
Research Article
Austin Alzheimers J Parkinsons Dis. 2014;1(1): 7.
Comparing Differences in ADL Outcomes for the STOMP Intervention for Dementia in the Natural Home Environment Versus a Clinic Environment
Ciro CA1*, Poole JL2, Skipper B3 and Hershey LA4
1Department of Rehabilitation Sciences, University of Oklahoma Health Sciences Center, USA
2Department of Occupational Therapy, University of New Mexico, USA
3Department of Family and Community Medicine, University of New Mexico, USA
44Department of Neurology, University of Oklahoma Health Sciences Center, USA
*Corresponding author: Ciro CA, Department of Rehabilitation Sciences, University of Oklahoma Health Sciences Center, 1200 N. Stonewall Ave, Oklahoma City, OK 73117, USA.
Received: August 26, 2014; Accepted: September 03, 2014; Published: September 04, 2014
Abstract
Background: Few studies have examined structured rehabilitation techniques for improving activities of daily living in people with mild-moderate dementia. We sought to examine the advantages to delivering the Skill-building through Task-Oriented Motor Practice (STOMP) intervention in the home environment (versus the clinic), hypothesizing that ADL improvement would be significantly better, time to meeting goals would be faster and fewer displays of behavior would be noted.
Methods: Compared results of two quasi-experimental studies of STOMP, one completed in the home, one completed previously in a clinic. Participants were English-speaking; community dwelling adults aged 50-90 diagnosed with mild-moderate dementia who could participate in an intensive rehabilitation program (5 days/week, 3 hours/day, for 2 weeks). Outcome measurements include examiner-observation of performance and proxy-report of performance and satisfaction with performance in patient-selected goals.
Results: No differences existed in the sociodemographic characteristics between the home and clinic groups where the groups were primarily white, married, had > high school education and had mild-moderate dementia. Results from the home group indicate that participants made significant improvement in ADL which was generally retained at the 90 day follow-up. These results were not significantly different than the clinic group. No significant advantages were noted for the home group in terms of time to meeting goals or exhibition of fewer behaviors.
Results: No differences existed in the sociodemographic characteristics between the home and clinic groups where the groups were primarily white, married, had > high school education and had mild-moderate dementia. Results from the home group indicate that participants made significant improvement in ADL which was generally retained at the 90 day follow-up. These results were not significantly different than the clinic group. No significant advantages were noted for the home group in terms of time to meeting goals or exhibition of fewer behaviors.
Discussion: The STOMP intervention appeared to work equally as well in the home and in the clinic. Future studies should continue to examine the benefits of massed practice using high-dose regimens.
Keywords: Dementia; Activities of daily living; Cognitive rehabilitation; Occupational therapy; Goal attainment scale
Introduction
People with Alzheimer’s disease and related dementias present with diverse cognitive and psychological deficits, yet all will report changes in how they function in daily activity [1,2]. Progressive loss in ADL is correlated with depression, anxiety and decreased quality of life for the person with dementia and increased burden for caregivers [3-5].
Despite the impact and progressive nature of ADL disability, we continue to lack standardized and effective treatments f or reversing ADL disability and delaying decline as the disease progresses [6,7]. New drug research is promising, but current drugs available to patients address short-term symptoms without modifying brain pathologies that cause ADL disability [8-10]. Previous behavioral research has focused on improving cognitive skills such as memory with little evidence of improvement in ADL [11]. Emerging research has broadened the focus to minimizing ADL disability through various forms of task-oriented training where individualized; therapy goals are practiced using the very tasks that people want to improve [6,12,13]. Results have indicated that people with mild dementia can improve in ADL performance, but transfer of the skill to and spontaneous initiation of the task within the natural environment is limited and few long term results are available [6,12].
We developed the Skill-building through Task-Oriented Motor Practice (STOMP) intervention to standardize the evaluation and delivery of task-oriented training for people with mildmoderate dementia using rehabilitation methods known to induce neuroplasticity in other progressive and non-progressive neurological populations [14,15]. Through our adaptation of the learned non-use phenomena as shown in Figure 1, we hypothesize that early disability in ADL is a negative behavioral response to errors in ADL performance and caregivers taking over tasks when only minimal supports may be needed to complete the tasks [16-18]. In people post-stroke, this phenomena is reversed by engaging the person in high-dose, taskoriented training which is shown to cause permanent change in neural circuits by creating new neural pathways and by-passing non-functioning circuits [15,19,20]. Through the power of neuroplasticity, we hypothesize that we can improve ADL performance and delay decline despite the progressive nature of dementia.
