
Research Article
Austin Alzheimers J Parkinsons Dis. 2023; 6(1): 1036.
Beneficial Effects of Ginkgoselect™ Phytosome™ on Cognitive Impairment and Oxidative Stress in Mild-Moderate Dementia
Ferrara S¹; Borgognone M²; Petrangolini G³; Antonella Riva³*
¹UOSD Centro Alzheimer Azienda Sanitaria Provinciale (ASP) Siracusa, Italy
²PL Pharma Srl, Italy
³Department of Research and Development, Indena SpA, Italy
*Corresponding author: Riva A Department of Research and Development, Indena SpA, Viale Ortles 12 - 20139 Milan, Italy. Tel: +39 02 57496 237 Email: antonella.riva@indena.com
Received: March 06, 2023 Accepted: April 06, 2023 Published: April 13, 2023
Abstract
In this study, we investigated for the first time the effects of a standardized extract from Ginkgo biloba leaves formulated with phospholipids Ginkgoselect™ Phytosome™, GBP) in combination with Standard Management (SM) in mild-moderate Alzheimer’s Disease (AD), focusing on the modulation of subject’s cognitive ability and oxidative status.
Participants with cognitive impairment freely decided to continue their SM protocol (donepezil 10 mg/day, rivastigmine 4.6 mg/day in patch and memantine 20 mg/day; n=37), or to receive GBP tablets 120 or 240 mg/day in combination with SM (n=43). Subjects were assessed at baseline and after 6 months for cognitive functions and exclusive mental abilities through specific neuropsychological tests, namely the Mini Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). We also evaluated the oxidative status through the determination of plasmatic derived reactive oxygen metabolites and anti-oxidant reserve through Plasma Antioxidant Test.
After 6 months of supplementation with GBP to SM, subjects reported a significantly higher improvement in cognitive and neuropsychological functions, compared to those receiving only SM. Moreover, participants in the GBP with SM group reported a more remarkable decrease in oxidative load and an increase in the concentration of antioxidant molecules compared to the control group.
Supplementation with GBP to SM demonstrates a positive effect in subjects with AD, helping them maintain or even increase their cognitive abilities, and improving their oxidative status both by reducing oxidative damage and by increasing the antioxidant reserve.
Keywords: Ginkgoselect phytosome; Cognition; Neuropsychological functions; Oxidative status; Anti-oxidant reserve
Introduction
Alzheimer’s Disease (AD) is the most common cause of dementia and one of the leading sources of morbidity and mortality in the aging population. It is a typical disease of the later years in life, roughly doubling in prevalence every 5 years after the age of 65 years and is significantly more common in women than men [1,2].
Classical symptoms of AD are difficulties with memory, language, problem-solving and other cognitive skills that, overall, limit subjects’ ability to perform everyday tasks and to self-care. The occurrence of these symptoms is associated with specific biological alterations in the brain, such as formation of neurofibrillary entanglement, senile plaques, amyloid deposition and synaptic loss [3]. The variable clinical presentation of AD is partially explained by the multifactorial nature of the disease, characterized by a complex interplay of genetic and environmental factors. The main recognized risk factors are advanced age, family history of the disease and presence of the APOE-e4 gene variant; however, other modifiable risk factors have been identified, such as cardiovascular diseases – which may affect cerebrovascular health – traumatic brain injury, diet and lifestyle, including education, social and cognitive engagement [2].
The most prevalent theory in AD pathogenesis is the β-amyloid (Aβ) hypothesis, which suggests that improperly cleaved Aβ precursor protein (βAPP) forms insoluble Aβ peptide aggregates in the brain, which eventually lead to neuronal damage and apoptosis [4]. Despite the important advances made in understanding the disease, no treatment, which can limit or stop the formation of these aggregates, has yet been developed. The only therapeutic options currently approved for mild to moderately severe AD are Acetylcholinesterase inhibitors (AChEI; donepezil, galantamine and rivastigmine) and the anti-glutaminergic memantine hydrochloride. These agents, however, are not curative but can only offer a temporary and symptomatic control of cognitive impairment, with variable responses and limited long-term effectiveness [2,5].
