
Special Article - Veterinary Research
Austin J Vet Sci & Anim Husb. 2016; 3(1): 1022.
Caseous Lymphadenitis (Pseudotuberculosis) in Camelids: A Review
U Wernery* and J Kinne
Central Veterinary Research Laboratory, Dubai, United Arab Emirates
*Corresponding author: U Wernery, Central Veterinary Research Laboratory, P.O. Box 597, Dubai, United Arab Emirates
Received: July 13, 2016; Accepted: September 20, 2016; Published: September 22, 2016
Abstract
Caseous Lymphadenitis (CLA) is one of the most important bacterial infections in livestock and can affect sheep, goat, cattle, camelids and equids. It is caused by Corynebacterium (C.) pseudotuberculosis and is characterized by abscessation of one or more superficial lymph nodes and sometimes causes infection of internal organs including mammary gland. The infection is spread by inhalation, ingestion or directly through wounds. CLA has been reported in Old World Camels (OWCs) from all camel rearing countries including in Australian feral dromedary population. The two South American tame camel species, llama and guanaco, are also affected by this disease in their countries of origin but also in the USA and especially in Europe in which they were introduced as companion animals. The virulence of the pathogen is attributed to its exotoxin phospholipase D (PLD) which is produced by all C. pseudotuberculosis strains. Two biotypes exist: ovine/caprine (serotype I or biotype ovis) and equine/bovine (serotype II or biotype bovis) and both have been identified in dromedaries using the nitrate reduction test. Hence, CLA-vaccines for camelids should include both sero types. Furthermore, Pulsed-Field Electrophoresis (PFGE) showed that camel isolates did not only differ from isolates of small ruminants, but also from each other thus making herd specific vaccines necessary. Morbidity of CLA may reach more than 90% in East African countries whereas mortality in Bactrian camels was reported to be 28%. The mortality rate in dromedaries is unknown. Both, young and adult camels are affected by the disease.
CLA is a chronic contagious disease and the intracellular bacterium forms abscesses in external and internal lymph nodes. These abscesses enlarge, may rupture and discharge infectious pus. The disease can cause severe economic losses. In sheep, the abscess often has the classical laminated “onion ring” appearance in cross section which has not been observed in camelids. Histologically several types of pyogranulomatous lesions in lymph nodes may display in one animal multiple abscesses, large central abscess with multiple smaller abscesses in the surrounding capsule and large single abscess with liquefactive necrosis.
A CLA vaccine using pure PLD as well as an antibody ELISA using protein A as conjugate has been developed at CVRL. A centrifuged and bacterial filtered Brain Heart Infusion broth (BHI) supernatant of a mixture of two CLA biotypes containing 750μg PLD gave a complete protection against a challenge with C. pseudotuberculosis. Antimicrobial treatment is often not successful and therefore an intravenous application of 20% sodium iodine diluted in 500ml NaCl as a single treatment has the best perspective of success.
Keywords: CLA; NWCs; OWCs; Vaccine; Serology
Introduction
Caseous Lymphadenitis (CLA) or pseudotuberculosis caused by Corynebacterium (C.) pseudotuberculosis is one of the most important bacterial infectious diseases in livestock. It affects sheep and goats worldwide and produces an ulcerative lymphangitis in cattle. It is widespread in Old World camels (OWCs) and the organism has also been isolated from abscesses of New World Camels (NWCs) [1]. The disease has been reported from Egypt and Asia as early as 1932 [2] and during the last century in OWCs from Egypt [3-5], India [6], United Arab Emirates [7], Iran [8], China [9-11], Kazakhstan [12], Ethiopia [13], Jordan [14] and Russia [15,16]. CLA cases have been observed in Europe not only in OWCs on the Canary Islands [17], but also in NWCs in Germany, Italy and Sweden [18-21].
The disease is endemic in horses in California and is named ‘Pigeon Fever’. It causes abscesses that lead horses’ chest to swell [22]. CLA is characterized by abscessation of one or more superficial lymph nodes and also may cause pneumonia, hepatitis, mastitis, arthritis, orchitis and meningitis [23]. Although very rare, the pathogen can cause human infections with the sheep/goat strain among farm and abattoir workers. Infected people show chronic lymphomegaly and normally require surgical treatment.
