
Review Article
Austin Alzheimers J Parkinsons Dis. 2014;1(2): 9.
Direct- And Spacer-Coupled Codrug Strategies for the Treatment of Alzheimer’s Disease
Fornasari E, Di Stefano A and Cacciatore I*
Department of Pharmacy, University “G. d’Annunzio” of Chieti-Pescara, Italy
*Corresponding author: Cacciatore I, Department of Pharmacy, University of Chieti-Pescara, via Dei Vestini 31, 66100 Chieti Scalo (CH), Italy.
Received: August 18, 2014; Accepted: October 01, 2014; Published: October 06, 2014
Abstract
Over the last years, the ‘multi-target-directed ligand’ strategy has been exploited by many researchers to develop novel attractive tools in the search for new agents for Alzheimer’s disease (AD). Small molecules that concurrently target and modulate AD multiple pathological factors can be synthesized using such strategy. This paper will mainly focus on direct- and spacer-coupled codrug approaches that we recently rationally used to design multifunctional molecules able to contrast oxidative stress, neuroinflammation, glutamate toxicity, and metal dyshomeostasis, as a function of the structural elements introduced in the chemical framework.
Although the potential use of these strategies needs further exhaustive studies, it may offer a promising therapeutic alternative for increasing neuronal protection and preventing AD progression.
Keywords: Alzheimer’s disease; Multi target direct ligand strategy; Codrug; Glutathione; Lipoic acid
Introduction
Alzheimer’s disease (AD) is a disabling brain disorder that is going to involve 100 million people all over the world by 2050 [1]. AD is marked by gradual memory loss and cognitive decline connected to a decrement of cholinergic neurons and acetylcholine (AChE) levels. Neuritic plaques – composed of amyloid- β protein (Aβ) – and Neurofibrillary Tangles (NFT) – formed by hyperphosphorylated tau proteins (t) – characterize AD affected brain [2]. Among different isoforms produced by the consecutive hydrolysis of the Aβ precursor (AβPP) mediated by β- and γ-secretases [3,4], the Aβ1-42 isoform in the brain intensifies the tendency for peptide aggregation [5] leading to a quickened formation of small Aβ oligomers [6,7]. In addition, after hyperphosphorylation, t-protein detaches from the microtubules and forms NFT intracellular neurotoxic aggregates [8]. These last, together with Aβ oligomers, are connected to neurotoxicity in AD brains.
The etiology of AD still remains obscure, but several events – for instance oxidative stress, Aβ plaques, metal dyshomeostasis, excitotoxicity, protein misfolding, and inflammatory processes – play a strategic role in the advancement of AD [9,10]. It is intricate to found the right succession of these events, but it was proved that oxidative damage is one of the initial pathological markers [11- 15]. Oxidative stress arises when the normal equilibrium between oxidative events and antioxidant defenses is interrupted either by deficiency of antioxidant enzymes or by incremented production of Reactive Oxygen Species (ROS).
In these pathological conditions, ROS react with lipids, proteins, nucleic acids, and other molecules thus altering their structure and function [16]. ROS generation in AD brains could be produced by high amounts of metal ions (Fe, Al, and Hg) or by Aβ aggregates [17]. In fact, the incubation of Cu(II), Zn(II), or Fe(III) with Aβ oligomers promotes the development of Aβ1-40 and Aβ1-42 deposits; notably, the deposition of fibrillar amyloid plaques is enhanced by Fe(III), while the formation of amorphous aggregates is induced by Cu(II) and Zn(II) [18]. Besides, Aβ plaques are responsible for inflammation in AD brains: in vitro studies on astrocytes have convincingly demonstrated that Aβ and APP activate glia in a dose- and timedependent manner, as measured by morphological response and expression of potent pro-inflammatory cytokines and TNF-α [19]. Neuroinflammatory processes that characterize AD might also be responsible for the depletion of Glutathione (GSH), the main tripetide able to counteract the oxidant injury [20,21]; effectively, some researchers found lowered GSH levels in AD brains [22,23]. Altogether, these data confirm that reduced antioxidant systems, metal ion dyshomeostasis, neuroinflammation, and Aβ aggregation generate a vicious circle closely related to the onset of AD.
Multi-Target Therapy for the Cure of AD
Current cure for AD focuses on symptomatic aspects of the pathology and four cholinesterase inhibitors (tacrine, donepezil, rivastigmine, and galantamine) and Memantine (MEM) are the only drugs approved by FDA. Acetylcholinesterase inhibitors enhance cholinergic neurotransmission through inhibition of acetylcholinesterase, thus decreasing the breakdown of ACh [24,25]. MEM - an uncompetitive N-methyl-D-aspartate (NMDA) antagonist - is the only drug employed in the therapy of moderate to severe dementia [26,27]. Nevertheless, all these drugs are not satisfactory to heal AD or to stop its progression. In fact, as AD is featured by a multifactorial nature, drugs are required to hit the pathology at different levels simultaneously [28].
In recent years, several medicinal chemistry approaches were proposed in the search for novel drug candidates [29]. Among them, the ‘Multi-Target-Directed Ligand’ (MTDL) strategy [30-33] is drawing attention of many researchers (Figure 1). Indeed, small molecules that concurrently target and modulate the AD pathological factors can be designed using this methodology. Hybrids and codrugs are examples of MTDLs [34]: the first ones are constituted by two diverse pharmacophores joined via a permanent bond, and that exert a dual effect resulting from a single chemical entity acting on different biological targets without undergoing enzymatic cleavage; on the other hand, codrugs consist of two or more pharmacophores direct-and/or spacer-coupled via a covalent chemical linkage that, after enzymatic biotransformation, explicate their biological effects. The major limitation of this approach is the requirement of welldesigned groups for the linker [34], whereas the main advantage is represented by the advancement of pharmacokinetic profiles of the single drugs. Figure 1 reports a schematic representation regarding the different ways of action of both hybrids and codrugs. Many examples of hybrids were proposed for the cure of AD and the most successful ones were reported in Table 1 [36-46].