More recently, new research pathways have been explored to block the formation of Aβ plaques by targeting more upstream processes [6-9]. One of the most interesting aspects currently under investigation is the role of neuroinflammation, as elderly people with AD display a chronic state of inflammation in the brain, which may influence AD progression. The effects of neuroinflammation are two-fold: on one side, the inflammatory activation stimulates microglia cells to kill adjacent neurons through the production of Reactive Oxygen Species (ROS) and proteolytic enzymes. On the other side, the inflammatory mediators enhance the formation of Aβ plaques, which, in turn, stimulate the production of cytokines from microglia cells, thus contributing to the creation of a vicious cycle of chronic and sustained inflammation [10,11]. Several studies have also highlighted the association between AD and neuronal oxidative stress, which manifests as damage to multiple cellular structures, eventually resulting in neuronal cell death [12].
Over the last two decades, various anti-inflammatory and anti-oxidant approaches have been investigated to prevent or delay AD progression, leading to mixed results [12,13]. Traditional and complementary remedies, such as phytochemical and nutraceuticals, have generated promising results in ameliorating cognition and preventing AD thanks to their anti-oxidant, anti-inflammatory and neuroprotective properties [14,15].
Ginkgo biloba, an indigenous tree of eastern Asia, has largely been used in traditional medicine for its health-promoting properties. The extract of G. biloba leaves contains two families of molecules, terpene lactones and ginkgo flavone glycosides, which present anti-oxidant and free radical scavenger properties, thus inhibiting lipid peroxidation and increasing cellular antioxidant defense mechanisms [16]. Moreover, the anti-oxidant and anti-inflammatory properties of G. biloba extract are reported to be synergistic to improve neurodegeneration related to retinal diseases [15]. The suggested mechanism of action is based on the ability of flavonoids to scavenge different free radicals, including ROS, and also the reactive nitrogen species as Nitric Oxide (NO) and ferryl ion species [17,18]. Regarding triterpenes, bilobalide and ginkgolides are able to increase cell enzymatic anti-oxidant mechanisms [19]. G. biloba extract has indeed shown cardio-protective, anti-asthmatic, antidiabetic and potent central nervous system activities, improving cognitive functions in elderly subjects who demonstrate memory loss [16,20,21]. A standardized G. biloba leaves extract (Ginkgoselect™) has been also formulated with phospholipids, to provide a food-grade delivery system (Ginkgoselect™ Phytosome™, GBP), which increases the absorption and bioavailability of the ginkgo-active components [22,23].
In this study, we investigated for the first time the effects of GBP, administered in combination with standard treatment in patients with AD, focusing on the modulation of subject’s cognitive ability and oxidative status.
Material and Methods
Population and Study Design
Overall, 80 participants with cognitive impairment, treated for at least 6 months with AChEI or memantine were enrolled in the study at the Centro Alzheimer of Azienda Sanitaria Provinciale in Siracusa (Italy). All study participants continued their Standard Management (SM) protocol, consisting of the administration of donepezil 10 mg/day, rivastigmine 4.6 mg/day in patch and memantine 20 mg/day (group A), or in addition to SM were supplemented with GBP tablets 120 or 240 mg/day (group B). Ginkgoselect™ Phytosome™ (Indena SpA) was supplied as Agheron™ tablets (120 mg each) kindly donated by PL Pharma (Ponte San Nicolò (PD), Italy).
The study was approved by the local Ethics Committee. The study was carried out in accordance with the relevant guidelines of the Declaration of Helsinki (1964), its amendments and the general principles of ICH Harmonised Tripartite Guidelines for Good Clinical Practice (ICH Topic E6, CPMP/ICH/135/95). At the beginning of the study, the written informed consent was obtained from all individual participants included in the study.