Economic losses due to CLA can be severe as many animals especially camelids are often valuable companion animals. In Australia, where several hundred thousand feral dromedaries are roaming through the interior, unsightly lymph node abscesses have been observed by many people. A similar picture is seen in East Africa, where more or less every dromedary exhibits swollen external lymph nodes. It is believed that a connection exists between browsing on thorny plants and CLA. Mortality rates of CLA in dromedaries in other countries than Europe where it can reach 15% are unknown and death always occurs in association with the spread of the pathogen into internal organs, mainly lung and liver.
Aetiology
CLA has been known under a variety of names like cheesy gland disease, ulcerative lymphadenitis, actinomycotic infection, pseudoactinomycosis, suppurative lymphadenitis and lymphadenitis. The French veterinarian Nocard was the first who described C. pseudotuberculosis in 1888. C. pseudotuberculosis is a short, irregular ovoid Gram-positive rod almost resembling a coccus. Smears from abscesses show a marked pleomorphism. Colonies are small, white and dry and can be surrounded by a narrow zone of haemolysis when sheep or ox blood is used. The plates should be incubated at 37oC for at least 48 hours. The facultative, intracellular bacterium multiplies in infected phagocytes and gets disseminated via lymph or blood to secondary sites where it causes abscesses of different size. The virulence of C. pseudotuberculosis is attributed to a major exotoxin, phospholipase D (PLD) which increases vascular permeability and also facilitates dissemination of the pathogen into lymph nodes where it inhibits chemotaxis and death of neutrophils as well as inactivation of complement [23]. Additionally, two other toxins, a toxic cell-wall lipid and a hemolysin are excreted by the pathogen. The toxic cellwall lipid is associated with the virulence of the bacterium and the hemolysin causes hemorrhages, increased vascular permeability enhancing bacterial invasion. So far, all isolated C. pseudotuberculosis isolates have produced PLD. Two biotypes are known: ovine/caprine and equine/bovine. From all of the CLA cases reported, only type I strain or biovar ovis was cultured. Only recently it was shown, that strains isolated from dromedaries in the United Arab Emirates and Kenya belong to both serotypes, serotype I (biotype ovis) and serotype II (biotype bovis) using the nitrate reduction test [24].
In some experimental trials with dromedaries, Afzal et al. [7] clearly showed that C. pseudotuberculosis strains which PLD genes were deleted are unable to produce lymph node abscesses. Over several years, more than 70 strains isolated from dromedaries in the United Arab Emirates and Kenya underwent different laboratory tests [25] at the Central Veterinary Research Laboratory (CVRL). It was found that with the exception of nitrate production, they reacted biochemically similar to known ruminant isolates. Similar biochemical results using API Coryne system (bioMérieux, France) were obtained by Connor et al. [26] investigating 45 ovine isolates and Tejedor et al. [17] testing the pathogen from dromedaries of the Canary Islands. Also Shen and Huang [27] came to the same conclusion when they compared isolated C. pseudotuberculosis strains from Bactrians and sheep biochemically from China and Mongolia from different areas. The strains showed the same biochemical patterns. Genotyping of C. pseudotuberculosis isolates were performed on UK sheep and dromedary isolates from Dubai using the Pulsed-Field Gel Electrophoresis (PFGE) with the Sfi an AscI restriction enzymes. This technique is applied for the separation of large DNA molecules and has made it easier to discriminate between strains thus linking isolates with clinical infections. The results showed that the strains examined did not only differ from isolates of small ruminants, but also from each other. It is therefore believed that genetically different C. pseudotuberculosis populations may exist on host-related basis and the use of autogenous herd-specific vaccines from other host species may not work. Also Braga [28] observed in his experimental infection trials in adult alpacas pathogenically distinct differences between different C. pseudotuberculosis strains.
CLA can affect entire dromedary herds and has also caused severe eradication problems in NWCs in Europe [1].