Hybrid
AChEI (IC50)
BuChE (IC50)
Inhibition
of Aß aggregation
Reference
Donezepil-Tacrine

0.27 ± 0.23 nM
66.3 ± 4.0
Aß1-40 = 46.1 %
[37]
AP2238

44 ± 0.006 nM
48.9 ± 3.7 mM
Aß1-42 > 50%
[38]
AP2469

8.60 ± 0.21 mM
124 ± 13 mM
Aß1-42 > 50%
[38]
Donezepil-Ebselen

97 ± 0.007 nM
-
Aß1-40 = 21.4 %
[39]
Tacrine-Dihydropyridine

105 ± 15 nM
> 100000
Aß1-42 = 34.9 %
[40]
Tacrine-Ferulic acid

10.9 nM
17.7 nM
-
[41]
Tacrine-8-hydroxyquinoline

7 0.50 ± 0.02 nM
8 1.0 ± 0.05 nM
6.5 ± 0.3 nM
55 ± 2 nM
19%
27%
[42]
Uperizine A-Tacrine

9 16.5 ± 0.3 nM
10 15.7 ± 0.9 nM
11 6.4 ± 0.8 nM
20.8 ± 2.6 nM
30.8 ± 2.0 nM
19.5 ± 1.9 nM
[43]
Galantamine-Memantine (Memagal)

1.16 ± 0.003 nM
-
[44]
Tacrine-Flurbiprofen

19.3 nM
3.7 nM
A? 1-40 = 31 %
[45]
Lipocrine

0.25 nM
10.8 nM
[46]
Memoquin

2.60 ± 0.48 nM
-
A? 1-40 28.3 ?M
A? 1-42 5.93 ?M
[47]
Table 1: Recent examples of the most promising hybrids proposed as MTDLs for the treatment of AD.
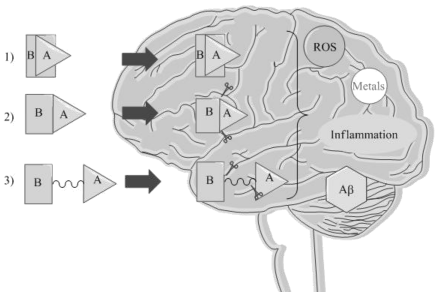
Figure 1: Schematic representation of MTDL strategy. 1) Hybrid molecule consists of two or more pharmacophores (A and B) fused to each other that do not require enzymatic activation to exert the biological effects; 2) Directcoupled codrug consists of two or more pharmacophores (A and B) linked to each other via a covalent bond that explicate the biological effects after enzymatic biotransformation; 3) Spacer-coupled codrug consists of two or more pharmacophores (A and B) linked to each other via a linker that is cleavable after enzymatic activity, releasing individually A and B able to operate at the active sites.
The codrug strategy has been widely used for the discovery of novel compounds in many therapeutic fields (i.e. anticancer, antibacterial, antiparkinson, antiviral), whereas it was less explored to discover novel MTDLs for AD [34].
Recently, Chen et al. developed the tacrine-silibinin codrug (16), obtained through conjugation of tacrine to silibinin, a natural compound provided with neuroprotective properties (Figure 2) [35]. This codrug, in addition to the decrease of epatotoxicity due to tacrine, resulted endowed with potent AChE and BuChE inhibition properties (IC50 = 53.9 and 49.7 nM, respectively).
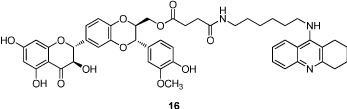
Figure 2: Structure of tacrine-silibinin codrug.
Because of the lack of literature data regarding AD codrugs, the present work is intended as an overview which mainly focuses on codrugs that we synthesized to simultaneously strengthen neuronal protection and avoid AD progression (Figure 3).
Ibuprofen-Glutathione, Ibuprofen-Lipoic Acid and Lipoic acid-GPE Codrugs against Oxidative Stress and Neuroinflammation in AD
Neuroprotective antioxidants constitute a promising class of molecules for the treatment of AD [47-50]. Among them, sulfurcontaining compounds [GSH, lipoic acid (LA), and other thiol derivatives] are able to hamper cell damage by counteracting ROS through different modes of action [51]. In this context, GSH behaves as direct or indirect scavenger of ROS in brain cells and works as substrate for enzymes implicated in detoxification processes [52]. GSH depletion is well documented in many neurodegenerative disorders, hence its restoration might be a potential strategy for the management of such pathologies. The uptake of GSH through the Blood-Brain-Barrier (BBB) is regulated by its membrane transporters; in fact, these last are capable of recognizing the GSH portion even when bound to other molecules which act as shuttles for the delivery of drugs inside the brain [53]. Alternatively, GSH levels can be reverted using GSH prodrugs, codrugs, and analogs, and cysteine derivatives. On the basis of our interest for short bioactive peptides, in 2007 we started to synthesize novel GSH-containing codrugs for the treatment of neurodegenerative diseases. Therefore, the direct-coupled codrug strategy was employed for the conjugation of ibuprofen to GSH (IBUGSH, 17) via an amide bond (Figure 3) [54]. AD affected patients found melioration following the treatment with IBU, as it specially reduces the a1-antichymotrypsin-mediated amyloidogenesis [55] and Aβ1-42 production, thus preventing the advancement of AD [56,57] in virtue of an allosteric modulation of γ-secretase activity. Due to the conjugation with GSH, IBU is dragged into the BBB explicating its antinflammatory activity directly on impaired neurons. Evaluation of IBU-GSH physico-chemical properties suggested that codrug 17 could be orally administered since it showed water solubility higher than 10 µg/mL and LogP value superior to 1.35 (Table 2); moreover, this LogP value is also appropriate for CNS penetration. Kinetic data revealed a good stability in simulated fluids (pH 1.3 and 7.4, t1/2 > 16 h), while in human plasma a slow bioconversion to IBU was observed (t1/2 > 0.7 h) (Table 3).