Study Outcomes
Subjects were visited at baseline and after 6 months to assess cognitive functions and exclusive mental abilities through specific neuropsychological tests, namely the Mini Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). The MMSE is the best-known short screening tool for cognitive impairment; it examines functions including registration, attention and calculation, recall, language, ability to follow simple commands and orientation; scores can indicate severe (≤9 points), moderate (10–18 points) or mild (19–23 points) cognitive impairment [24]. The MoCA is a brief cognitive screening tool that encompasses eight cognitive domains (visuo-spatial, executive, naming, memory, attention, language, abstraction, delayed recall and orientation) and generates a total score of maximum 30 points; a score <26 is considered as having neurocognitive impairment [25].
In addition, subjects’ blood samples were collected both at baseline and after 6 months to evaluate the oxidative status, through the derived reactive oxygen metabolites (d-ROMs) test [26], and the anti-oxidant reserve (concentration of water-soluble antioxidants) through the Plasma Antioxidant Test (PAT) (Free Radical Analytical System [FRAS]) [27].
Statistical Analysis
All data were analyzed by descriptive statistics only, due to the pilot nature of the study. Furthermore, a specific analysis was also conducted to compare treatment response among females and males. We used the repeated measures two-way ANOVA (treatment, time) to evaluate response to treatment during the study, followed by Bonferroni test for “ad hoc” assessments. P values<0.05 on 2-sided test were considered as statistically significant.
Results
Overall, 80 subjects were included in the study; participants presented homogeneous characteristics in terms of age (mean age: 72.7 years; range: 62–76); sex (43 females and 37 males) and education (mean number of years of schooling 7.25; range 6–9).
A total of 37 participants (22 females and 15 males) were treated with SM only, while 43 (21 females and 22 males) were also supplemented with GBP (SM+GBP). Seven patients (five females and two males) in the SM group and seven patients (two females and five males) in the SM+GBP group discontinued treatment during the course of the study and were excluded from the analysis.
As shown in Figure 1, after 6 months of observation, subjects in the SM group reported a moderate decline in execution skills, as shown by the reduction of the MoCA mean score, which decreased from 12.63 to 12.26. Furthermore, no difference was recorded in the cognitive ability, as observed by the maintenance of the MMSE score. They also showed a considerable decrease in the concentration of water-soluble antioxidants, which were reduced from 1627.03 to 1589.86 units (1 unit: 1.4 μmol/L of ascorbic acid) (Figure 2). No significant change was reported in the d-ROMs test and the oxidative status remained in the moderate range (from 341 to 400 units; 1 unit: 0.08 mg H2O2/dl) both at baseline and after 6 months (Figure 3).
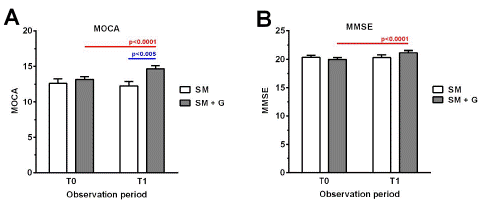
Figure 1: MMSE (A) and MoCA (MMSE (A) and MoCA (B) in participants treated with Standard Management vs standard management (SM)+GBP (G) at baseline (T0) and after 6 months of treatment (T1).) in participants treated with Standard Management vs standard management (SM)+GBP (G) at baseline (T0) and after 6 months of treatment (T1).
Conversely, subjects who were supplemented with also GBP reported beneficial effects in all the parameters under analysis. From a cognitive point of view, we observed an increase in both the MMSE (mean change from baseline vs 6 months: 19.97 vs 21.16) and the MoCA score (13.16 vs 14.66) (Figure 1A & B). This group also benefited from a reduction in the oxidative status, which decreased from 440.91 to 365.94 units (d-ROMs) (Figure 3), and showed a considerable improvement in the antioxidant reserve that changed from a status of high oxidative stress at baseline (151.44 units) to normal values after 6 months of treatment (2037.58 units; PAT) (Figure 2).