Epidemiology
The infection is spread via ingestion, inhalation or directly through wounds. Affected camels often concurrently suffer a severe tick infestation (Hyalomma dromedarii) from which C. pseudotuberculosis is often isolated. Additionally, mucous membranes of the oral cavity might be damaged by acacia thorns and or by dry and hard stems of desert plants. Following its entry through the skin or mucous membrane, the pathogen is phagocytosed by leukocytes and transported by lymph or blood to the predilection sites. CLA is a chronic disease. In sheep and goats, the incubation period ranges from 25 to 40 days whereas in camelids the incubation period is much longer. Abscesses can form even after more than 6 months. Some reports show that dromedaries become infected even after more than a year after the last positive CLA case was observed in a herd. This indicates that either carriers exist or the environment is contaminated.
Scarce information is available about age distribution, morbidity and mortality. CLA occurred for the first time in Saudi Arabia in 1989 in two herds comprising of 2500 camels. In total 15% of adult dromedaries developed numerous abscesses at the predilection sides and biovar ovis was isolated from multiple external and internal abscesses mainly in lung and liver and from ticks [29]. CLA was detected in 10% of 339 adult camels in Egypt [4]. Again serotype I, Corynebacterium ovis was isolated. The same percentage of 10% of CLA infected dromedaries were found by Domenech [13] in Ethiopia and in Jordan 8% of adult dromedaries were affected with multiple muscle and subcutaneous abscesses at various locations of the body [14]. When 107 dromedaries were slaughtered at Oseem abattoir in Egypt, 62 (60%) showed typical lesions of CLA [5]. Many visits were done by one of the authors (UW) to investigate CLA in dromedaries of the northern part of Kenya. Over 90% of several hundred dromedaries adspected during these visits showed swollen external lymph nodes of which many had burst. It was found that CLA also occurred in very young dromedaries at the age of 3 to 4 months. Their dams were also heavily infected and it seems that maternal antibodies did not protect young offsprings from CLA. Several researchers have described CLA in Bactrian camels. Chen et al. [9] investigated the disease in Gansu province in China. The disease is known as “Haas” and between 1975 to 1981, 2843 Bactrian camels were affected of which 28% died. Han et al. [11] used a C. pseudotuberculosis formalin – alum vaccine in the field and during a 3-year period, the CLA morbidity decreased from 27% to 6%. Also, in the Gansu province Wu [10] isolated the pathogen from 12% of Bactrian camels and Samartsev [12] has seen single CLA cases in young and adult Bactrians and sometimes real outbreaks in Western Kazakhstan in 1936.
CLA does not only affect OWCs but also has been reported from NWCs from many different parts of the world. The disease appeared in Europe for the first time in camelids in 2003 after CLA was introduced into the sheep industry in the United Kingdom in 1991 [19]. In Europe, mortality in OWCs can reach 15% and in NWCs 22% mainly when internal organs are involved. In Bactrians, a mortality rate of 28% has been described by Han et al. [11]. Not only on the Canary Island of Spain, CLA has affected the camel tourist industry also on mainland Europe. Many dromedaries and Bactrians purchased from animal traders for breeding purposes were affected by the disease which also spread to other animals when introduced into herds. Increased intermingling of animals of different species for show performance and trade pose a severe risk for the spread of CLA.
In Italy Beghelli et al. [20] reported an outbreak of CLA in central Italy after 54 alpacas were imported from Germany into an established herd of 28 animals. Despite all efforts to control the outbreak, it spread to other premises. Imported alpacas from Germany also caused considerable problems in an alpaca herd in Sweden 2003 due to a severe CLA infection [20].
Several authors have reported CLA in Peru in NWCs even at altitudes above 4000m. Natural infections with the Corynebacterium pathogen in Andean alpacas produced mastitis and abscesses in superficial lymph nodes of the bodies as well as abscess formation mainly in renal lymph nodes. In alpaca mastitis cases, it is assumed that the pathogen is disseminated through milk to suckling crias and not through skin wounds (shearing).
Cases of CLA have also been reported in North American camelids but they are rare and treatment was often successful through antimicrobial therapy and excision of the abscesses.