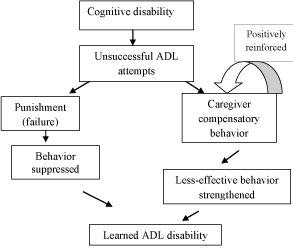
Figure 1: Development of Learned ADL Disability in Dementia (Adapted from Lillie &Mateer [18]).
In a previous pilot study, we demonstrated that the STOMP intervention delivered in a clinic environment was useful for improving ADL performance and results were maintained at the 90-day follow-up [21]. However, we also noted some decline in participants which we hypothesized was in part due to problems with transferring learning to the home environment. Therefore, we sought to examine the outcomes of delivering the STOMP intervention in the home environment and then to compare those results with our earlier clinic results. We hypothesized that STOMP delivered in the home would be result in 1) significantly higher post-intervention ADL scores with better retention of ADL at 90 days; 2) goals being met more quickly, and finally, 3) fewer behavioral disruptions during the intervention.
Methods
Research design
The home study was a quasi-experimental pre-post design comparing the impact of delivering the STOMP intervention in the natural home environment. Two universities, the University of Oklahoma Health Science Center (OUHSC) and University of New Mexico (UNM) received a collaborative grant to complete a two-site study. The data for participants from the clinic study were collected through a quasi-experimental, pre-post design conducted in an OUHSC laboratory in 2012.
Participants
Participants in the home study met the following inclusion criteria: 1) community-dwelling English speaking adult (50-90 years old); 2) living with someone (spouse, friend, relative, caregiver, etc) in a residential setting or assisted living who could provide informed consent; 3) diagnosed with dementia with exclusions (see exclusion criteria); 4) MMSE score >10 and ≥ 25; 5) able to understand and follow one step commands; 6) will have functional movement of one arm; 7) participant or family member can identify three goal areas related to self-care or home management; 8) able to participate in 3 hours of daily intervention in their home environment for 2 consecutive weeks (excluding weekend). Participants and legally authorized representatives [5 per site] were recruited through geriatric medicine collaborators, newspaper ads, radio/TV spots, campus wide emails and presentations at local chapters of the Alzheimer’s Association support group.
Eligibility screening and consent were conducted by the PIs (CAC, JLP), in the participant’s home, with their legally-authorized representative. Participants provided assent for inclusion. Participant inclusion criteria and recruitment methods for the clinic study were the same except that people needed to be able and willing to come to a laboratory for daily intervention.
Measures
Sociodemographic information/medical history was collected using a standard intake form. The Mini-Mental Status Examination (MMSE) consists of 30 points grouped into 7 domains such as orientation, recall, language, attention and visual construction. When compared to a reliable and valid dementia rating scale, a MMSE cutoff point for mild dementia (21-25) yields an acceptable kappa=.62, P<.001; a MMSE cut-point for moderate dementia (>10 and <20) yields a kappa=.79, p<.001. [22].
TheCornell Scale for Depression in Dementia (CSDD) is a 19- item scale that measures the presence of depression through semistructured interviews with the participant and caregiver. The items are scored on a scale of 0–2, where 0=absent, 1=mild or intermittent and 2=severe symptoms [23]. A cut-off score of 7 yields a sensitivity of .90 and a specificity of .75 for identifying major depression in people with mild-moderate dementia [24]. Internal consistency (α=.81) is good in a dementia population [23].
The Caregiver Burden Scale (CBS) is a 22 –item caregiver assessment of burden perception that includes items for health, personal, social or financial well-being [25]. Caregivers rate statements of burden on a continuous scale of “0” indicating never and “4” indicating nearly always. Amount of burden is indicated by adding scores where 0–20 =minimal to no burden; 21-40= moderate burden and >40 =moderate to severe burden. For caregivers of people with dementia, internal consistency is good (α=.82), and test-retest reliability is acceptable for varying time frames [25,26].
The Canadian Occupational Performance Measure (COPM) is a semi-structured interview tool for prioritizing areas of functional performance deficit [27]. The client identifies tasks that are most important and then reports their performance on each task on a scale of 1-10 (1=worst, 10=best). Clinically significant change in a pre-post intervention program is = 2. The COPM has been used across the lifespan with people with a variety of disabilities to include stroke, dementia and traumatic brain injury. In adults with >1 impairment in function, test-retest reliability is adequate (ICC = 0.67) [28].
