobacteriosis impaired the swimming performance of delta smelt held in captivity [47]. In this context, debilitated movements associated with mycobacterial and other microbial infections may likely predispose the smelt to predation in the SFE precluding collection and analysis of infected fish.
Conclusion and Future Direction
This study improves our current knowledge regarding the prevalence of smelt harboring infections in anthropogenically impacted sites, and across sampling months and salinity regions in the SFE. Impacts of infections on wild fish populations have been perceived as insignificant to catastrophic [34] as stressor effects are expectedly more severe in captive environments [48]. Although the risks of harboring subclinical infections are currently unknown in wild delta smelt, documenting spatial and temporal variations of infection severity will provide the essential foundation for information critical for uncovering potential stressors that may trigger the activation of the subclinical state to full blown mycobacteriosis in the SFE. In the Chesapeake Bay, mycobacterial epizootics in various fish species were viewed as a syndromic sentinel of altered environmental conditions [13], providing highly relevant implication on fish health in the SFE. Data analyses of various health indicators (e.g. histopathology, condition indices, nutritional status, enzymatic and reproductive biomarkers) and water quality from the fish health project will be evaluated for potential sublethal effects (e.g. growth and reproduction) of latent mycobacterial infections in delta smelt. Our current results may lend support to management and conservation efforts associated with emerging infections for this threatened species including potential relationship with broader effects on critical habitats in the SFE altered by anthropogenic impacts.
Acknowledgment
The Ecosystem Restoration Program Grant #E118304 and US Geological Survey Grant #G12AC20079 provided funding for this study. The State of California, Department of Fish and Wildlife (CDFW), National Marine Fisheries Service, US Fish and Wildlife Service, and US Bureau of Reclamation are also acknowledged. We thank our collaborators at CDFW: Randy Baxter and Steve Slater for their logistic support on delta smelt collections and Carol Atkins for grant management. AHP personnel are greatly appreciated: Georgia Ramos for DNA extraction of smelt tissues, Michael Park for plasmid DNA extraction, Gary Wu and Ching Teh for archiving samples, Andrew Petersen and Christopher Tai for field sampling, and Susan Yun at SVM/EPM for virus isolation. Our colleagues Bruce G. Hammock, Tomofumi Kurobe, and Scott Foott, including one anonymous reviewer, are greatly appreciated for providing critical reviews of the manuscript.
References
- Service RF. Environmental restoration. Delta blues, California style. Science. 2007; 317: 442-445.
- Glibert PM. Long-term changes in nutrient loading and stoichiometry and their relationships with changes in the food web and dominant pelagic fish species in the San Francisco Estuary, California. Rev Fish Sci. 2010; 18: 211-232.
- Mount J, Bennett W, Durand J, Fleenor W, Hanak E, Lund J, et al. Aquatic ecosystem stressors in the Sacramento-San Joaquin Delta Public Policy Institute of California. 2012.
- Sommer TR, Armor C, Baxter R, Breuer R, Brown L, Chotkowski M, et al. The collapse of pelagic fishes in the upper San Franciso Estuary. Fisheries. 2007; 32: 270-277.
- Moyle PB. Inland Fishes of California. Berkeley: University of California Press. 2002.
- Bennett WA. Critical assessment of the delta smelt population in the San Francisco Estuary, California. San Francisco Estuary Watershed Sci. 2005; 3: 1-73.
- Foott SJ, Bigelow J. Pathogen survey, gill NaK ATPase activity and leucocyte profile of adult delta smelt. Calif Fish Game. 2010; 96: 223-231.
- Antonio-Baxa D, Swanson C, Cech J, Doroshov S, Hedrick RP. Prevalence of Mycobacterium spp. in delta smelt Hypomesus transpacificus in Sacramento-San Joaquin estuary in California. Calif Fish Game. 2000; 86: 233-243.
- Teh S, Baxa DV, Acu&nTilde;a S, Kurobe T, Javidmehr A, Krithika S. Quarterly Report: Fall low salinity habitat (FLaSH) fish health study: Contrasts in health indices, growth and reproductive fitness of delta smelt and other pelagic fishes rearing in the low salinity zone and Cache Slough regions. Science Division, Bay-Delta Office, U.S. Bureau of Reclamation, Sacramento, CA. 2013; 1-96.