Codurg
Chemical hydrolysis
Human plasma
Rat Plasma
t˝ (h)
pH 1.3
kobs (h-1)
pH 1.3
t˝ (h)
pH 7.4
kobs (h-1)
pH 7.4
t˝ (h)
kobs (h-1)
t˝ (h)
kobs (h-1)
17
>16
n.a.
>16
n.a.
>0.75
0.91±0.02
n.a.
n.a.
18
>85
n.a.
>85
n.a.
3.005±0.135
0.23±0.01
1.137±0.023
0.66±0.02
19
>85
n.a.
>85
n.a.
2.020±0.057
0.35±0.01
0.842±0.025
0.84±0.02
20
>85
n.a.
>85
n.a.
1.922±0.070
0.36±0.01
0.718±0.0018
0.97±0.02
21
58.75±1.76
0.0118±0.0002
217.17±2.35
0.0032±0.0001
3.14±0.08
0.22±0.03
0.085±0.004
8.15±0.46
22
55.0±2.4
0.013±0.001
120±1
0.005±0.001
1.57±0.08
0.44±0.02
immediate
n.a.
23
6.32±0.09
0.29±0.01
30.2±1.1
0.023±0.010
5.72±0.23
0.12±0.02
0.99±0.04
0.70±0.02
24
>7
n.a.
15.37±0.32
0.045±0.001
8.50±0.39
0.081±0.001
n.a.
n.a.
25
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
a Values are the means of ± three experiments; n.a.: not available.
Table 3: Kinetic data for chemical and enzymatic hydrolysis of codrugs 17-25 at 37 °Ca.
Apart from its ROS scavenging activity, IBU-GSH was also assayed for its ability to antagonize the harmful effects of Aβ1-40 in an in vivo experimental model. In behavioral tests of long-term spatial memory, rats treated with codrug 17 (23 µmol/Kg for 28 days) showed a significant improvement compared to the controls (rat treated only with Aβ1-40, IBU + Aβ1-40, GSH + Aβ1-40, respectively). Behavioral results were strengthened by histochemical analysis, showing a lower expression of Aβ plaques and pyknotic cells in Aβ- infused rat cerebral cortex [54,58].
In the two-year period 2010-2012, we synthesized other codrugs containing IBU conjugated to LA via an amide bond (IBU-LA, 18-20) (Figure 3) using the spacer-coupled codrug strategy [59- 62]. LA has proved to be a ROS scavenger, a chelant of metals, a GSH precursor [63] and a good BBB-crossing agent [64]. For the synthesis of codrugs 18-20 we selected polyamine chains of different length as chemical spacers, as they are involved in many signaling pathways regulating the expression of different proteins [65]. Oral administration of codrugs 18-20 could be suitable since their water solubility is higher than 10 µg/mL and LogP values are superior to 1.35 (Table 2); in addition, cLogP values (> 4) favor their BBB permeation. The pharmacokinetic profiles of codrugs 18-20 revealed a high stability (t1/2 > 85 h) in aqueous buffer solutions (pH 1.3 and 7.4) and in human plasma (t1/2 > 2 h) (Table 3). Furthermore, IBU-LA codrugs were hydrolyzed more rapidly in brain tissue (t1/2 > 13 min) than in human plasma, indicating that these new entities might allow targeted delivery of the parent drugs to damaged neurons [59]. DPPH and DOM experiments evidenced free radical scavenging activities of codrugs 18-20.
In Aβ1-40–infused rat cerebral cortex codrug 18 treatment showed neuroprotective activity at the dose of 10 mg/Kg for 28 days; it resulted able to decrease aggregation of Aβ plaques and expression of Aβ1-40 proteins [60], as well as limit NOS activity [61] that is augmented in Aβ1-40-perfused rat brains, as reported by Limon et al [68]. In the same experimental model, neuroprotective and antiapoptotic roles of codrug 18 on neuroglobin (Ngb) levels have been investigated [62] since Ngb deficiency is associated with age-related neurodegeneration [67]. IBU-LA (18) displayed a significant restoration of Ngb levels in Aβ-infused rat cerebral cortex respect to the control through p-Akt and p–CREB recruitment [62].
In 2012 we conjugated LA, via direct-coupled codrug strategy, to another endogenous neuroprotective tripeptide, glycyl-L-prolyl- L-glutamic acid (GPE) that is able to guarantee the release of acetylcholine and dopamine from rat cortex and striatum through stimulation of dopaminergic neurotransmission (Figure 3). In AD experimental models, the neuroprotective effect of GPE seems to be connected to the regulation of intracellular Ca2+ signaling and programmed neuronal death [68].
