
Review Article
Austin Alzheimers J Parkinsons Dis. 2015; 2(1): 1023.
Depression Preceding Parkinson’s Disease Onset
Nagayama H* and Kimura K
Department of Neurological Science, Graduate School of Medicine, Japan
*Corresponding author: Hiroshi Nagayama, Department of Neurology, Nippon Medical School, 1-1-5 Sendagi Bunkyo-ku, Tokyo 113-8603, Japan
Received: February 26, 2015; Accepted: August 08, 2015; Published: August 13, 2015
Abstract
Parkinson’s disease (PD) is characterized by many non-motor symptoms, some of which appear prior to motor symptom onset. This preceding period is called the preclinical stage, and some symptoms such as depression are observed during this period. In this report, we review the characteristics of depression observed during the preclinical stage of PD. We included studies retrieved from a MEDLINE search of the following: (1) published between 1970 and March 2014, (2) included Medical Subject Heading (MESH) terms including Parkinson’s disease and MESH terms for depression. There were 18 reports that mentioned preclinical depression or depression-related disorders in PD. Most of these reports were case-control studies and a few were cohort studies; there were no prospective studies. PD diagnosis criteria were variable, and some reports did not include these criteria. The diagnosis of depression was also variable, and was based on questionnaires, interviews, medical record reviews, and database searches. Most of these reports indicated that depression preceding PD onset was a significant risk factor for developing PD, with odds ratios of 1.50–3.40. In particular, a history of depression within 5 years of PD diagnosis correlated with PD development. These results should be interpreted with caution because the criteria for depression and PD varied among studies; however, depression preceding the onset of PD may be a risk factor for developing PD.
Keywords: Parkinson’s disease; Depression; Preclinical stage; Serotonin
Abbreviations
PD: Parkinson’s Disease; NMS: Non-motor Symptom; MESH: Medical Subject Heading; DSM: Diagnostic and Statistical Manual of Mental Disorders; H-ICDA: Hospital International Classification of Diseases Adapted; ICPC: International Classification of Primary Care; ICD: International Classification of Disease; 5-HIAA: 5-hydroxyindoleacetic Acid
Introduction
Symptoms other than motor symptoms have recently been characterized in Parkinson’s disease (PD): non-motor symptoms (NMSs). Some NMSs appear prior to the onset of motor symptoms, such as a kinesis, tremor, and rigidity [1]. The onset of PD is usually marked by the onset of motor symptoms; therefore, the preceding period between the onset of non-motor and motor symptoms is called the preclinical stage. Some of the NMSs observed in the preclinical stage of PD are constipation, hyposmia, rapid eye movement (REM) sleep behavior disorder (RBD), and depression [2].
Psychotic symptoms are reportedly one of the main NMSs observed in PD. In 1913, the correlation between PD and personality was studied [3]; most patients with PD were diligent and socially respectable people. Subsequently, the personality of patients with PD was described as depressive, introversive, and prudent [4–10]. These personality traits likely became apparent during the preclinical stage of PD. Patrick and Levy first reported on depression in patients having PD in 1922 [11]. Today, it is known that the prevalence of depression in patients with PD is higher than that in the general population, and the main symptom is a hedonic [12]. Depression and PD may share a common pathophysiological mechanism [13- 15]. Moreover, some reports suggested that a history of depression is related to developing PD [16-32]. In this report, we review whether preclinical depression may be a risk factor for developing PD, and how preclinical depression is related to the pathophysiological mechanisms involved in developing PD.
Literature search
Our literature search encompassed psychological terms meaning depression because the criteria and/or definition of depression as changed over time. This review will focus only on research that examined the association between PD and preceding depression. We determined whether previous review reports existed regarding the association between preclinical depression and PD by executing a MEDLINE search for all reviews of PD and risk factors from 1970 to March 2014. Results of reviews that met the assessment criteria are reported along with additional relevant results. We used the following Medical Subject Heading (MESH) terms in our search: Parkinson’s disease and one or more of the following, including depression, depressive disorder, affective disorder (psychotic), bipolar disorder, and adjustment disorder. We included reports that clearly mentioned preclinical depression in the examined PD cohort; however, clinical diagnostic criteria for depression or PD were irrelevant. If depression was indicated in the report even just a little, we adopted the reports as widely as possible, and the contents were commented respectively.