- Teh S, Baxa DV, Javidmehr A, Krithika S, Ramos G, Wu G. Progress Report: Contrasts in health indices of delta smelt reared in the low salinity zone and Cache Slough regions in Summer 2012-2013 (Summer townet delta smelt health study). 2014; 1-25.
- Teh S, Baxa DV, Javidmehr A, Kurobe T, Krithika S. Annual Report 2013: Fall low salinity habitat (FLaSH) fish health study: Contrasts in health indices, growth, and reproductive fitness of delta smelt and other pelagic fishes rearing in the low salinity zone and Cache Slough regions. 2014; 1-105.
- Teh S, Baxa DV, Javidmehr A, Kurobe T, Krithika S. Progress Report: Studies assessing factors that influence the spawning migration behavior and reproductive condition of delta smelt (Hypomesus transpacificus) in the Sacramento-San Joaquin Delta. Science Division, Bay-Delta Office, U.S. Bureau of Reclamation, Sacramento, CA. 2014; 1-32.
- Teh S, Baxa DV, Javidmehr A, Kurobe T, Krithika S. Annual Progress Report: Studies assessing factors that influence the spawning migration behavior and reproductive condition of delta smelt (Hypomesus transpacificus) in the Sacramento-San Joaquin Delta. Science Division, Bay-Delta Office, U.S. Bureau of Reclamation, Sacramento, CA. 2014; 1-46.
- Nigrelli R, Vogel H. Spontaneous tuberculosis in fishes and in other cold-blooded vertebrates with special reference to Mycobacterium fortuitum Cruz from fish and human lesions. Zool. 1963; 48: 131-144.
- Frerichs GN. Mycobacteriosis: Nocardiosis. Inglis V, Roberts RJ, Bromage NR, editors. In: Bacterial Diseases of Fish. Blackwell Scientific Publications. 1993; 219-235.
- Bruno DW, Griffiths J, Mitchell CG, Wood BP, Fletcher ZJ, Drobniewski FA, et al. Pathology attributed to Mycobacterium chelonae infection among farmed and laboratory-infected Atlantic salmon Salmo salar. Dis Aquat Org. 1998; 33: 101-109.
- Rhodes MW, Kator H, Kotob S, van Berkum P, Kaattari I, Vogelbein W, et al. A unique Mycobacterium species isolated from an epizootic of striped bass (Morone saxatilis). Emerg Infect Dis. 2001; 7: 896-899.
- Kane AS, Stine CB, Hungerford L, Matsche M, Driscoll C, Baya AM. Mycobacteria as environmental portent in Chesapeake Bay fish species. Emerg Infect Dis. 2007; 13: 329-331.
- American Fisheries Society? Fish Health Section. FHS Blue Book: Suggested Procedures for the Detection and Identification of Certain Finfish and Shellfish Pathogens. 2007 edn. FHS, Bethesda, Maryland. 2007.
- Noga EJ. Fish disease diagnosis and treatment. Missouri: Mosby-Yearbook, Inc. 1996.
- Klinger RE, Francis-Floyd R. Submission of fish for diagnostic evaluation. 2013.
- Brady YJ, Vinitnantharat S. Viability of bacterial pathogens in frozen fish. J Aquat Anim Health. 1990; 2: 149-150.
- World Health Organization. Guidelines for the collection of clinical specimens during field investigation of outbreaks. 2000.
- Goede RW, Barton BA. Organismic indices and an autopsy-based assessment as indicators of health and condition of fish. Adams SM, editor. In: Biological Indicators of Stress in Fish. American Symposium 8, American Fisheries Society, Maryland. 1990; 8: 93-108.
- Cunningham CO. Molecular diagnosis of fish and shellfish diseases: present status and potential use in disease control. Aquaculture. 2002; 206: 19-55.
- Purcell MK, Getchell RG, McClure CA, Garver KA. Quantitative polymerase chain reaction (PCR) for detection of aquatic animal pathogens in a diagnostic laboratory setting, J Aquat Anim Health. 2011; 23: 148-161.
- Miller LP, Crawford JT, Shinnick TM. The rpoB gene of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1994; 38: 805-811.
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-speci?c gap penalties and weight matrix choice. Nucleic Acids Res. 1994; 22: 4673-4680.
- Bolick A, True K. US Fish and Wildlife Service. 2011.