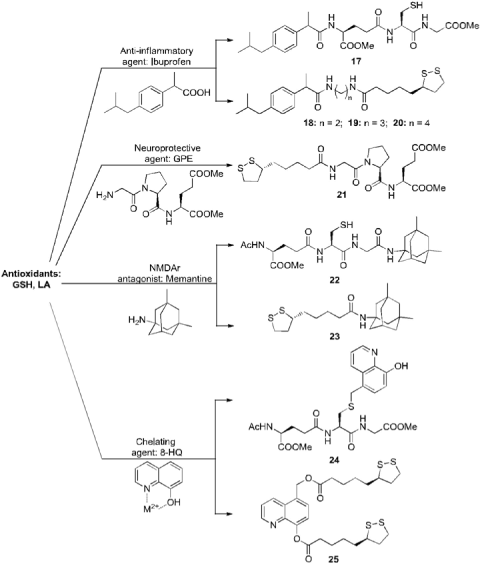
Figure 3: Direct- and spacer-coupled codrug strategy applied to the synthesis
of multifunctional ligands for the treatment of AD.
LA-GPE (21) showed an adequate pharmacokinetic profile owing to a good chemical stability at pH 1.3 (t1/2 = 58 h ), 7.4 (t1/2 = 217 h ), and human plasma (t1/2 > 3 h ), exhibiting good lipophilicity (logP = 1.51) and water solubility (8.65 mg/mL) (Tables 2-3). Additionally, LA-GPE has been classified as a high CNS permeable compound (Pe > 4 x 10-6 cm s-1) using Parallel Artificial Membrane Permeability Assay (PAMPA-BBB), a non-cell based assay to predict the ability of the codrug to diffuse through BBB (Table 2). At the concentration of 100 µM LA-GPE displayed neuroprotective activity against H2O2 in SH-SY5Y human neuroblastoma cells through a mechanism not yet defined. Overall, these findings suggest that IBU-GSH, IBU-LA, and LA-GPE may protect against oxidative stress generated by ROS and reduce AD inflammatory state.
Codrug
Water Solubilitya
Lipophilicity
PAMPA-GI Pe(10-6 cm s-1)b
PAMPA-BBB Pe(10-6 cm s-1)b
(mg/mL)
LogPa
cLogP
LogK0
pH 5.0c
pH 6.5 c
pH 7.4 c
pH 7.4 c
Classificationd
17
0.0103 ± 0.9 x 10-3
2.70 ± 0.20
3.02 ± 0.72
3.300 ± 0.140
n.a.
n.a.
n.a.
n.a.
n.a.
18
0.02 ± 0.9 x 10-3
n.a.
4.43 ± 0.53
2.239 ± 0.090
n.a.
n.a.
n.a.
n.a.
n.a.
19
0.02 ± 0.2 x 10-3
n.a.
4.95 ± 0.51
2.387 ± 0.095
n.a.
n.a.
n.a.
n.a.
n.a.
20
0.01 ± 0.4 x 10-3
n.a.
5.71 ± 0.50
2.719 ± 0.057
n.a.
n.a.
n.a.
n.a.
n.a.
21
8.65 ± 0.35
1.51 ± 0.02
1.43 ± 0.01
n.a.
n.a.
6.05
12.63
6.45
CNS +
22
0.43 ± 0.02
2.55 ± 0.01
1.67 ± 0.74
0.610 ± 0.030
n.a.
n.a.
n.a.
1.8
CNS -
23
0.02 ± 0.004
4.20 ± 0.06
5.23 ± 0.46
2.660 ± 0.090
0.51
2.57
10
3.47
CNS +/-
24
12 ± 0.42
n.a.
n.a.
n.a.
7.5
12.9
16
2.8
CNS +/-
25
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
a Values are the means of ± three experiments
b Artificial membrane permeability coefficient
c pH of both donor and acceptor compartments
d ‘CNS + ’ (high BBB permeation predicted), Pe (10-6 cm s-1) > 4.0; ‘CNS+ /- ’ (BBB uncertain permeation), Pe (10-6 cm s-1) from 4.0 to 2.0 ‘CNS _ ’
(low BBB permeation predicted), Pe (10-6 cm s-1) < 2.0; The calculated cLogP was determined using ACD LogP software package, version 4.55 (Advanced Chemistry Development Inc., Toronto, Canada); n.a.: Not Available
Table 2: Physico-chemical properties of codrugs 17-25.