Goal areas established through the COPM were formatted for individualized measure using Goal Attainment Scaling (GAS). The GAS is an individualized measure of marking goal achievement to track within-subject longitudinal change and allow comparison within a group of people that will have different goals/ interventions. Using an ordinal measure (-2, -1, 0, 1, 2), the researcher breaks the goal down into five possible outcomes post-intervention where “0” equals the intended goal (determined after researcher observation); negative scores represent “much less” and “somewhat less” than the expected outcome and positive scores represent “somewhat more” and “much more” than expected outcome. A T score is calculated that represents the person’s performance on their goals. A T score of 50 indicates expected performance, below 50, less than expected and above 50, more than expected. The GAS in combination with the COPM has been used to successfully measure clinical change in people with traumatic brain injury and dementia [29,30].
At the end of each hour of the 3 hour intervention, interventionists completed three behavioral tracking forms and one intervention fidelity monitoring form. The first form tracked the number of repetitions completed for each task. A second form tracked the amount of time spent on each task within a session. A third form tracked the frequency of specific neuropsychiatric behaviors such as wandering, delusions, hallucinations, inappropriate activity (e.g., taking off clothing unrequested or inappropriate sexual behavior), purposeless activity, verbal outbursts, physical threats or violence, agitation, sleepiness, tearfulness, anxiety or phobias. The fidelity monitoring form required the interventionist to check the use of all of the hypothesized active elements of STOMP (family-centered goals, therapeutic use of self, repetition, blocked practice, verbal praise between steps, and errorless learning). These forms were developed by the first author and have been used previously in STOMP studies [21,30].
Procedures
Prior to recruitment and the start of the home-based intervention, the second author and three occupational therapists, two in Oklahoma and one in New Mexico received 40 hours of certification and training for the STOMP intervention. Up to one month before the intervention, the PIs went to the home to complete baseline descriptive measures. One –two weeks prior to the intervention, the treating OT went to the home to obtain baseline COPM measures and to observe ADL goals in order to create GAS outcomes. When appropriate, up to $250 of adaptive equipment was ordered to support performance of any of the three goals chosen by the participant/family. The caregiver was asked to sign a contract of willingness to complete ADLs using STOMP methods as appropriate for the task. The week following the intervention, the PIs went to each participant’s home and observed performance of the 3 family-identified goals in the context of the home environment to obtain GAS scores. Caregiver perceptions of participant performance in the 3 goals were noted by COPM scores. Every 30 days, we called caregivers to provide an opportunity to ask questions and maintain the relationship to minimize loss to followup. Three months after the end of the intervention, the PIs returned to the participant’s home to collect 90-day follow-up data.
In the comparison group, clinic participants were also assessed in their home environments. The only difference in care was that the intervention was completed in the first author’s laboratory, the Occupational Performance Laboratory (OPaL). The Opal is an 880 square foot lab divided into four rooms which are contextuallydesigned to look like an apartment. Graduate OT students supervised by the first author delivered the clinic intervention after 40 hours of training/certification in the STOMP intervention.
Intervention
Each hour of training focused on one of three family-identified goals. The hour was structured to deliver “massed practice” where repetitions of the task are high and the rest breaks were limited to 10min/hour as described in other motor learning studies [31,32]. “Practice-able” steps for each COPM goal were developed by the OT and included any compensations or adaptive equipment that might support performance of the ADL task. The delivery of taskoriented training incorporated multi-component features of motor learning to include: 1) 3 hours of STOMP intervention, 5x /week for 2 consecutive weeks (excluding the weekend); 2) error less learning in which participants were prevented from making mistakes and 3) verbal praise after each step. For example, one participant practiced making phone calls using a “photo phone” in which pictures are placed on a phone and rather than dialing a number, the participant pushes the photo of a person to call them. Steps for using a photo phone were created to include pick up the receiver, push a picture, speak to person and then hang the phone up. These steps were practiced under errorless practice conditions for as many times as tolerated within the hour devoted to that goal. To enhance transfer of training to the caregivers, we invited caregivers to watch the intervention daily and required hands-on training of the intervention one day/week.
Data analysis
Means, standard deviations, and ranges were calculated for continuous variables and frequency counts were calculated for nominal variables. T-tests or Fisher’s exact tests compared the home and clinic groups on all demographic variables. Chi-square or Fisher’s exact tests was used to examine differences in frequency of neuropsychiatric behaviors. GAS T-scores were calculated usinga previously-described formula [29]. Repeated measures analysis of were used to test for main effect of group (home, clinic) and time (pre-intervention, post-intervention, 90- month follow-up) for the COPM performance scores, COPM satisfaction scores and GAS-T scores. Appropriate post-hoc tests were conducted as needed.