Outline of previous reports
There were 18 reports that mentioned depression or disorders including depression preceding PD onset [16–33]; one was a review [33]. The limited number of reports may be due to difficulty evaluating events that precede PD onset. The oldest report was from 1972. However, most reports were from the 1990s to the early 2000s. The review [33] systematically and precisely covered many studies; however, it did not include all the studies that we included in this report. Most previous reports were case-control studies [16–28], a few were cohort studies [29–32], and there were no prospective studies. In most of the case-control studies, PD patients were enrolled in the each hospital or region, and the risk of depression for the developing PD was compared with sex and age matched controls. The cohort study used a regional registration system/database(e.g., general practitioner/public hospital system or National Health Insurance Research Database), which contained10,000 to more than 100,000 subjects to calculate the prevalence of a history of depression in patients with PD [29,31] or the prevalence of newly developed PD in patients with depression [30,32]. However, even in the latter studies, the prevalence of newly developed PD was determined retrospectively in a patient cohort diagnosed with depression during a certain period (i.e., not a prospective study). The control group in these studies was a non-PD group of the same cohort; however, the control group in one study consisted of individuals diagnosed with another disease, such as diabetes mellitus or osteoarthritis [29].
The PD diagnostic methods were variable. Explicit diagnostic criteria were used in several reports [22-24,26]: Criteria for Diagnosing Parkinson’s Disease described by Calne [34,22], British Brain Bank criteria for the clinical diagnosis of PD[23,26,35,36], and Research diagnostic criteria for Parkinson’s disease described by Ward and Gibb [37,24]. In some studies, diagnostic criteria were included [16,17,19,21,28,29,32] or a diagnosis was made based on the combination of cardinal symptoms included in other reports [18,20,25,27,30,31].
Depression diagnostic methods were also variable. A history of depression was detected by a lack of unification, as determined using questionnaires [20-22], interviews [16-18,24,26,28], medical record reviews [19,23,25], and hospital or regionally based registration database searches[29-32]. A few reports evaluated depression and other psychiatric disorders (i.e., disorders that should be clearly distinguished from depression) as a single group. Kessler [16,17] and Raj put et al. [19] described some psychiatric disorders, including depression, as a nervous breakdown or psychoneurosis/ psychosomatic disorder, respectively. In the former, the term nervous breakdown included a wide range of psychiatric disorders, such as a manic state and neurosis. In the latter, psychoneurosis/psychosomatic disorders included hysteria and irritable bowel syndrome, in addition to depression. Diagnostic criteria of these psychiatric disorders, including depression, were not described.
Questionnaire and interview results were retrospectively evaluated and classified according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the Hospital International Classification of Diseases Adapted (H-ICDA) [38] in some reports [18,25,27,28,32]. However, the remaining reports [20-22,24,26] simply stated whether the questionnaire or interview results indicated the presence of depression, without any explicit criteria. Disorders (e.g., depression, mood disorder, or depressive state) differed among most database cohort studies [23,29-31], even though objective disorders were classified according to the International Classification of Primary Care (ICPC) [39] or International Classification of Disease (ICD). Furthermore, Yang et al. reported a correlation between developing PD and a history of antidepressant administration [21]. Therefore, because the diagnosis of depression and the meaning of the term “depression” were diverse, the results of these previous reports should be interpreted with caution.
The risk of developing PD in patients with a history of depression
Past reports were divided into two types. The first group included reports in which the main objective was to determine epidemiological factors involved in developing PD, and where psychiatric symptoms, including depression, were evaluated as a part of these factors [16,17,19-22,24,27]. The second group comprised reports that only focused on psychiatric disorders surrounding the depression, and where the relationship between the risk of developing PD and a history of psychiatric depression was evaluated [18,23,25,26,28-32].
A. Reports that evaluated depression as an epidemiological factor related to developing PD: Most of these reports were casecontrol studies from the 1970s–1990s. The odds ratio summary is shown in (Figure 1). Some of these studies evaluated disorders other than depression, as mentioned above [16,17,19]; therefore, we excluded them in (Figure 1). These four out of five of reports, indicated that a history of depression was a significant risk factor for developing PD (odds ratio: 1.54–3.40) [20-22,24,27]. Behari et al. divided their cohort into two groups according to the preceding period of depression before the onset of PD, and a history of depression was a significant risk factor only in the group whose preceding period was 10 years or shorter [27].