Glutathione-Memantine and Lipoic Acid- Memantine Codrugs Against Oxidative Stress and NMDA Excitotoxicity in AD
Another approach for the treatment of AD is to block glutamatergic neurotransmission [69] since an overactivation of NMDA receptors (NMDAr), followed by high intracellular concentrations of Ca2+, is responsible for excitotoxicity. Therefore, NMDAr antagonists can be useful for the cure of pathologies characterized by an overstimulation of NMDAr [70]. As NMDAr antagonist, Memantine (MEM) was approved in 2004 by FDA for the management of late-stage cases of AD; its mechanism of action is based on the modulation of Ca2+ influx leaving the channel relatively open for neurotransmission at low stimulation rates [71]. These considerations gave the rationale for developing a new series of codrugs that completed the pharmacological activities of MEM and antioxidant molecules
Therefore, in 2013 we combined the antiglutamatergic properties of MEM to the radical scavenging activities of antioxidant agents, such as GSH and LA, synthesizing two novel anti-AD codrugs named GSH-MEM (22) and LA-MEM (23) (Figure 3) [69]. Codrugs 22-23 were obtained by joining GSH or LA, respectively, to MEM via an amide bond using the direct-coupled codrug strategy. GSH-MEM possesses a good water solubility (0.43 mg/mL), whereas LA-MEM seems to be less water soluble (0.02 mg/mL), in agreement with the higher lipophilicity of LA (Table 2). The water solubility and LogP suggested a good absorption profile of codrug 22 after oral administration (Table 2). GSH-MEM showed a good stability toward gastrointestinal hydrolysis (t1/2 = 55 h) compared to LA-MEM (t1/2 > 6 h) (Table 3). On the contrary, in rat and human plasma GSHMEM underwent rapid bioconversion into its constituents (t1/2 > 1 h in human plasma and immediate hydrolysis in rat plasma), while LA-MEM displayed a good enzymatic stability in both enzymatic environments (t1/2 > 5 h in human plasma and > 1 h in rat plasma). Codrug 23 was classified as greatly BBB-permeable (Pe = 3.47 x 10-6 cm s-1) while codrug 22 as weakly BBB-permeable (Pe = 1.80 x 10-6 cm s-1), suggesting a good penetration through the BBB and a targeted delivery of LA directly to sites damaged by oxidative stress (Table 2). The antioxidant profiles of 22-23 were confirmed by testing them on GL15 cellular population, an astroglial-like cell line used as an in vitro model of astrocytes. At the concentration of 10 µM, both codrugs were able to contrast the intracellular ROS produced by H2O2 in GL15 cells similarly to GSH and LA. LA-MEM significantly inhibits Aβ1-42 aggregation interfering with the Aβ aggregation process, as evidenced by a ThT fluorimetric in vitro assay [73]. These favorable properties make LA-MEM a promising candidate to be screened in vivo for its anti-AD activities.
Glutathione-8-Hydroxyquinoline and Bis- Lipoyl-8-Hydroxyquinoline Codrugs Against Oxidative Stress and Metal Dyshomeostasis in AD
High levels of metal ions [Cu(II), Zn(II), and Fe(III)] have been found in Aβ plaques of AD brains. Particularly, Cu(II) and Zn(II), bind to Aβ peptides facilitating Aβ aggregation. Moreover, Cu(II) and Fe(III), either unbound and bound to Aβ peptides, promote overproduction of ROS, well-known for their toxicity [72]. Many reports support the hypothesis that the modulation of biometals in the brain represents a promising therapeutic strategy for the treatment of AD [73-75]. Recently, novel inhibitors able to complex metals were developed with the aim of inhibiting Aβ aggregation [76-78].
Lately, to search chemical tools skilled in reducing oxidative stress and modulating metal dyshomeostasis in AD patients, we designed novel codrugs potentially able to interfere with metal-Aβ and metal-ROS interactions. We focused on molecular combinations obtained by joining GSH and/or LA, as antioxidant molecules (Figure 3), with 8-hydroxyquinoline (8-HQ) that – chelating Cu(II), Fe(III), and Zn(II) – prevents the precipitation of Aβ plaques [79-81].
GS(HQ)H (24) is a water soluble (12 mg/mL) codrug, stable in human plasma with a t1/2 > 8 h and BBB-permeable (Pe = 2.8 x 10-6 cm s-1) (Tables 2-3) [82]. In in vitro experiments on SH-SY5Y human neuroblastoma cells – differentiated with Retinoic Acid (RA) to obtain a cholinergic phenotype – codrug 24, at the concentration of 1 µM, showed a marked prevention of cellular death against H2O2- induced oxidative stress. Moreover, the experimental KD values demonstrated that 24 could be able to remove Cu(II) and Zn(II) from the Aβ peptide, leaving unmodified physiological pools of Cu(II) and Zn(II) in vivo. The KD value obtained for the Cu(II)/ligand system is 280 pmol/L, whereas the one calculated for Zn(II)/ligand system is 0.57 µmol/L, within the range suggested by Faller and Hureau [83]. The synergic effect of GSH and HQ resulted evident when the two molecules were combined respect to the single compounds. By globally evaluating the activity profile, GS(HQ)H could be one of the earliest chelanting codrugs to test on Aβ-infused rats.
In parallel, codrug LA-HQ-LA (25), obtained combining the antioxidant and neuroprotective properties of LA and chelating activities of 8-HQ, was synthesized by linking via two ester bonds the 5-hydroxymethyl-8-hydroxyquinoline to LA (Figure 3) [84]. Codrug 25, due to its elevated lipophilicity, is able to cross the BBB and possessing two ester bonds of different strength, might slowly and continuously release LA and HQ directly inside the brain. Our outcomes showed that LA-HQ-LA resulted in significant neuroprotective and antioxidant effects against H2O2-induced neurotoxicity in human neuroblastoma SH-SY5Y cells differentiated with RA. The metal chelating properties and the pharmacokinetic profile of codrug 25 are currently under study in our research laboratory.
Conclusion
The complexity of AD is suggested to arise from multiple pathological factors, such as oxidative stress, free radicals, metal dyshomeostasis, and neuroinflammation; all these factors contribute in a different manner to the onset of AD. To simultaneously contrast these features, in the last years we have rationally designed novel codrugs by joining antioxidant portions to molecules endowed with antinflammatory, antiglutamatergic, or metal chelating activities into a single framework. These codrugs could diminish the oxidative damage caused by mitochondrial free radicals and delay the degenerative process related to excessive deposition of Aβ, metal accumulation, or glutamate excitotoxicity. These properties highlight that our multifunctional ligands could be interesting in the search for new disease-modifying agents for AD. In our upcoming research, we intend to widen this work by testing in vivo all the synthesized codrugs and preparing nanotechnologic formulations to improve their pharmacokinetic profiles, thus rendering them attractive tools for future pharmacological applications.