To ascertain whether earlier goal attainment was achieved when the STOMP intervention was delivered in the home compared to the clinic environment, we tallied the number of days from day 1 of the intervention to day of goal attainment for each goal and then calculated a mean goal attainment score for each participant. Goal attainment was defined as meeting GAS goals consistently 2 days in a row that is achieving a GAS score of > 0 for 2 days in a row. We then placed participants into 3 groups based on goal attainment (all 3 goals attained, 2 goals attained, 1 goal attained). A Fisher’s exact test compared the home and clinic environment by goal attainment group. A Cohen’s kappa was used to examine inter-rater reliability between the three clinicians delivering STOMP. Proportions were calculated for interventionist adherence to all seven active ingredients of STOMP (family-centered goals, task-oriented training, ≥ 2 repetitions each session, blocked practice, verbal praise between steps, errorless learning and therapeutic relationship). All analyses were completed using SAS 9.3 (Cary, NC).
Results
Within the home sample, there were no significant differences between OUHSC and UNM groups by age, cognitive status or depression. However, the OUHSC group had a significantly higher mean caregiver burden score (M=40.8) than the UNM group (M=28.6; p=.04). Table 1 shows the baseline demographics of both the home and clinic samples. There were no significant differences between the clinic and home groups for any of the demographic variables. Participants in both home and clinic groups showed moderate cognitive involvement and moderate caregiver burden. Goal choices were varied widely as expected and included use of cell phone/computer/television remote controls, medication management, writing checks, cooking small meals and participation in previous leisure activities such as needle work. All participants in both groups attended 100% of sessions and 100% were available for the 90-day follow-up.
Characteristics
Home (N = 10)
% (n)
Clinic (N = 6)
% (n)
Mean age years (SD)
78.9 (6.6)
74.7 (10.1)
Gender
Female
Male
40 (4/10)
60 (6/10)
50 (3/10)
50 (3/10)
Ethnicity % Caucasian
80 (8/10)
83.3 (5/10)
Caretaker
Spouse
Daughter/son
70 (7/10)30 (3/10)
83.3 (5/10)
16.7 (1/10)
Marital status % married
70 (7/10)
100 (6/6)
Education
< 12 years
High School Graduate
Post- High School Education
10 (1/10)
10 (1/10)
80 (8/10)
16.7 (1/10)
16.7 (1/10)
66.7 (4/10)
Mean Mini Mental Status Exam (SD)
18.3 (3.2)
20.0 (4.0)
Mean Cornell Depression Scale (SD)
3.7 (2.5)*
5.2 (4.4)*
Mean Caregiver Burden Scale (SD)
34.7 (10.7)†
45.8 (12.3)†
Mean Years memory loss (SD)
4.9 (3.7)
7.3 (5.0)
Type of Dementia
Probable Alzheimer’s
Vascular
Lewy-Body
Fronto-temporal
50 (5/10)
40 (4/10)
10 (1/10)
NA
66.7 (4/6)
16.7 (1/6)
NA
16.7 (1/6)
History Arthritis
80 (8/10)
50 (3/6)
History Depression
50 (5/10)
66.7 (4/6)
History Anxiety
20 (2/10)
33.3 (2/6)
History Stroke
20 (2/10)
33.3 (2/6)
History Falls
50 (5/10)
50 (3/6)
Needing help with using phone
100 (10/10)
66.7 (4/6)
Needing help with walking distances
60 (6/10)
66.7 (4/6)
Needing help with shopping food/clothes
80 (8/10)
66.7 (4/6)
Needing help with housework
70 (7)
50 (3/6)
Needing help with money management
100 (10)
100 (6/6)
Needing help feeding self
0
16.7 (1/6)
Needing help dressing self
30 (3/10)
16.7 (1/6)
Needing help with grooming
20 (2/10)
50 (3/6)
Needing help getting in/out of bed
10 (1/10)
0
Needing help bathing self
20 (2/10)
16.7 (1/6)
Needing help preparing meals
70 (7/10)
83.3 (5/6)
Needing help with toileting
20 (2/10)
16.7 (1/6)
Needing help remembering appointments/ following a schedule
90 (9/10)
100 (6/6)
Needing help managing medications
90 (9/10)
66.7 (4/6)
*Score of greater than 7 indicate depression; † Scores of 21 – 40 = mild to moderate burden; scores of 41-60 = moderate to severe burden.