- National Oceanic and Atmospheric Administration (NOAA), Office of Response and Restoration. Assessment of environmental contaminants associated with the National Defense Reserve Fleet in Suisun Bay, California. 2009.
- Lafferty KD, Holt RD. How should environmental stress affect the population dynamics of disease? Ecol Lett. 2003; 6: 654-664.
- Marcogliese DJ. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev Sci Tech. 2008; 27: 467-484.
- Harvell D, Altizer S, Cattadori IM, Harrington L, Weil E. Climate change and wildlife diseases: when does the host matter the most? Ecology. 2009; 90: 912-920.
- Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009; 90: 888-900.
- Parikka 1, Hammarén MM, Harjula SK, Halfpenny NJ, Oksanen KE, Lahtinen MJ, et al. Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathog. 2012; 8: e1002944.
- Falkinham JO, Nichols G, Bartram J, Dufour A, Portaels F. Natural ecology and survival in water of mycobacteria of potential public health significance. Bartram J, Cotruvo JA, Dufour A, Rees G,Pedley S, editors. In: Pathogenic Mycobacteria in Water: A Guide to Public Health Consequences, Monitoring and Management. London: IWA Publishing. 2004; 15-25.
- Thompson B, Adelsbach T, Brown C, Hunt J, Kuwabara J, Neale J, et al. Biological effects of anthropogenic contaminants in the San Francisco Estuary. Environ Res. 2007; 105: 156-174.
- Kuivila KM, Hladik M. Understanding the occurrence and transport of current-use pesticides in the San Francisco Estuary Watershed. San Francisco Estuar Watershed Science. 2008; 6.
- Hedrick RP. Relationships of the host, pathogen and environment: implications for diseases of cultured and wild fish populations. J Aquat Anim Health. 1998; 10: 107-111.
- Belas R, Faloon P, Hannaford A. Potential applications of molecular biology to the study of fish mycobacteriosis. Annu Rev Fish Dis. 1995; 5: 133-173.
- Kimmerer WL, Gross ES, MacWilliams ML. Is the response of estuarine nekton to freshwater flow in the San Francisco Estuary explained by variation in habitat volume? Estuar Coast. 2009; 32: 375-389.
- Karvonen A, Rintamäki P, Jokela J, Valtonen ET. Increasing water temperature and disease risks in aquatic systems: climate change increases the risk of some, but not all, diseases. Int J Parasitol. 2010; 40: 1483-1488.
- Sakanari JA, Reilly CA, Moser M. Tubercular lesions in Pacific coast populations of striped bass. Trans Amer Fish Soc. 1983; 112: 565-566.
- Hedrick RP, McDowell T, Groff J. Mycobacteriosis in cultured striped bass from California. J Wildl Dis. 1987; 23: 391-395.
- Höner zu Bentrup K, Russell DG. Mycobacterial persistence: adaptation to a changing environment. Trends Microbiol. 2001; 9: 597-605.
- Manabe YC, Bishai WR. Latent Mycobacterium tuberculosis-persistence, patience, and winning by waiting. Nat Med. 2000; 6: 1327-1329.
- Hernandez-Pando R, Jeyanathan M, Mengistu G, Aguilar D, Orozco H, Harboe M, et al. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet. 2000; 356: 2133-2138.
- Parrish NM, Dick JD, Bishai WR. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998; 6: 107-112.
- Parikka M, Hammarén MM, Harjula SK, Halfpenny NJ, Oksanen KE, Lahtinen MJ, et al. Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathog. 2012; 8: e1002944.
- Moyle PB, Herbold B, Stevens DE, Miller LW. Life history of delta smelt in the Sacramento-San Joaquin Estuary, California. Trans Am Fish Soc. 1992; 121: 67-77.
- Riley SC, Munkittrick KR, Evans AN, Krueger CC. Understanding the ecology of disease in Great Lakes fish populations. Aquat Ecosyst Health. 2008; 11: 321-334.
- Swanson C, Baxa DV, Young PS, Cech JJ, Hedrick RP. Reduced swimming performance in delta smelt infected with Mycobacterium spp. J Fish Biol. 2002; 61: 1012-1020.
- Wedemeyer GA, Meyer FP, Smith L. Environmental Stress and Fish Diseases. Snieszko SF, Axelrod HR, editors. In: Diseases of Fishes, TFH Publications, New Jersey. 1976; 73-79.