Clinical Signs and Pathology
In small ruminants as well as in camelids, infection with C. pseudotuberculosis induces the development of superficial abscesses localized mainly in the cephalic, prescapular and prefemoral lymphnodes (Figure 1).
Figure 1: Development of superficial abscesses localized mainly in the
cephalic, prescapular and prefemoral lymphnodes.
Pathognomonic in camels are cold, closed painless abscesses up to the size of a lemon or orange in the external lymph nodes (Figure 2).

Figure 2: Pathognomonic in camels are cold, closed painless abscesses up
to the size of a lemon or orange in the external lymph nodes.
In sheep and goats, the abscess develops a laminated (onion ring) pattern [30]. This pathological changes have never been described in camelids [3]. In camelids three different types of pyogranulomatous lesions have been observed:
- Single abscess, when ripe and opened, the abscess extrudes thick, white-cream like pus (Figure 3).
- Large central abscess with multiple small abscesses in the peripheral connective tissue capsule (Figure 4).
- Multiple abscesses and no central abscess (Figure 5).
- Central necrotic core of pus
- Several layers of immune cells (macrophages and lymphocytes)
- Fibrous capsule which encircles the pyogranuloma
- Remove all infected animals immediately
- Disinfect and clean stables and pens rigorously, remove the dung, bedding and topsoil from pens, move the herd immediately into a newly erected burma
- Animal housing should be free from wire and other causes of skin trauma
- External parasites must be controlled
- Purchase of animals should only be allowed from herds with no history of abscessation
- Serological screening of the entire herd and removal of reactors, no serological positive animal should be detected after the second screening
- Wernery U, J Kinne, Schuster RK. Camelid Infectious Disorders. OIE Book. 2014; 163-173.
- Carpano M. disease in Egypt and Asia. Boll Sez Ital Sci Inst Microbiology. 1932; 4: 108.
- Nashed SM, Mahmoud AZ. Microbiological and histopathological studies for rare cases of Corynebacterium infection in camel. Assiut Vet Med J. 1987; 18: 83-86.
- Abou-Zaid AA, Selim AM, Yousef FH, Abd El-Samea MM. Lymphadenitis in camels. 2nd Vet Med Cong Zagazig. 1994; 600-607.
- El-Sergany MA, Sofy MM, Lotfi H, Hassanain MA, Nassar AM, Laila A, et al. Lymphadenitis in Egyptian camels with special reference to bacteriological and parasitological affections. Egypt J. Comp. Path. Clinic Path. 1991; 4: 25-45.
- Purohit NR, Chouhan DS, Choudhary RJ. Lymphangitis in the camel. Agri-Practice. 1985; 6: 23-24.
- Afzal M, Sakir M, Majid Hussain. Corynebacterium pseudotuberculosis infection and lymphadenitis (Toloa or Mala) in the camel. Tropical Animal Health and Production. 1996; 28: 158-162.
- Esterabadi AH, Entessar F, Hedayati H, Narimani AA, Sadri M. Isolation of Corynebacterium pseudotuberculosis from camel in Iran. Arch Inst Razi. 1975; 27: 61-66.
- ChenJJ, Han ZY, Shang YZ, Caimude. Gansu J. Anim. Sci. and Vet Med Suppl. 1984; 51-54.
- Wu JG. Pustules in camel. Gansu J. Anim Sci and Med. 1987; 3: 4-5.
- Han ZY, Chen JG, Shang YZ. Experiment on immunization against corynebacteriosis of the Bactrian camel. Acta Agriculturae Universitatis, Gansu. 1983; 2: 47-58.
- Samartsev AA. Infectious pustular dermatitis in camels. Proc. of Kazakh Res. Vet Inst. 1950; 5: 190-197.
- Domenech J. Étude bactériologigne de Corynebacterium pseudotuberculosis et de Corynebacterium pyogens isoléz chez le dromadaire en Ethiopie. Rev Elev Méd. Vét Pays trop. 1980; 33: 123-126.
- Hawari AD. Corynebacterium pseudotuberculosis infection (Caseous Lymphadenitis) in camels (Camelus dromedarius) in Jordan. American Journal of Anim and Vet Sci. 2008; 3: 68-72.