Table 1: Baseline characteristics of home and clinic groups.
Within sample comparisons (OKC versus UNM)
Within the home sample, there were no significant differences between the OUHSC group and UNM group for initial and final COPM performance scores. However, post-intervention performance scores were significantly different between the groups where UNM participants had a higher mean score (M=8.7; SD: 0.7) than the OUHSC group (M=7.4; SD 0.8; p=.02). Additionally, there were no significant differences between the OUHSC and UNM groups on COPM satisfaction scores across time. For GAS scores, there was a significant difference in post-intervention scores where OUHSC participants had a higher mean GAS score (M=72.3; SD=11.7) compared to the UNM participants (M=55.2; SD 5.2; p=.02). There were no differences between the OUHSC and UNM participants in 90-day GAS means.
Between sample comparisons (home versus clinic)
Table 2 shows the means and standard deviations for the COPM and GAS-T scores. Intervention effects were assessed using the GENMOD procedure (SAS). For the COPM performance scores, there were no significant group (p = 0.93) or interaction (p =0.77) effects but there was a significant overall effect for time (p = .003). COPM performance scores improved significantly from pre -to post-intervention (p < .001). Scores decreased significantly from post intervention to the 3 month follow-up (p = 0.01) and remained significantly higher than pre-intervention (p=0 .001). On the COPM satisfaction scores, there were no significant group (p = 0.45) or interaction (p =0.94) effects but there was a significant overall effect for time (p = .004). Participants showed significant improvements in COPM satisfaction scores from pre to post intervention (p < .001). Scores decreased from post intervention to the 3 month follow-up but the decrease was not significant (p = 0.18) and scored remained significantly higher than at pre-intervention (p < .001).
Outcome
Pre-intervention
Post Intervention
3 month follow-up
Home
Clinic
Home
Clinic
Home
Clinic
M (SD)
M (SD)
M (SD)
M (SD)
M (SD)
M (SD)
COPM
Performance
3.0 (1.3)
3.4 (1.0)
8.0 (1.0)
7.9 (1.5)
7.0 (1.3)
6.9 (1.4)
Satisfaction
3.5 (1.8)
2.7 (2.0)
8.3 (1.1)
7.9 (1.7)
7.7 (1.6)
7.2 (2.3)
GAS-T
5.0 (0.6)
5.5 (0.5)
63.8 (12.4)
69.1 (13.8)
55.2 (20.4)
53.3 (24.7)
Table 2: Participant Scores on the COPM and GAS-T by Group and Assessment Period.
For the GAS-T scores, there were no significant group (p = 0.76) or interaction (p =0.73) effects but there was a significant overall effect for time (p = .003). GAS-T scores improved significantly from pre -to post- intervention (p < .001). Scores decreased from post intervention to the 3 month follow-up but did not decrease significantly (p = 0.14) and remained significantly improved from pre-intervention (p < .001).
Table 3 shows goal attainment by group. Only one person in the home group did not attain success with at least 2 goals. All people in the clinic group attained at least 2 goals. There was no difference as to which group achieved success sooner (p= 0.41). Table 4 highlights the neuropsychiatric behaviors exhibited during the STOMP intervention by group. There were no significant differences in the total of behaviors exhibited or in specific behaviors except for anxiety which was higher in the clinic (p=.03). Ad hoc analysis reveals that one participant in the clinic group scratched her head excessively when anxious which was also a ritual at home. Several behaviors that were tracked (delusions, hallucinations, physical threats) were not exhibited by any of the participants in either group.
Number of goals attained
Home % (N)
Clinic % (N)
1 goal attained
10 (1)
0
2 goals attained
10 (1)
33.3 (2)
3 goals attained
80 (8)
66.7 (4)
Total
100
100
Table 3: Goal Attainment by Group.
Behavior
Home (N = 10)
Clinic (N = 6)
P value*
Total number of behaviors
65
86
ns
Anxiety
5
35
.03
Depression
10
7
ns
Agitation
0
10
ns
Verbal outburst
19
15
ns
Wandering
3
0
ns
Purposeless activity
28
19
ns
Inappropriate activity
0
1
Ns
*Wilcoxon two-sample tests
Table 4: Frequency of Neuropsychiatric Behaviors Exhibited During Intervention by Group.