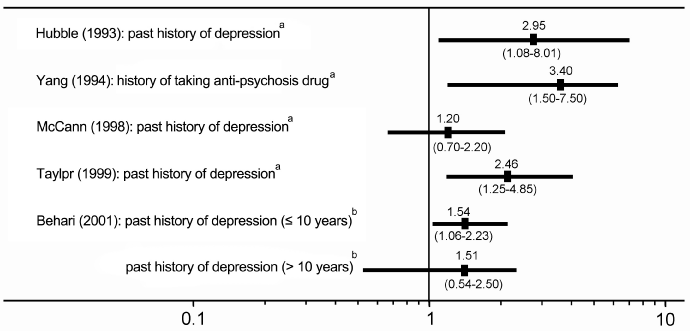
Figure 1: History of depression is a risk factor for developing Parkinson’s
disease (PD) [1].
Summary of reports that evaluated depression as one of the epidemiological
factors related to developing to Parkinson’s disease. The results of five
reports are shown [Reference No: 20-22,24,27]. The results from reports that
contained disorders other than depression were excluded. All five reports
were case-control studies; age- and sex-adjusted odds ratios and 95%
confidence intervals are shown. Diagnosis was based on questionnaires/
interviews (a) and the Diagnostic and Statistical Manual of Mental Disorders
(DSM)-III (b).
How important depression is as a risk factor? Although the risk factors examined differed among studies, common risk factors found in more than two studies were: (1) family history of PD and (2) depression. A family history of PD was a significant risk factor in all five reports, with an odds ratio of 2.88–9.98. Moreover, common protective factors found in more than two studies were: (1) smoking and (2) the use of well water. Smoking was a significant protective factor in three out of four reports, with an odds ratio of 0.19–0.99. One report suggested that the use of well water was a risk factor for developing PD [27]; conversely, the use of well water was a significant protective factor in two out of four reports, with an odds ratio of 0.70–0.96.
As mentioned above, although the definition of or criteria for depression varied among studies, many reports suggested that a history of depression may be an important risk factor for developing PD.
B. Reports that evaluated depression in the preclinical PD phase: Most of these reports were published in the early 2000s, and almost all were cohort studies. In the seven reports published after the year 2000 [25,26,28-32], the depression diagnosis was based on DSM-III or ICPC criteria. The odds ratio summary is shown in (Figure 2). In six out of seven of these reports, preceding depression was a significant risk factor for developing PD, with an odds ratio of 1.5–3.24. However, as described above, one study included a control group that had diseases such as diabetes mellitus and osteoarthritis [29]. There was a only one report that found no significance between the preceding depression and developing PD, however, this report only included patients that had a major depressive episode according to the DSM-III [28].
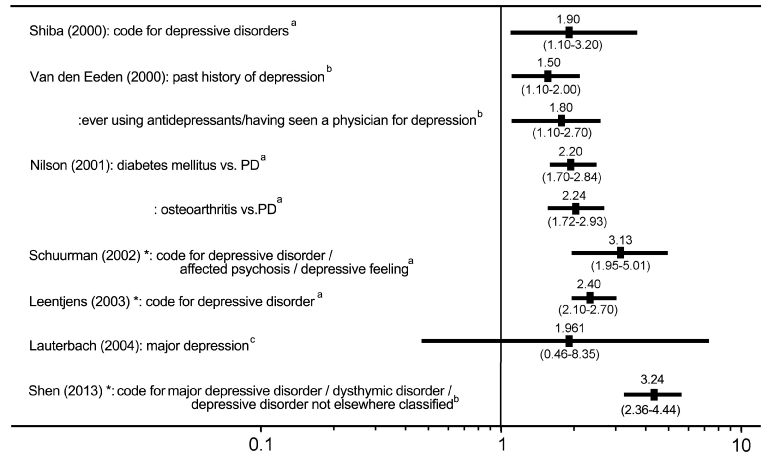
Figure 2: History of depression as a risk factor for developing Parkinson’s
disease [2].
Summary of reports that evaluated depression in the preclinical phase of
Parkinson’s disease. The result of seven reports in which the odds ratios
were calculated are shown [Reference No: 25,26,28-32]. These reports
include cohort (*) and case control studies. Age- and sex-adjusted odds ratios
and 95% confidence intervals are shown. Diagnosis was based on the code
for specific criteria (a), interview (b), and DSM-III (c).