- Spesivtseva NA, Noskov AI. Epizootic lymphangitis in camels. Trudy Vses. Inst Vet Sanit Ectoparasit. 1959; 14: 86.
- Dalling T, Robertson A, Boddie G, Spruell J. Diseases of camels. In the Int. Encyclopaedia of Vet Med, W. Green and Son, Edinburgh. 1966; 1: 585.
- Tejedor MT, Martin JL, Corbera JA, Schulz U, Gutierrez C. Pseudotuberculosis in dromedary camels in the Canary Islands. Trop. Anim. Hlth Prod. 2004; 36: 459-462.
- Kobera R, Poehle D. Case report in South American Camelids in Germany. In: Proc. 4th European Symposium on SACs and DECAMA. European Seminar, 7. – 9.10, Goettingen, Germany 151. 2004.
- Muenchau B. Pseudotuberculosis bei Kamelen. In Proc of DGZWE. 2006; 61-73.
- Beghelli D, D’Slterio GL, Severi G, Moscati L, Pezzotti G, Foglini A, et al. Evaluation of the immune response to vaccination against C. pseudotuberculosis in an alpaca herd in Italy. Preliminary results. In: 4th European Symposium in South American Camelids/DECAMA European Seminar, Goettingen, Germany. 2004; 7- 9.
- Norgren T. Corynebacterium pseudotuberculosis in alpacas. 2008.
- Promed. Pigeon Fever, equine USA (Oregon). 2007.
- Markey B, Leonard F, Archambault M, Cullinane A, Macguire D. Clinical Veterinary Microbiology, Elsevier. 2013; 135 -145.
- Berlin M. Corynebacterium pseudotuberculosis bei Kamelen – Epidemiologie undPhasen der Impfstoffherstellung. Bachelor of Science Thesis (B. Sc.) Beuthhochschule für Technik Berlin – University of Applied Science. 2015; 1-43.
- Wernery U. Caseous Lymphadenitis (Pseudotuberculosis) in camelids. J. Camel Pract and Research. 2012; 19: 21-27.
- Connor KM, Quirie MM, Baird G, Donachie W. Characterization of United Kingdom isolates of Corynebacterium pseudotuberculosis using pulsed-field gel electrophoresis. J Clinical Microbiology. 2000; 38: 2633-2637.
- Shen BY, Huang DS. Comparison of biochemical reactions of some pseudotuberculosis in strains from corynebacteriotic camels in different districts of China. Xinjiang Anim. Sci. and Tech. 1981; 4: 26-30.
- Braga WU. Protection in alpacas against Corynebacterium pseudotuberculosis using different bacterial components. Veterinary Microbiol. 2007; 119: 297-303.
- Radwan AI, El-Magawry S, Hawari A, Al-Bekairi SI, Rebleza RM. Corynebacterium pseudotuberculosis infection in camels (Camelus dromedaries) in Saudi Arabia. Trop Anim Health Prod. 1989; 21: 229-230.
- Pépin M, Paton MW. Caseous lymphadenitis in sheep and goats. In: Infectious and Parasitic Diseases of Livestock. Eds. Lefèvre PC, Blancou J. Chermette R. und Uilenberg G. Lavoisier. 2010; 1151-1163.
- Paule BJA, Meyer R, Moura Costa LF, Bahia RL, et al. Three-phase partitioning as an efficient method for extraction/concentration of immunoreactive excreted-secreted proteins of Corynebacterium pseudotuberculosis. Protein Expression and Purification. 2004; 34: 311-316.
- Seyffert N, Guimaraes AS, Pacheco LGC, Portela RW, Bastos BL, Dorella FA, et al. High seroprevalence of caseous lymphadenitis in Brazilian goat herds revealed by Corynebacterium pseudotuberculosis secreted proteins-based ELISA. Research in Vet Science. 2009; 88: 50-55.
- Berlin M, Joseph M, Jose S, Raghavan R, Syriac G, Paily N, et al. Production of a Caseous Lymphadenitis Vaccine for dromedaries. J Camel Pract and Res. 2015; 22: 163-168.