Post-training, all interventionists received 90% and above on tests examining understanding of the intervention. Cohen’s kappa for agreement between raters was (k=.79). Interventionist adherence to active ingredients was high 91.5% –100%.
Discussion
We sought to examine potential advantages to delivering the STOMP intervention in the home versus the clinical environment. Much like our clinic-based study, we found that when STOMP was delivered in the home environment, people with mild-moderate dementia demonstrated improvement in ADL activities postintervention which was largely retained at 90 days. We found no differences in performance between the home and clinic groups— both improved and retained performance. In both groups, our outcomes using the COPM indicate that our change scores were clinically significant as demonstrated by a ≥ 2 point change [33]. The participants in the home group did not demonstrate an advantage in meeting goals sooner or in a reduction of behaviors which was low in both groups.
Unique to dementia research, we found that high-intensity, short duration, massed practice of meaningful ADL delivered either in the home or clinic shows promise for ADL recovery in people with mildmoderate dementia. Other researchers have examined meaningful ADL training in the home environment [6,7]. Graff et al examined a home-based ADL retraining regimen with an emphasis on caregiver training using the Assessment of Process Skills to examine differences in observed performance in ADL [7]. After 5 weeks of training (2 hours/week), participants demonstrated improvement in ADL performance which was retained at 90 days [7]. In parallel, we also examined observed performance but additionally examined caregiver report of performance and satisfaction using the COPM to corroborate the caregiver’s perception of daily performance over time with the rater’s cross-sectional 90 day evaluation. Similarly, Clare et al. examined a home-based, cognitive rehabilitation program in which people chose their own goals using the COPM and then participated in an 8 week program of task-oriented training and cognitive domain specific retraining [6]. Post-intervention ADL outcome measurements were self-reported COPM scores and therapist observation using percent of goal obtainment (fully achieved, partially achieved or not achieved). They found statistically significant improvement in patient-reported COPM scores that did not reach clinical significance (≥ 2 point change). Of note, they also did not use the COPM to examine 6 month outcomes so long term follow-up on ADL performance is not available. In contrast to that study, we asked caregivers to rate performance and satisfaction with performance using the COPM to improve the reliability of scores over time (as compared to people with memory loss). Self-report versus proxy report has been studied and discussed extensively in the literature with pros and cons for both methods noted [34-36]. Recent findings do suggest that when a performance-based assessment of ADL is used as the standard, then proxy report is more highly correlated with performance results than self-report in people with cognitive impairments [37]. Of importance is that all three studies have used performance-based measure to examine ADL outcomes after ADL intervention which is trend that strengthens the reliability and validity of ADL intervention results [6,7,30].
Critical to the evaluation of STOMP is further examination of the need for massed practice [38]. In STOMP, participants completed massed practice of three goals over a three hour period (one goal/ hour with 10 minutes of rest). We explicitly designed this portion of the intervention to align with current tenets of neuroplasticity which suggest that repetition and task specificity facilitate permanent behavioral changes, in this case performance of ADL [14,15]. In fact, hundreds of repetitions may be needed to optimize function in people post stroke but this remains unstudied in dementia and we could find no other study in which massed practice was used in people with dementia [39-41]. Other ADL research has examined dosages of 1-2 hours/week and positive ADL changes have been documented [6,7]. Unknown is the extent to which these changes last beyond 90 days; lasting change may represent that the intervention facilitated neuroplastic events that supported ADL function. Post-hoc analyses for our study do provide results of interest related to amount of practice. Participants who continued to practice the ADL the way it was practiced either in the home or in the clinic, either maintained or improved in the ADL task that they practiced. Participants who stopped practicing generally demonstrated worsening performance though performance did not decrease to baseline levels at the 90 day follow-up. Clare et al. also remarked that those participants who engaged in more therapy between intervention sessions showed larger increases in COPM scores than those that did no extra practice [6]. The necessity of massed practice for people with dementia may be dependent on their neuroplastic response to these therapies which are yet to be measured.
Because massed practice has not been documented in people with dementia, we thought it necessary to consider their behavioral responses to massed practice. Would engaging in highly-repetitive tasks for long periods prove stressful or irritating? While we could find a number of studies examining interventions to minimize negative behaviors in people with dementia [42-44], we could find no other rehabilitation interventions that directly examined frequency of negative behaviors in response to the intervention. Taking a broad approach to the measurement of stress during an intervention, we reported on a variety of behaviors and reported very few behaviors in either setting and no difference in the number of behaviors by setting. Certainly this might be explained in part by emerging evidence suggests that older adults are more motivated for interventions driven by self-selected goals [6,45,46]. On the other hand, we also need to examine the STOMP intervention with samples of people with existing behavioral difficulties to better understand the response of massed practice in this select population.