Characteristics of depression that preceded PD development
As mentioned above, it is difficult to determine common characteristics of preceding depression because of the variations in previous studies. However, we will discuss common characteristics of preceding depression that were reported in multiple previous studies.
A. Latency b period between the onset of depression and onset of PD: A more recently onset of depression tended to have significant correlation with PD development [25,27,31]. Shiba et al. reported that a history of depression was a significant risk factor for developing PD; however, if the latency was greater than 5, 10, or 20 years, a significant correlation was not observed [25]. A relatively large number of patients had a latency of 5 years or less between the onset of depression and the onset of PD; half of patients were depressed within the previous 10 years (Figure 3). Leentjens also reported that the mean lag time between the onset of depression and the onset of PD was 10.1±10.4 (standard deviation) years (1 month to 36years) [31]; however, there were 7, 10, and 14 patients with 1, 2, and 3 year lag times. Therefore, the majority of patients had a shorter lag time. Behari et al. reported that depression preceding the onset of PD by less than 10 years was a significant risk factor for developing PD; however, a depression diagnosis that preceded PD onset by more than 10 years was not (Figure 1). These results indicate that a history of depression, particularly close in time to PD onset (less than about 5 years), correlated with PD development.
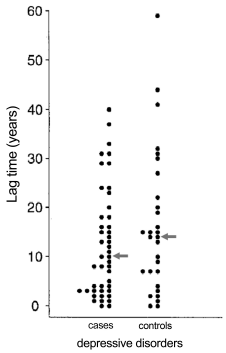
Figure 3: Lag time between the onset of depression and Parkinson’s disease
motor symptoms [25].
The Hospital International Classification of Diseases Adapted (H-ICDA)
classified the type of depression as depressive disorder. The control group
consisted of age- and sex-matched patients without Parkinson’s disease who
had the same depressive disorder. An arrow denotes the median. The peak
number of patients experiencing Parkinson’s disease symptoms occurred
within 5 years after the onset of depression.
B. The relationship between depression and dementia: Patients having PD with low mini-mental state examination (MMSE) scores tended to have depression [40]. Therefore, dementia should be evaluated when depression is discussed in PD. However, only two reports of preceding depression evaluated/excluded dementia [19,23]. In both reports, preceding depression was a significant risk factor regardless of the presence or absence of dementia. Although it is difficult to conclude based solely on these two reports, dementia likely has little influence on depression preceding the onset of PD.
C. The influence of medication for the treatment of depression: Some antidepressants, such as tricycle antidepressants, induce Parkinsonism. One report evaluated the correlation between past antidepressant use and developing PD [21]. Shiba et al. also reported the influence of a history of depression medication usage on developing PD [25]. In this report, the prevalence of a medication history of was higher in controls than in cases (48.3% vs. 36.2), and therefore they concluded that a depression-medication history did not influence the development of PD. However, it is difficult to make a conclusion based on a single report.
D. The correlation between preceding other psychiatric disorders and development PD: Many reports indicated that a history of depression was a significant risk factor for developing PD. Are psychiatric disorders other than depression also a risk factor, or is this a specific phenomenon in depression? It is very important to consider other psychiatric disorders when attempting to elucidate the common etiology between PD and depression.
A few reports examined the influence of a history of other psychiatric disorders on developing PD. However, the disorders evaluated varied among studies, including: psychosis (schizophrenia, manic-depressive state, paranoia, and hallucinatory psychosis) [19], personality disorders [19,25], anxiety [25,28], phobia [28], bipolar disorders [25,28], somatoform disorders [25], obsessive disorders [29], and alcohol abuse [28]. In these disorders, preceding anxiety [25] and some types of phobia [28] were related to developing PD. The latter study included few patients and lacked precise evaluation [28]; however, preceding anxiety, particularly of recent onset, was significantly related to later PD onset in the former report [25]. However, it is difficult to conclude regarding these psychiatric disorders, as the terms and criteria (i.e., manic-depressive state vs. bipolar disorders) changed according to the study date, and the number of enrolled patients was limited.