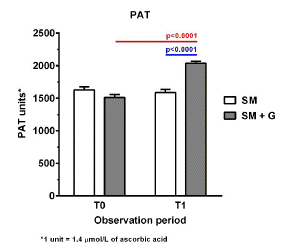
Figure 2: Antioxidant reserve (PAT) in participants treated with Standard Management (SM) vs Standard Management (SM)+GBP (G) at baseline (T0) and after 6 months of treatment (T1).
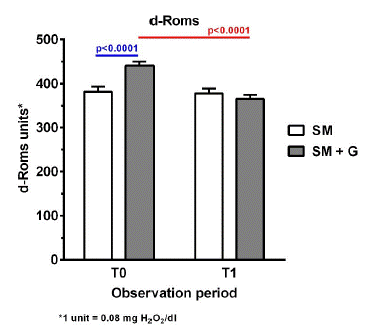
Figure 3: Oxidative stress (d-ROMS) in participants treated with Standard Management (SM) vs standard management (SM)+GBP (G) at baseline (T0) and after 6 months of treatment (T1).
The comparative analysis between females and males showed no relevant differences in response to treatment according to sex (Figure 4).
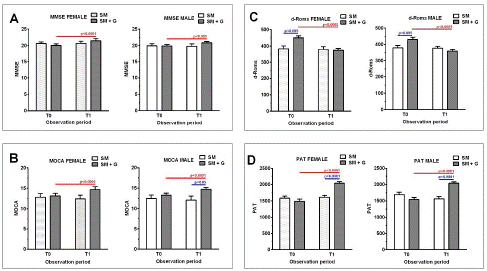
Figure 4: (A) MMSE score, (B) MoCA score, (C) d-ROMS results and (D) PAT results among female (left) and male (right) treated with Standard Management (SM) vs Standard Management (SM)+GBP (G) at baseline (T0) and after 6 months of treatment (T1).
Discussion
Overall, the results of this study show that the supplementation of Gingko biloba extract formulated in phospholipids is a highly beneficial tool in addition of the pharmacological standard therapy, demonstrated a positive effect in subjects with mild moderate AD, helping them maintain or even increase their cognitive abilities, and improving their oxidative status both by reducing oxidative damage and by increasing the antioxidant reserve.
The results of our study further confirm the limited effectiveness of currently available options in the treatment of AD. In this pilot study, we observed that patients treated with AChEI and memantine experienced a slight decline in execution skills measured by the MoCA, while cognitive abilities were substantially maintained after 6 months of treatment, according to the MMSE. The supplementation with GBP to the SM conversely showed positive results in terms of cognition, improving subjects’ status on both cognitive scales (Figure 4). Notably, previous studies have reported the beneficial effects of G. biloba leaves extract on cognition and memory both in healthy subjects and in patients with dementia [21,28,29]. In particular, a randomized controlled trial showed that the administration of G. biloba extract effectively improved cognition, neuropsychiatric symptoms and functional abilities in patients with AD or vascular dementia [30].
In terms of oxidative status, as shown by the d-ROMS and PAT results, standard treatment for AD has no considerable effect in reducing oxidative damage. After 6 months of SM, participants showed only a minor decrease in oxidative stress and a deterioration of the antioxidant reserve (Figures 2 & 3). On the other hand, the supplementation with GBP showed a remarkable decrease in the oxidative load, whose mean values were reduced from a condition of high (400–500 units; d-ROMs) to moderate (341–400 units; d-ROMs) oxidative stress. In addition, participants showed a substantial increase in the concentration of antioxidants, which after 6 months of treatment reached normal values (2000–2200 units; PAT). These positive results are associated with the antioxidant properties of GBP. The free radical scavenging activity of flavonoids contained in GBP may help to stabilize ROS, thus protecting neuronal cells from oxidative stress-induced damage, limiting AD progression. The importance of oxidative damage in the development and progression of AD has been suggested in multiple studies, and antioxidants have been recognized as part of the therapeutic strategies for AD treatment [12].
Of note, GBP presents additional important properties that may help prevent or limit AD progression. In particular it has been shown to promote blood flow and provide capillary protection, which might have a positive impact on brain vasculature, a fundamental component for a normal functioning brain [31].