Acknowledgement
The authors would like to thank Italian Ministry of University and Research (MIUR) for the financial support.
References
- Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer's disease. Arch Med Res. 2012; 43: 600-608.
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature. 1999; 399: A23-31.
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992; 359: 322-325.
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992; 258: 126-129.
- Jarrett JT, Berger EP, Lansbury PT Jr. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993; 32: 4693-4697.
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002; 416: 535-539.
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004; 44: 181-193.
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001; 65: 391-426.
- Jellinger KA. General aspects of neurodegeneration. J Neural Transm Suppl. 2003; 101-144.
- Lombet A, Zujovic V, Kandouz M, Billardon C, Carvajal-Gonzalez S, Gompel A, et al. Resistance to induced apoptosis in the human neuroblastoma cell line SK-N-SH in relation to neuronal differentiation. Role of Bcl-2 protein family. Eur J Biochem. 2001; 268: 1352-1362.
- Polidori MC, Griffiths HR, Mariani E, Mecocci P. Hallmarks of protein oxidative damage in neurodegenerative diseases: focus on Alzheimer's disease. Amino Acids. 2007; 32: 553-559.
- Markesbery WR. The role of oxidative stress in Alzheimer disease. Arch Neurol. 1999; 56: 1449-1452.
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003; 53 Suppl 3: S26-36.
- Moreira PI, Honda K, Quan L, Aliev G, Oliveira CR, Santos MS, et al. Alzheimer's Disease and Oxidative Stress: The Old Problem Remains Unsolved. Curr Med Chem. 2005; 5: 51-62.
- Farooqui T, Farooqui AA. Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev. 2009; 130: 203-215.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39: 44-84.
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997; 23: 134-147.
- Cricthton RR, Dexter DT, Ward RJ. Metal based neurodegenerative diseases - From molecular mechanism to therapeutic strategies. Coordin Chem Rev. 2008; 252: 1189-1199.
- Gilgun-Sherki Y, Melamed E, Offen D. Anti-inflammatory drugs in the treatment of neurodegenerative diseases: current state. Curr Pharm Des. 2006; 12: 3509-3519.
- Cardinaux JR, Allaman I, Magistretti PJ. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia. 2000; 29: 91-97.
- Stefanis L, Park DS, Friedman WJ, Greene LA. Caspase-dependent and -independent death of camptothecin-treated embryonic cortical neurons. J Neurosci. 1999; 19: 6235-6247.
- Gu M, Owen AD, Toffa SE, Cooper JM, Dexter DT, Jenner P, et al. Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J Neurol Sci. 1998; 158: 24-29.
- Liu H, Harrell LE, Shenvi S, Hagen T, Liu RM. Gender differences in glutathione metabolism in Alzheimer's disease. J Neurosci Res. 2005; 79: 861-867.
- Pohanka M. Acetylcholinesterase inhibitors: a patent review (2008 - present). Expert Opin Ther Pat. 2012; 22: 871-886.
- Hyde C, Peters J, Bond M, Rogers G, Hoyle M, Anderson R, et al. Evolution of the evidence on the effectiveness and cost-effectiveness of acetylcholinesterase inhibitors and memantine for Alzheimer's disease: systematic review and economic model. Age Ageing. 2013; 42: 14-20.
- Hellweg R, Wirth Y, Janetzky W, Hartmann S. Efficacy of memantine in delaying clinical worsening in Alzheimer's disease (AD): responder analyses of nine clinical trials with patients with moderate to severe AD. Int J Geriatr Psychiatry. 2012; 27: 651-656.
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006; : CD003154.
- Bolognesi ML, Matera R, Minarini A, Rosini M, Melchiorre C. Alzheimer's disease: new approaches to drug discovery. Curr Opin Chem Biol. 2009; 13: 303-308.
- Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008; 51: 347-372.
- León R, Garcia AG, Marco-Contelles J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer's disease. Med Res Rev. 2013; 33: 139-189.
- Zhang HY. One-compound-multiple-targets strategy to combat Alzheimer's disease. FEBS Lett. 2005; 579: 5260-5264.
- Muñoz-Torrero D, Camps P. Dimeric and hybrid anti-Alzheimer drug candidates. Curr Med Chem. 2006; 13: 399-422.
- Van der Schyf CJ, Mandel S, Geldenhuys WJ, Amit T, Avramovich Y, Zheng H, et al. Novel multifunctional anti-Alzheimer drugs with various CNS neurotransmitter targets and neuroprotective moieties. Curr Alzheimer Res. 2007; 4: 522-536.
- Van der Schyf CJ, Mandel S, Geldenhuys WJ, Amit T, Avramovich Y, Zheng H, et al. Novel multifunctional anti-Alzheimer drugs with various CNS neurotransmitter targets and neuroprotective moieties. Curr Alzheimer Res. 2007; 4: 522-536.
- Chen X, Zenger K, Lupp A, Kling B, Heilmann J, Fleck C, et al. Tacrine-silibinin codrug shows neuro- and hepatoprotective effects in vitro and pro-cognitive and hepatoprotective effects in vivo. J Med Chem. 2012; 55: 5231-5242.