Hypothesis for the etiology of depression preceding the onset of PD
A. Monoamine hypothesis: The biochemical assessment of depression began after antidepressants were introduced clinically. In the 1950s, an agent with anti depressive properties was discovered by chance [41,42]: tricycle antidepressants and monoamine oxidase inhibitors were subsequently introduced clinically. These drugs typically mimic the effects of serotonin and nor epinephrine [41,42]; therefore, the monoamine hypothesis was introduced in the 1960s. It is now known that abnormalities in monoamines (e.g., serotonin) are not the singular cause for the path physiology of depression. However, serotonin abnormalities are a major cause of depression, as selective serotonin reuptake inhibitors (SSRIs) are effective for the treatment of depression.
The serotonin level was reportedly reduced in PD, regardless of the presence of depression [43]. Furthermore, 5-hydroxyindoleacetic acid (5-HIAA) was reduced in the cerebrospinal fluid of patients having PD with depression (Figure 4) [44]. Therefore, some reports suggested that serotonin abnormalities caused the preceding depression [18,27,30].
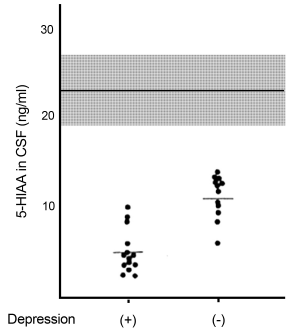
Figure 4: 5-Hydroxyindoleacetic acid (5-HIAA) concentrations in the
cerebrospinal fluid (CSF) of patients with Parkinson’s disease and depression
[44].
Serotonin was reduced in patients with Parkinson’s disease. However, the
concentration of 5-HIAA, a serotonin metabolite, was more reduced in the
cerebrospinal fluid of patients with Parkinson’s disease and depression than
in those without depression. The mean and normal ranges of control are
represented by the horizontal line and gray zone, respectively.
B. Preclinical phase of PD: Because serotonin abnormalities are a potential cause of preceding depression, the relationship between serotonin abnormalities and the PD preclinical pathophysiological state should be considered. A lag of 5 years between the onset of depression and PD strongly correlated with development of PD. It is unclear what change advances the preclinical stage of PD. In 1983, Calne and Langston reported a hypothesis regarding preclinical degeneration in PD [45]. This hypothesis suggested that the number of dopaminergic neurons is gradually reduced according to age after nigrostriatal dopaminergic neurons are damaged (e.g., by environmental toxin exposure). Then, when the number of neurons was reduced to less than 20–30%, clinical symptoms of PD appeared. According this hypothesis, the PD preclinical period occurred over a relatively long period. The same results were found in [18F]-dopa positron emission tomography (PET) studies in the 1990s, and the preclinical period was considered to last about 40 years [46,47]. However, with advances in device precision, PET studies published after the late 1990s suggested that the preclinical period was 5–10 years [48,49]. This preclinical period was likely responsible for the 5-year lag between the onset of depression and the onset of PD [25].
C. Preclinical period and serotonergic damage: Recently, Braak et al. classified pathological features of the PD brain according to the distribution of Lewy bodies (i.e., Braak stages) [50]. Braak stage 3 is characterized by pathological changes that extend into the midbrain, and the emergence of motor symptoms. Stages 1 and 2 are pre symptomatic. Pathological changes are limited to the medulla oblongata and olfactory bulb in stage 1. The changes progress to involve the raphe nuclei, gig a to cellular reticular nucleus, and locus coeruleus in stage 2. Because the raphe nuclei are the serotonergic nuclei of origin, these changes may cause preceding depression before PD development. However, it is unlikely that this is the only cause of depression preceding PD development because the prevalence of developing PD following depression is very low in comparison with the degree of raphe nuclei damage.
Conclusion
Many reports suggested that depression preceding the onset of PD was a significant risk factor for developing PD, with odds ratios of 1.50–3.40. In particular, a history of depression within 5 years prior to PD onset correlated with PD development. However, these results must be interpreted with caution, because the diagnostic criteria for depression and PD varied among studies, as did the enrolled patients and controls. Furthermore, half of the reports were case-control studies, and there were no prospective studies.
It is unclear if depression and PD share a common pathophysiological mechanism or not. Although it may be difficult to systematically interpret there ports of depression preceding PD development, preceding depression and PD may share a common pathophysiological mechanism, as many report a positive relationship.