The last thing to point out to our clinical study is certainly relative to women’s response. The increased prevalence of AD among women likely arises through a combination of factors, including sex chromosomes, hormones, brain structure, together with gender and life experiences [32]. These factors are being explored as part of a new direction in AD’s research. The use of GBP improved significantly cognitive abilities and antioxidant reserve without gender specific effects that is showing a beneficial support also in enrolled women.
No relevant side effects has been reported and it is very important considering that this supplementation should be chronic and in a frail population.
This study has some limitations. The first is the small sample size and the short supplementation period taking into consideration the chronic nature of degenerative dementia. Also a follow-up period is missing.
Regarding the strengths of this study, we should underline that GBP is a food supplement highly standardized and supported with a good bio-absorption profile of Ginkgo biloba principles. This more bioavailable food-grade delivery system in phospholipids could be potentially able to support the improvement of neurodegeneration when associated with the standard management.
Conclusion
The results of our study suggest that the use of GBP as adjuvant to current standard management is safe and may improve cognitive functionality in AD progression reducing both the oxidative load (d-ROMS) and the depletion of the antioxidant reserve (PAT). Further clinical studies, possibly randomized and double-blind, with a larger number of subjects, are though advised.
Author Statements
Conflicts of Interest
SF declares no conflict of interest. MB is employee of PL Pharma Srl. GP and AR are Indena’s employees.
Funding
This research did not receive any specific grant from funding agencies in the public or not-for-profit sectors.
Acknowledgement
We would like to express our gratitude to Dr. Paola Misiano, together with Polistudium srl in the person of Aashni Shah and Ambra Corti for their valuable editorial support; Dr. Mauro A.M Carai for his precise statistical analysis; Dr. Paolo Morazzoni for his scientific support in the revision of the clinical protocol.
References
- Prince M, Albanese E, Guerchet M, Prina M. World Alzheimer Report 2014: Dementia and Risk Reduction an Analysis of Protective and Modifiable Factors. 2014.
- Alzheimer’s Association. Alzheimer’s Association Report 2021.
- De Oliveira J, Kucharska E, Garcez ML, Rodrigues MS, Quevedo J, et al. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells. 2021; 10: 2581.
- Hampel H, Hardy J, Blennow K, Chen C, Perry G, et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol Psychiatry. 2021; 26: 5481–5503.
- Passeri E, Elkhoury K, Morsink M, Broersen K, Linder M, et al. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int J Mol Sci. 2022; 23: 13954.
- Grimaldi LME, Zappala G, Iemolo F, Castellano AE, Ruggieri S, et al. A pilot study on the use of interferon beta-1a in early Alzheimer’s disease subjects. J Neuroinflammation. 2014; 11: 30.
- Di Meco A, Li JG, Blass BE, Abou-Gharbia M, Lauretti E, et al. 12/15-Lipoxygenase inhibition reverses cognitive impairment, brain amyloidosis, and tau pathology by stimulating autophagy in aged triple transgenic mice. Biol Psychiatry. 2017; 81: 92–100.
- Mendiola-Precoma J, Berumen LC, Padilla K, Garcia-Alcocer G. Therapies for prevention and treatment of Alzheimer’s disease. Biomed Res Int. 2016; 2016: 2589276.
- Kulshreshtha A, Piplani P. Current pharmacotherapy and putative disease-modifying therapy for Alzheimer’s disease. Neurol Sci. 2016; 37: 1403–1435.
- Halliday G, Robinson SR, Shepherd C, Kril J. Alzheimer’s disease and inflammation: a review of cellular and therapeutic mechanisms. Clin Exp Pharmacol Physiol. 2000; 27: 1–8.
- Ho GJ, Drego R, Hakimian E, Masliah E. Mechanisms of cell signaling and inflammation in Alzheimer’s disease. Curr Drug Targets Inflamm Allergy. 2005; 4: 247–256.