- U Wernery. Production of a Caseous Lymphadenitis Vaccine for dromedaries. J. Camel Pract. and Res. 2015; 22: 163-168.
- Bergin TJ. Corynebacterium pseudotuberculosis and “Mala” lymphadenitis in camels. In FAO: The Camel development and research. Proc Kuwait Seminar, Kuwait. 1986; 10: 20-23.
- Johnson B, Dietrich F, Petrorsky N, Kinne J, Wernery R, Wernery U. Characterization of adjuvants for use in dromedary immunization. J Camel Pract and Res. 2015; 22: 33-48.

Figure 3: Single abscess, when ripe and opened, the abscess extrudes thick,
white-cream like pus.
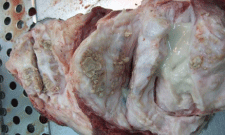
Figure 4: Large central abscess with multiple small abscesses in the
peripheral connective tissue capsule.
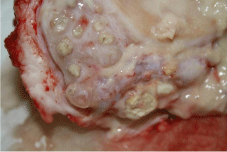
Figure 5: Multiple abscesses and no central abscess.
Microscopic lesions of the pyogranulomatous C. pseudotuberculosis abscess are composed of:
Nashed and Mahmoud [3] and Abou-Zaid et al. [4] described in detail accompanied by pictures histological lesions of CLA in Egyptian dromedaries. From the abscesses they isolated C. ovis and from some of them also Staphylococcus aureus and Streptococcus spp. Closed abscesses were sterile. Like in our investigation Nashed and Mahmoud [3] classified the lesions into two distinct types: multiple focal areas with caseous central necrosis and large central lymph node areas with liquefactive necrosis. On the other hand, Abou-Zaid et al. [4] presented the alterations as serous lymphadenitis, suppurative lymphadenitis and chronic suppurative lymphadenitis which is the sequence of lesion development from acute to chronic stages.
A few cases have been seen in dromedaries whereby the abscess breaks through the ribs and the organisms enter the lung producing severe bronchopneumonia with pulmonary caverns (Figure 6).
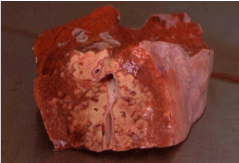
Figure 6: Abscess breaks through the ribs and the organisms enter the lung
producing severe bronchopneumonia with pulmonary caverns.
Serology
Several different serological tests have been tried for the diagnosis of CLA but none has been validated for camelids. They include haemagglutination, haemagglutination inhibition, Agar Gel tests and ELISAs. Most of these tests have a low sensitivity. However, a new indirect double antibody sandwich ELISA, an Interferon Gamma assay and a Western Blot are said to possess a high specificity and sensitivity at herd level in goats and sheep. The most effective of these tests detect antibodies to the PLD exotoxin.
In several European countries with increasing number of camelids, a program exists to test all NWCs regularly on a voluntary basis for antibodies to CLA. During these investigations it was observed that more than 40% of tested NWCs in some areas showed antibodies to C. pseudotuberculosis without showing any clinical signs. It confirms that a high number of NWCs had come in contact with the pathogen or are carriers without showing clinical signs.
Many antigen preparations have been used in the ELISA tests such as whole cell extract, cell wall antigens, PLD, cell supernatant and recombinant exotoxin. The most specific diagnostic test seems to be the ELISA based on recombinant PLD expressed in E.coli.
Direct ELISAs for the detection of IgG antibodies in sera from sheep and goats with CLA are commercially available for example from Hyphen, BioMed, France, ELITEST CLA. This test detects antibodies with the help of HRP-labelled mouse monocloncal antigoat/ sheep conjugate. These ELISAs are not validated for use in camelid.
Paule et al. [31] and Seyffert et al. [32] used a non-commercial ELISA for the detection of antibodies to CLA after extraction and concentration of immunoreactive C. pseudotuberculosis proteins. Wernery [25] and Berlin et al. [33] developed an in house ELISA on the basis of the commercially available ELITEST. For dromedaries the anti-goat/sheep conjugate was replaced by protein A and this test is currently routinely used to detect CLA antibodies in dromedaries with encouraging results [34].