- Camps P, Formosa X, Galdeano C, Gómez T, Muñoz-Torrero D, Scarpellini M, et al. Novel donepezil-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. J Med Chem. 2008; 51: 3588-3598.
- Tarozzi A, Bartolini M, Piazzi L, Valgimigli L, Amorati R, Bolondi C, et al. From the dual function lead AP2238 to AP2469, a multi-target-directed ligand for the treatment of Alzheimer’s disease. Pharma Res Per. 2014; e00023.
- Luo Z, Sheng J, Sun Y, Lu C, Yan J, Liu A, et al. Synthesis and evaluation of multi-target-directed ligands against Alzheimer's disease based on the fusion of donepezil and ebselen. J Med Chem. 2013; 56: 9089-9099.
- Marco-Contelles J, León R, de los Ríos C, Samadi A, Bartolini M, Andrisano V, et al. Tacripyrines, the first tacrine-dihydropyridine hybrids, as multitarget-directed ligands for the treatment of Alzheimer's disease. J Med Chem. 2009; 52: 2724-2732.
- Chen Y, Sun J, Fang L, Liu M, Peng S, Liao H, et al. Tacrine-ferulic acid-nitric oxide (NO) donor trihybrids as potent, multifunctional acetyl- and butyrylcholinesterase inhibitors. J Med Chem. 2012; 55: 4309-4321.
- Fernández-Bachiller MI, Pérez C, Gonzélez-Muñoz GC, Conde S, López MG, Villarroya M, et al. Novel tacrine-8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimers disease, with neuroprotective, cholinergic, antioxidant, and copper-complexing properties. J Med Chem. 2010; 53: 4927-4937.
- Gemma S, Gabellieri E, Huleatt P, Fattorusso C, Borriello M, Catalanotti B, et al. Discovery of huperzine A-tacrine hybrids as potent inhibitors of human cholinesterases targeting their midgorge recognition sites. J Med Chem. 2006; 49: 3421-3425.
- Simoni E, Daniele S, Bottegoni G, Pizzirani D, Trincavelli ML, Goldoni L, et al. Combining galantamine and memantine in multitargeted, new chemical entities potentially useful in Alzheimer's disease. J Med Chem. 2012; 55: 9708-9721.
- Chen Y, Sun J, Peng S, Liao H, Zhang Y, Lehmann J. Tacrine-flurbiprofen hybrids as multifunctional drug candidates for the treatment of Alzheimer's disease. Arch Pharm (Weinheim). 2013; 346: 865-871.
- Rosini M, Andrisano V, Bartolini M, Bolognesi ML, Hrelia P, Minarini A, et al. Rational approach to discover multipotent anti-Alzheimer drugs. J Med Chem. 2005; 48: 360-363.
- Bolognesi ML, Cavalli A, Melchiorre C. Memoquin: a multi-target-directed ligand as an innovative therapeutic opportunity for Alzheimer's disease. Neurotherapeutics. 2009; 6: 152-162.
- Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: A promising therapeutic intervention in neurodegenerative disease. Free Radic Res. 2011; 45: 888-905.
- Di Matteo V, Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Curr Drug Targets CNS Neurol Disord. 2003; 2: 95-107.
- Cacciatore I, Cornacchia C, Baldassarre L, Fornasari E, Mollica A, Stefanucci A, et al. GPE and GPE analogues as promising neuroprotective agents. Mini Rev Med Chem. 2012; 12: 13-23.
- Cornacchia C, Cacciatore I, Baldassarre L, Mollica A, Feliciani F, Pinnen F. 2,5-diketopiperazines as neuroprotective agents. Mini Rev Med Chem. 2012; 12: 2-12.
- Moosmann B, Behl C. Antioxidants as treatment for neurodegenerative disorders. Expert Opin Investig Drugs. 2002; 11: 1407-1435.
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000; 62: 649-671.
- Kannan R, Kuhlenkamp JF, Jeandidier E, Trinh H, Ookhtens M, Kaplowitz N. Evidence for carrier-mediated transport of glutathione across the blood-brain barrier in the rat. J Clin Invest. 1990; 85: 2009-2013.
- Pinnen F, Sozio P, Cacciatore I, Cornacchia C, Mollica A, Iannitelli A, et al. Ibuprofen and glutathione conjugate as a potential therapeutic agent for treating Alzheimer's disease. Arch Pharm (Weinheim). 2011; 344: 139-148.
- Morihara T, Teter B, Yang F, Lim GP, Boudinot S, Boudinot FD, et al. Ibuprofen suppresses interleukin-1beta induction of pro-amyloidogenic alpha1-antichymotrypsin to ameliorate beta-amyloid (Abeta) pathology in Alzheimer's models. Neuropsychopharmacology. 2005; 30: 1111-1120.
- Pasqualetti P, Bonomini C, Dal Forno G, Paulon L, Sinforiani E, Marra C, et al. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer's disease. Aging Clin Exp Res. 2009; 21: 102-110.
- Zara S, De Colli M, Sozio P, Cacciatore I, Bosco D, Di Stefano A, et al. Ibuprofen-Glutathione conjugate as anti-inflammatory and anti-apoptotic agent in rat brain infused withβ Amyloid (1-40). J Cytol Histol. 2014; 5: 249.
- Sozio P, D'Aurizio E, Iannitelli A, Cataldi A, Zara S, Cantalamessa F, et al. Ibuprofen and lipoic acid diamides as potential codrugs with neuroprotective activity. Arch Pharm (Weinheim). 2010; 343: 133-142.