- Jurcău MC, Andronie-Cioara FL, Jurcău A, Marcu F, Ţiț DM, et al. The Link between Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation in the Pathophysiology of Alzheimer’s Disease: Therapeutic Implications and Future Perspectives. Antioxidants (Basel). 2022; 11: 2167.
- Ardura-Fabregat A, Boddeke EWGM, Boza-Serrano A, Brioschi S, Castro-Gomez S, et al. Targeting neuroinflammation to treat Alzheimer’s disease. CNS Drugs. 2017; 31: 1057-1082.
- Cooper EL, Ma MJ. Alzheimer disease: Clues from traditional and complementary medicine. J Tradit Complement Med. 2017; 7: 380-385.
- Martínez-Solís I, Acero N, Bosch-Morell F, Castillo E, González-Rosende ME, et al. Neuroprotective Potential of Ginkgo biloba in Retinal Diseases. Planta Med. 2019; 85: 1292-1303.
- Naik SR, Pilgaonkar VW, Panda VS. Neuropharmacological evaluation of Ginkgo biloba Phytosomes in rodents. Phytother Res. 2006; 20: 901-5.
- Liu XP, Luan JJ, Goldring CE. Comparison of the antioxidant activity amongst Gingko biloba extract and its main components. Zhong Yao Cai. 2009; 32: 736-40.
- Wang J, Zheng M, Chen L, Liu Z, Zhang Y, et al. Rapid screening, separation, and detection of hydroxyl radical scavengers from total flavonoids of Ginkgo biloba leaves by chromatography combined with molecular devices. J Sep Sci. 2016; 39: 4158-4165.
- Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci. 2008; 73: R14–R19.
- Pietri S, Maurelli E, Drieu K, Culcasi M. Cardioprotective and anti-oxidant effects of the terpenoid constituents of Ginkgo biloba extract (EGb 761). J Mol Cell Cardiol. 1997; 29: 733-42.
- Gauthier S, Schlaefke S. Efficacy and tolerability of Ginkgo biloba extract EGb 761® in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clin Interv Aging. 2014; 9: 2065-77.
- Mauri PL, Simonetti P, Gardana C, Minoggio M, Morazzoni P, et al. Liquid chromatography/atmospheric pressure chemical ionization mass spectrometry of terpene lactones in plasma of volunteers dosed with Ginkgo biloba L. extracts. Rapid Commun Mass Spectrom. 2001; 15: 929–34.
- Carini M, Aldini G, Rossoni G, Morazzoni P, Facino RM. Complexation of Ginkgo biloba extract with phosphatidylcholine improves cardioprotective activity and increases the plasma antioxidant capacity in the rat. Planta Med. 2001; 67: 326-30.
- Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12: 189‐98.
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53: 695-699.
- Kilk K, Meitern R, Härmson O, Soomets U, Hõrak P. Assessment of oxidative stress in serum by d-ROMs test. Free Radic Res. 2014; 48: 883-9.
- Titov VN, Krylin VV, Dmitriev VA, Iashin IaI. Plasma antioxidant activity--a test for impaired biological functions of endoecology, exotrophy, and inflammation reactions. Klin Lab Diagn. 2010; 7: 3-14.
- Kaschel R. Specific memory effects of Ginkgo biloba extract EGb 761 in middle-aged healthy volunteers. Phytomedicine. 2011; 18: 1202-7.
- Nash KM, Shah ZA. Current perspectives on the beneficial role of Ginkgo biloba in neurological and cerebrovascular disorders. Integr Med Insights. 2015; 10: 1-9.
- Ihl R, Tribanek M, Bachinskaya N; GOTADAY Study Group. Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761® in Alzheimer’s disease and vascular dementia: results from a randomised controlled trial. Pharmacopsychiatry. 2012; 45: 41-6.
- D’Alessandro A, Caroli A, D’Alessandro A, Mandolesi D, Venosi S, et al. Utilizzo del Ginkgo biloba fitosoma nella malattia venosa cronica degli arti inferiori: studio pilota. Acta Phlebologica. 2015; 16: 83-92.
- Jessica L Podcasy, C Neill Epperson. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016; 18: 437-446.