Treatment and Control
The following procedures should come immediately into effect when a CLA case has been detected in a camel herd:
In small ruminants, animals with enlarged lymph nodes are culled. This procedure is not practical with camelids. Corynebacteria are very sensitive to penicillin, tetracyclines and cephalosporines but the fibrous capsule and the pus in the abscess prevents the medication from reaching the bacteria. Antimicrobial treatment is often not rewarding and other treatment methods should be considered. Since erythromycin is more able to penetrate tissues, Bergin [35] proposed a combination of penicillin and erythromycin to treat pseudotuberculosis in camels. Other possibilities of treating CLA in camels is the intravenous injection of 20ml of Dimethyl Sulfoxide (DMSO) in combination with 20-25ml of Baytril for 12 days or intravenous injection of 20% sodium iodine in 500 ml NaCl as a single treatment which can be repeated 2 to 3 weeks later. The abscesses may eventually subside with no relapse. Purohit et al. [6] used corticosteroids and systemic antibiotics with little success and therefore injected intramuscularly a Lugol’s solution for 5 days with the following doses: 100ml, 100ml, 90ml, 80ml and 70ml. Samartsev [12] applied with good success 2 to 5% creolin and iodine lotions topically. However, in cases with multiple abscesses, surgical and antibiotic treatments are recommended (Figure 7).
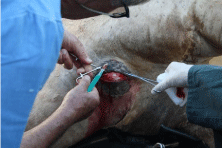
Figure 7: In cases with multiple abscesses, surgical and antibiotic treatments
are recommended.
Subcutaneous ripe abscesses can be lanced and cleaned out on a daily basis with iodine solution. It is of paramount importance to provide strict aseptic methods, destroying contaminated equipment and disinfection of instruments used.
Vaccination
Vaccines against CLA for sheep and goats are commercially available, but are not licensed in countries in which they are not produced. Therefore, for their use in NWCs for example a special permit is needed. Additionally, sheep and goat lymphadenitis vaccines are not used in camels because the protective doses are not adopted for camels. Therefore, they are not available for camelids. These commercial vaccines do not provide complete protection against the development of abscess formation but a significant reduction in the number of abscesses. The vaccines are formulated from concentrated formalin – inactivated C. pseudotuberculosis culture supernatant containing PLD, ultra-filtrated C. pseudotuberculosis antigens; Clostridium perfringens type D and C and Cl. tetani. Attenuated mutant vaccines are also available. One of the formalin-inactivated vaccines has been used in dromedaries during a trial vaccination but had to be abandoned due to the development of granulomas of different sizes at the injection site (Figure 8).

Figure 8: Development of granulomas of different sizes at the injection site.
The granuloma development after vaccination was the reason why researchers from CVRL embarked on a project to find the most suitable adjuvants for dromedaries [36]. Additionally, several scientists have started research on the production of autogenous vaccines against CLA mainly in NWCs [18,20,28] with different results. They used cell wall proteins, toxin components and killed bacteria of C. pseudotuberculosis from alpaca origin and muramyl dipeptide as adjuvant. From vaccination experiments with these autogenous vaccines which were also used in dromedaries [25], it was shown that the exotoxin PLD plays the major role in the development of CLA indicating the need to block or neutralize PLD by vaccination. No abscesses developed in an experimental vaccination and challenge trial in alpacas when they were vaccinated with a CLA vaccine containing high level of toxin (PLD 500μg/ml, 28). These vaccines were formulated from concentrated formalininactivated C. pseudotuberculosis culture supernatants containing PLD. Berlin et al. [33] embarked on a different approach by using only PLD from non-formalin inactivated culture supernatant containing no C. pseudotuberculosis bacteria or any bacterial cell wall protein. The mixture of 2 CLA biotypes (ovine/caprine and equine/bovine) containing 750μg PLD per dromedary gave a complete protection against a challenge dose containing 4.0x10³cfu/ml of an ovine/caprine biotype. Sero conversion was tested with a newly established indirect ELISA using PLD as antigen and protein A as conjugate.
References