Our results should be interpreted cautiously due to study limitations. First we are comparing the results of two quasiexperimental projects, neither of which used randomized sampling to enroll participants. Sample sizes were small and while we were able to detect differences within groups, may have been too small to detect differences between groups. All examiners were aware of the treatment and potential outcomes which may have biased results. All of our participants from both the home and clinic samples have been primarily white and educated which limits broad generalization.
Conclusion
In summary, we found no advantages to delivering the STOMP intervention in the home versus the clinic as both resulted in significant improvement in ADL. Toleration of massed practice by people with dementia appears good as evidence by very few displays of negative behavior over the course of the intensive, two-week intervention. The STOMP intervention holds promise as an evidence-informed model for the evaluation and treatment of ADL disability in people with mild-moderate dementia both in the clinic and in the home environment. Caregiver training in the STOMP intervention holds promise for extending ADL independence beyond the intervention. Future randomized-controlled trials will test the superiority of STOMP over other currently used behavioral or medical intervention for reducing ADL disability in people with dementia.
Acknowledgement
This project was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences for the National Institutes of Health through Grant Number 8UL1TR000041 and in part by the Oklahoma University Health Sciences Center. We would like to thank Janice Knoefel, MD, and the occupational therapists assisting in Albuquerque and Oklahoma City (Deepa Santhanan, MS, MOTR/L, Sydney Mann, MOTR/L and Amanda Lowrance, MOTR/L.
References
- Ciro CA. Maximizing ADL performance to facilitate aging in place for people with dementia. Nurs Clin North Am. 2014; 49: 157-169.
- Thies W, Bleiler L, Alzheimer's Association. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013; 9: 208-245.
- Crespo M, Hornillos C, de Quirós MB. Factors associated with quality of life in dementia patients in long-term care. Int Psychogeriatr. 2013; 25: 577-585.
- Li H, Zhang H, Huang H, Wang Y, Huang H. Caring burden and associated factors of care providers for senile dementia patients in an urban-rural fringe of Fuzhou City, China. Aging Clin Exp Res. 2012; 24: 707-713.
- Bruvik FK, Ulstein ID, Ranhoff AH, Engedal K. The quality of life of people with dementia and their family carers. Dement Geriatr Cogn Disord. 2012; 34: 7-14.
- Clare L, Linden DE, Woods RT, Whitaker R, Evans SJ, Parkinson CH, et al. Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: a single-blind randomized controlled trial of clinical efficacy. Am J Geriatr Psychiatry. 2010; 18: 928-939.
- Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Rikkert MG. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. BMJ. 2006; 333: 1196.
- Corbett A, Pickett J, Burns A, Corcoran J, Dunnett SB, Edison P, et al. Drug repositioning for Alzheimer's disease. Nat Rev Drug Discov. 2012; 11: 833-846.
- Man BY-W, Chan H-M, Leung C-H, Chan DS-H, Bai L-P, Jiang Z-H, et al. Group 9 metal-based inhibitors of β-amyloid (1–40) fibrillation as potential therapeutic agents for Alzheimer's disease. Chemical Science. 2011; 2: 917-921.
- Citron M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov. 2010; 9: 387-398.
- Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early-stage Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev. 2003; : CD003260.
- Bier N, Provencher V, Gagnon L, Van der Linden M, Adam S, Desrosiers J. New learning in dementia: transfer and spontaneous use of learning in everyday life functioning. Two case studies. Neuropsychol Rehabil. 2008; 18: 204-235.
- Hubbard IJ, Parsons MW, Neilson C, Carey LM. Task-specific training: evidence for and translation to clinical practice. Occup Ther Int. 2009; 16: 175-189.
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008; 51: S225-239.
- Rossi F, Gianola S, Corvetti L. Regulation of intrinsic neuronal properties for axon growth and regeneration. Prog Neurobiol. 2007; 81: 1-28.
- Pulvermüller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001; 32: 1621-1626.
- Taub E, Crago JE, Uswatte G. Constraint-induced movement therapy: A new approach to treatment in physical rehabilitation. Rehabilitation Psychology. 1998; 43: 152-170.
- Lillie R, Mateer CA. Constraint-based therapies as a proposed model for cognitive rehabilitation. J Head Trauma Rehabil. 2006; 21: 119-130.