- Zara S, Rapino M, Sozio P, Di Stefano A, Nasuti C, Cataldi A. Ibuprofen and lipoic acid codrug 1 control Alzheimer's disease progression by down-regulating protein kinase C e-mediated metalloproteinase 2 and 9 levels inβ-amyloid infused Alzheimer's disease rat model. Brain Res. 2011; 1412:79-87.
- Zara S, Di Stefano A, Nasuti C, Rapino M, Patruno A, Pesce M, et al. NOS-mediated morphological and molecular modifications in rats infused with Aß (1-40), as a model of Alzheimer's disease, in response to a new lipophilic molecular combination codrug-1. Exp Gerontol. 2011; 46: 273-281.
- Zara S, De Colli M, Rapino M, Pacella S, Nasuti C, Sozio P, et al. Ibuprofen and lipoic acid conjugate neuroprotective activity is mediated by Ngb/Akt intracellular signaling pathway in Alzheimer's disease rat model. Gerontology. 2013; 59: 250-260.
- Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997; 29: 315-331.
- Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997; 22: 359-378.
- Melchiorre C, Bolognesi ML, Minarini A, Rosini M, Tumiatti V. Polyamines in drug discovery: from the universal template approach to the multitarget-directed ligand design strategy. J Med Chem. 2010; 53: 5906-5914.
- Limón ID, Díaz A, Mendieta L, Chamorro G, Espinosa B, Zenteno E, et al. Amyloid-beta(25-35) impairs memory and increases NO in the temporal cortex of rats. Neurosci Res. 2009; 63: 129-137.
- Szymanski M, Wang R, Fallin MD, Bassett SS, Avramopoulos D. Neuroglobin and Alzheimer's dementia: genetic association and gene expression changes. Neurobiol Aging. 2010; 31: 1835-1842.
- Cacciatore I, Baldassarre L, Fornasari E, Cornacchia C, Di Stefano A, Sozio P, et al. (R)-a-Lipoyl-Glycyl-L-Prolyl-L-Glutamyl dimethyl ester codrug as a multifunctional agent with potential neuroprotective activities. Chem Med Chem. 2012; 7: 2021-2029.
- Massoud F, Gauthier S. Update on the pharmacological treatment of Alzheimer's disease. Curr Neuropharmacol. 2010; 8: 69-80.
- Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006; 23: 2611-2622.
- Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer's disease: preclinical evidence. Int J Geriatr Psychiatry. 2003; 18: S23-32.
- Sozio P, Cerasa LS, Laserra S, Cacciatore I, Cornacchia C, Di Filippo ES, et al. Memantine-sulfur containing antioxidant conjugates as potential prodrugs to improve the treatment of Alzheimer's disease. Eur J Pharm Sci. 2013; 49: 187-198.
- Lee S, Zheng X, Krishnamoorthy J, Savelieff MG, Park HM, Brender JR, et al. Rational design of a structural framework with potential use to develop chemical reagents that target and modulate multiple facets of Alzheimer's disease. J Am Chem Soc. 2014; 136: 299-310.
- Mounsey RB, Teismann P. Chelators in the treatment of iron accumulation in Parkinson's disease. Int J Cell Biol. 2012; 2012: 983245.
- Perez CA, Tong Y, Guo M. Iron Chelators as Potential Therapeutic Agents for Parkinson's Disease. Curr Bioact Compd. 2008; 4: 150-158.
- Man BY-W, Chan H-M, Leung C-H, Chan DS-H, Bai L-P, Jiang Z-H, et al. Group 9 metal-based inhibitors of Β-amyloid (1-40) fibrillation as potential therapeutic agents for Alzheimer's disease. Chem Sci. 2011; 2: 917-921.
- Ma DL, He HZ, Leung KH, Chan DS, Leung CH. Bioactive luminescent transition-metal complexes for biomedical applications. Angew Chem Int Ed Engl. 2013; 52: 7666-7682.
- Ma DL, Chan DS, Ma VP, Leung KH, Zhong HJ, Leung CH. Current advancements in Aβ luminescent probes and inhibitors of Aβ aggregation. Curr Alzheimer Res. 2012; 9: 830-843.
- Bongarzone S, Bolognesi ML. The concept of privileged structures in rational drug design: focus on acridine and quinoline scaffolds in neurodegenerative and protozoan diseases. Expert Opin Drug Discov. 2011; 6: 251-268.
- Kayyali R, Pannala AS, Khodr H, Hider RC. Comparative radical scavenging ability of bidentate iron (III) chelators. Biochem Pharmacol. 1998; 55: 1327-1332.
- Dickens MG, Franz KJ. A prochelator activated by hydrogen peroxide prevents metal-induced amyloid Beta aggregation. Chembiochem. 2010; 11: 59-62.
- Cacciatore I, Cornacchia C, Fornasari E, Baldassarre L, Pinnen F, Sozio P, et al. A glutathione derivative with chelating and in vitro neuroprotective activities: Synthesis, physicochemical properties, and biological evaluation. Chem Med Chem. 2013; 8: 1818-1829.
- Faller P, Hureau C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-beta peptide. Dalton Trans. 2009; 1080-1094.
- Cacciatore I, Fornasari E, Baldassarre L, Cornacchia C, Fulle S, Di Filippo ES, et al. A potent (R)-alpha-bis-lipoyl derivative containing 8-hydroxyquinoline scaffold: Synthesis and biological evaluation of its neuroprotective capabilities in SH-SY5Y human neuroblastoma cells. Pharmaceuticals 2013; 6: 54-69.