References
- de Lau LM, PJ, Hofman A, Breteler MM. Subjective complaints precede Parkinson disease: the Rotterdam study. Arch Neurol. 2006; 63: 362-365.
- Tolosa E, Compta Y, Gaig C. The premotor phase of Parkinson’s disease. Parkinsonism Relat Disord. 2007; 13: 2-7.
- Camp CD. Paralysis Agitans, multiple sclerosis and their treatment. White WA, Jelliffe SE, Kimpton H, editors. In: Modern treatment of nervous and mental disease. Lea &Febiger, 1913; 651-657.
- Glosser G, Clark C, Freundlich B, Kliner-Krenzel L, Fla-herty P, Stern M. A controlled investigation of current and premorbid personality:characteristics of Parkinson ’s disease patients. Mov Disord. 1995; 10: 201-206.
- Fujii C, Harada S, Ohkoshi N, Hayashi A, Yoshizawa K. Cross-cultural traits for personality of patients with Parkinson ’s disease in Japan. Am J Med Genet. 2000; 96: 1-3.
- Hubble JP, Venkatesh R, Hassanein RE, Gray C, Koller WC. Personality and depression in Parkinson ’s disease. J Nerv Ment Dis. 1993; 181: 657-662.
- Jacobs H, Heberlein I, Vieregge A, Vieregge P. Personality traits in young patients with Parkinson ’s disease. Acta Neurol Scand. 2001; 103: 82-87.
- Eatough VM, Kempster PA, Stern GM, Lees AJ. Premorbid personality and idiopathic Parkinson’s disease. Adv Neurol. 1990; 53: 335-337.
- Poewe W, Karamat E, Kemmler GW, Gerstenbrand F. The premorbid personality of patients with Parkinson’s disease: a comparative study with healthy controls and patients with essential tremor. Adv Neurol. 1990; 53: 339-342.
- Smythies JR. The previous personality in Parkinsonism. J Psychosom Res. 1967; 11: 169-171.
- Patrick HT, Levy DM. Parkinson’s disease: a clinical study of 146 cases. Arch Neurol Psychiatry. 1922; 7: 711-720.
- Lemke MR. Depressive symptoms in Parkinson's disease. Eur J Neurol. 2008; 15: 21-25.
- Gareri P, De FP, De SG. Neuropharmacology of depression in aging and age-related diseases. Ageing Res Rev. 2002; 1: 113-134
- Robinson RG, Chemerinski E, Jorge R. Pathophysiology of secondary depressions in the elderly. J Geriatr Psychiatry Neurol. 1999; 12: 128-136.
- Taylor AE, Saint-Cyr JA, Lang AE, Kenny FT. Parkinson’s disease and depression. A critical re-evaluation. Brain. 1986; 109: 279-292.
- Kessler II. Epidemiologic studies of Parkinson's disease. II. A hospital-based survey. Am J Epidemiol. 1972; 95: 308-318.
- Kessler II. Epidemiologic studies of Parkinson's disease. 3. A community-based survey. Am J Epidemiol. 1972; 96: 242-254.
- Santamara J, Tolosa E, Valles A. Parkinson's disease with depression: a possible subgroup of idiopathic parkinsonism. Neurology. 1986; 36: 1130-1133.
- Rajput AH, Offord KP, Beard CM, Kurland LT. A case-control study of smoking habits, dementia, and other illnesses in idiopathic Parkinson's disease. Neurology. 1987; 37: 226-232.
- Hubble JP, Cao T, Hassanein RE, Neuberger JS, Koller WC. Risk factors for Parkinson's disease. Neurology. 1993; 43: 1693-1697.
- Yang J, Wu Z, Lou X. A case-control study on risk factors in etiology of Parkinson's disease. Zhonghua Liu Xing Bing Xue Za Zhi. 1994; 15: 6-9.
- McCann SJ, LeCouteur DG, Green AC, Brayne C, Johnson AG, Chan D, et al. The epidemiology of Parkinson's disease in an Australian population. Neuroepidemiology. 1998; 17: 310-317.
- Gonera EG, van't Hof M, Berger HJ, van Weel C, Horstink MW. Symptoms and duration of the prodromal phase in Parkinson's disease. Mov Disord. 1997; 12: 871-876.