- van Paasschen J, Clare L, Woods RT, Linden DE. Can we change brain functioning with cognition-focused interventions in Alzheimer's disease? The role of functional neuroimaging. Restorative Neurology and Neuroscience. 2009; 27: 473-491.
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2007; CD006073.
- Ciro C, Dao H, Anderson M, Robinson C, Hamilton T, Hershey L. Improving daily life skills in people with dementia: Testing the STOMP intervention model. Journal of Alzheimer's disease and Parkinsonism. Under review.
- Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006; 14: 139-144.
- Müller-Thomsen T, Arlt S, Mann U, Mass R, Ganzer S. Detecting depression in Alzheimer's disease: evaluation of four different scales. Arch Clin Neuropsychol. 2005; 20: 271-276.
- Vida S, Des Rosiers P, Carrier L, Gauthier S. Depression in Alzheimer's disease: receiver operating characteristic analysis of the Cornell Scale for Depression in Dementia and the Hamilton Depression Scale. J Geriatr Psychiatry Neurol. 1994; 7: 159-162.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980; 20: 649-655.
- Seng BK, Luo N, Ng WY, Lim J, Chionh HL, Goh J, et al. Validity and reliability of the Zarit Burden Interview in assessing caregiving burden. Ann Acad Med Singapore. 2010; 39: 758-763.
- Law M, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollack N. Canadian Occupational Performance Measure. Ottawa, Canada: CAOT Publications; 1990.
- Eyssen IC, Beelen A, Dedding C, Cardol M, Dekker J. The reproducibility of the Canadian Occupational Performance Measure. Clin Rehabil. 2005; 19: 888-894.
- Doig E, Fleming J, Kuipers P, Cornwell PL. Clinical utility of the combined use of the Canadian Occupational Performance Measure and Goal Attainment Scaling. Am J Occup Ther. 2010; 64: 904-914.
- Ciro CA, Hershey LA, Garrison D. Enhanced task-oriented training in a person with dementia with Lewy bodies. Am J Occup Ther. 2013; 67: 556-563.
- van Halteren-van Tilborg IA, Scherder EJ, Hulstijn W. Motor-skill learning in Alzheimer's disease: a review with an eye to the clinical practice. Neuropsychol Rev. 2007; 17: 203-212.
- Dick MB, Hsieh S, Dick-Muehlke C, Davis DS, Cotman CW. The variability of practice hypothesis in motor learning: does it apply to Alzheimer's disease? Brain Cogn. 2000; 44: 470-489.
- McColl MA, Paterson M, Davies D, Doubt L, Law M. Validity and community utility of the Canadian Occupational Performance Measure. Can J Occup Ther. 2000; 67: 22-30.
- Rubenstein LZ, Schairer C, Wieland GD, Kane R. Systematic biases in functional status assessment of elderly adults: effects of different data sources. J Gerontol. 1984; 39: 686-691.
- Magaziner J, Zimmerman SI, Gruber-Baldini AL, Hebel JR, Fox KM. Proxy reporting in five areas of functional status. Comparison with self-reports and observations of performance. Am J Epidemiol. 1997; 146: 418-428.
- Desai AK, Grossberg GT, Sheth DN. Activities of daily living in patients with dementia: clinical relevance, methods of assessment and effects of treatment. CNS Drugs. 2004; 18: 853-875.
- Mitchell MB, Miller LS, Woodard JL, Davey A, Martin P, Burgess M, et al. Regression-based estimates of observed functional status in centenarians. Gerontologist. 2011; 51: 179-189.
- Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. 5th edn. Champaign, IL: Human Kinetics. 2011.
- Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehabil Neural Repair. 2010; 24: 620-635.
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002; 125: 773-788.
- Boyd L, Winstein C. Explicit information interferes with implicit motor learning of both continuous and discrete movement tasks after stroke. J Neurol Phys Ther. 2006; 30: 46-57.
- Bharwani G, Parikh PJ, Lawhorne LW, VanVlymen E, Bharwani M. Individualized behavior management program for Alzheimer's/dementia residents using behavior-based ergonomic therapies. Am J Alzheimers Dis Other Demen. 2012; 27: 188-195.
- Mossello E, Ridolfi A, Mello AM, Lorenzini G, Mugnai F, Piccini C, et al. Animal-assisted activity and emotional status of patients with Alzheimer's disease in day care. Int Psychogeriatr. 2011; 23: 899-905.