- Taylor CA, Saint-Hilaire MH, Cupples LA, Thomas CA, Burchard AE, Feldman R. Environmental, medical, and family history risk factors for Parkinson's disease: a New England-based case control study. Am J Med Genet. 1999; 88: 742-749.
- Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Anxiety disorders and depressive disorders preceding Parkinson's disease: a case-control study. Mov Disord. 2000; 15: 669-677.
- Van den Eeden SK, Tanner CM, Popat RA, Bernstein AL, Nelson LM. The risk of Parkinson’s disease associated with head injury and depression: a population-based case control study. Neurology. 2000; 54: A347.
- Behari M, Srivastava AK, Das RR, Pandey R. Risk factors of Parkinson's disease in Indian patients. J Neurol Sci. 2001; 190: 49-55.
- Lauterbach EC, Freeman A, Vogel RL. Differential DSM-III psychiatric disorder prevalence profiles in dystonia and Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2004; 16: 29-36.
- Nilsson FM, Kessing LV, Bolwig TG. Increased risk of developing Parkinson's disease for patients with major affective disorder: a register study. Acta Psychiatr Scand. 2001; 104: 380-386.
- Schuurman AG, van den Akker M, Ensinck KT, Metsemakers JF, Knottnerus JA, Leentjens AF, et al. Increased risk of Parkinson's disease after depression: a retrospective cohort study. Neurology. 2002; 58: 1501-1504.
- Leentjens AF, Van den Akker M, Metsemakers JF, Lousberg R, Verhey FR. Higher incidence of depression preceding the onset of Parkinson's disease: a register study. Mov Disord.2003; 18: 414-418.
- Shen CC, Tsai SJ, Perng CL, Kuo BI, Yang AC. Risk of Parkinson disease after depression: a nationwide population-based study. Neurology. 2013 22; 81: 1538-1544.
- Ishihara L, Brayne C. A systematic review of depression and mental illness preceding Parkinson's disease. Acta Neurol Scand. 2006; 113: 211-220.
- Calne DB, Snow BJ, Lee C: Criteria for diagnosing Parkinson’s disease. Ann Neurol. 1992; 32: 125-S127.
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988; 51: 745-752.
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55: 181-184.
- Ward CD, Gibb WR. Research diagnostic criteria for Parkinson's disease. Adv Neurol. 1990; 53: 245-249.
- Commission on Professional and Hospital Activities. Hospital Ad-aptation of ICDA-H-ICDA, 2nd ed. Ann Arbor, MI: Commission on Professional and Hospital Activities. 1973.
- Lamberts H,Wood M. International classification of primary care. Oxford: Oxford University Press; 1987; 19: 433-435
- Tandberg E, Larsen JP, Aarsland D, Cummings JL: The occurrence of depression in Parkinson’s disease. Arch Neurol. 1996; 53: 175-179.
- Slattery DA, Hudson AL, Nutt DJ. Invited review : the evolution of antidepressant mechanisms. Fundam Clin Pharmacol. 2004; 18: 1-21.
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiologyof depression.Neuron.2002; 34: 13-25.
- Birkmayer W, Birkmayer JD. Dopamine action and disorders of neurotransmitter balance. Gerontology. 1987; 33: 168-171.
- Kostic VS, Djuricic BM, Covickovic-Sternic N, Bumbasirevic L, Nikolic M, Mrsulja BB. Depression and Parkinson's disease: possible role of serotonergic mechanisms. J Neurol. 1987; 234: 94-96.
- Calne DB, Langston JW. Aetiology of Parkinson's disease. Lancet. 1983; 2: 1457-1459.
- Bhatt MH, Snow BJ, Martin WR, Pate BD, Ruth TJ, Calne DB. Positron emission tomography suggests that the rate of progression of idiopathic parkinsonism is slow. Ann Neurol. 1991; 29: 673-677.
- Vingerhoets FJ, Snow BJ, Lee CS, Schulzer M, Mak E, Calne DB. Longitudinal fluorodopa positron emission tomographic studies of the evolution of idiopathic parkinsonism. Ann Neurol. 1994; 36: 759-764.
- Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson's disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry. 1998; 64: 314-319.
- Hilker R, Schweitzer K, Coburger S, Ghaemi M, Weisenbach S, Jacobs AH, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol. 2005; 62: 378-382.
- Braak H, Ghebremedhin E, Rb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004; 318: 121-134.